Abstract
Background
High serum CA19-9 is usually caused by pancreaticobiliary malignancies, but it has also been found in a tiny minority of calculous cholecystitis patients.
Aims
To clarify the relationship between calculous cholecystitis and serum CA19-9.
Methods
Clinical data of calculous cholecystitis patients with high serum CA19-9 (high group, n = 20) and normal serum CA19-9 (normal group, n = 40) who underwent cholecystectomy were analyzed. Serum CA19-9 of high group were followed-up and gallbladder specimens were analyzed by immunohistochemistry.
Results
Serum CA19-9 in the high group ranged from 105 to 1635 U/ml, of which 30% exceeded 1000 U/ml. Follow-up results showed that 20 patient’s serum CA19-9 returned to normal after cholecystectomy, including 4 closely followed-up patients whose serum CA19-9 recovered within one month. Immunohistochemical results revealed that CA19-9 was mildly positive only in mucosal epithelial cells in the normal group, but positive in mucosal epithelial cells, vascular endothelial cells, and intercellular substances in the high group, accounting for high serum CA19-9.
Conclusion
Serum CA19-9 is proved to be associated with calculous cholecystitis for the first time, so that clinicians should consider calculous cholecystitis associated CA19-9 elevation in the clinic practice besides other CA19-9 related diseases.
Introduction
In 1979, CA19-9 was discovered in human colorectal cancer cell lines by Koprowski et al. [Citation1]. After that, a series of studies showed that serum CA19-9 has high specificity and sensitivity for digestive tract tumors [Citation2–4], especially pancreatic cancer and gallbladder cancer [Citation5,Citation6]. Therefore, serum CA19-9 has long been used as a tumor marker to assist cancer diagnosis and to assess prognosis of patients [Citation7–9]. But we have occasionally observed in clinical practice that high serum CA19-9 can be detected in patients with calculous cholecystitis, one of the most common benign surgical diseases. However, there is no solid evidence to demonstrate the causality between calculous cholecystitis and serum CA19-9.
This study mainly focused on the perioperative changes of serum CA19-9 in calculous cholecystitis patients to clarify the causality between serum CA19-9 and calculous cholecystitis. In addition, immunohistochemistry (IHC) will be used to explore the origin of high serum CA19-9 in calculous cholecystitis.
Materials and methods
Patients
Between January 2018 and October 2021, a total of 20 calculous cholecystitis patients with high serum CA19-9 (CA19-9 value was three times higher than normal) were selected from clinical data browser. Then calculous cholecystitis patients with normal serum CA19-9 (CA19-9 < 34U/ml) at the same time were randomly selected according to the ratio of 1:2 (). The inclusion criteria were as follows: (a) patients underwent detailed laboratory and imaging examinations such as blood routine, blood biochemistry, B-ultrasound (BUS) and magnetic resonance imaging (MRI) within 1 week before cholecystectomy (CCY); (b)patients were without liver dysfunction, choledocholithiasis, or pancreatitis; (c) patients did not have the neoplastic disease; (d)patients had received CCY and their final diagnoses has been confirmed by pathological examinations.
Clinical data and tissue samples collection
The clinical data of each patient were collected, including general information, radiological investigation, serological data (serum CA19-9 was detected by electrochemiluminescence), and pathological examination. In addition, 26 gallbladder specimens were collected from the Department of Pathology of the First Affiliated Hospital of Anhui Medical University, including 3 cases of normal gallbladder (gallbladder cholesterol polyps), 3 cases of serum CA19-9 positive gallbladder cancer,10 cases of calculous cholecystitis with high serum CA19-9, 10 cases of calculous cholecystitis with normal serum CA19-9, of which normal gallbladder and gallbladder cancer were used as negative and positive control, respectively. This study was approved by the Ethics Committee of the First Affiliated Hospital of Anhui Medical University, and informed consent form was signed with patients.
Morphological observations
To observe the morphology of gallbladder mucosa and pathological changes caused by calculous cholecystitis, gallbladder tissue was embedded, fixed, and sectioned. Then the sections were stained with hematoxylin and eosin. Finally, the integrity of gallbladder mucosa was observed and photographed under an optical microscope.
Immunohistochemistry
Tissue sections were dewaxed using xylene and then hydrated in alcohol, peroxidase was removed using 3% hydrogen peroxide. The antigen was exposed in microwave using citrate buffer and the sections were incubated with primary antibody (CA19-9, MXB biotechnologies, China) at 4 °C overnight. After PBS washing for three times, the sections were treated with secondary antibody for 30 min at 37 °C. The sections were treated with SABC for 30 min at 37 °C, then the reaction was visualized by using DAB. The stained sections were stained again with hematoxylin.
The stained slides were scored by three researchers according to the following criteria: (1) The intensity of staining was scored as 0 (absent),1 (weak), 2 (moderate) and 3 (intense); (2) The proportion of stained areas were scored as 0 (<1%), 1 (1–10%), 2 (10–50%) and 3 (>50%). If the total score (proportion + intensity) was > 3, expression was defined as positive, and if the score was = 6, it was regarded as strong positive.
Statistical analysis
All clinical data were recorded in Excel. The t-test and chi-square test were performed to compare the clinical data of the high group with the normal group.
Data management and analysis were performed using SPSS version 26.0 and P-value <0.05 was considered statistically significant.
Results
Clinical features of patients with high serum CA19-9
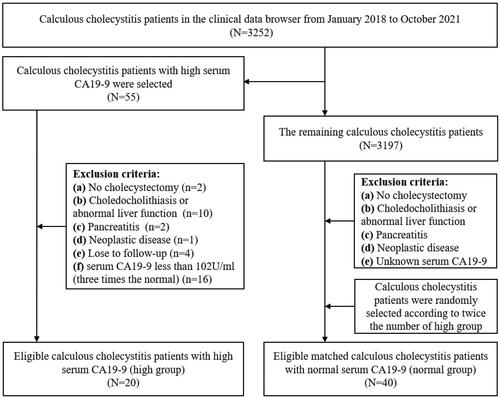
Serum CA19-9 ranged from 105 U/ml to 1635 U/ml in 20 patients with high serum CA19-9 (high group), of which 30% exceeded 1000 U/ml, and serum CA19-9 of all patients with normal serum CA19-9 (normal group) was lower than 34 U/ml (). Furthermore, analysis of clinical data showed that high group had a significantly higher proportion of gallbladder wall thickening than normal group (p < 0.001) and the average gallbladder wall thickness in the two groups was 5.53 ± 2.22 and 2.16 ± 1.78 mm (p < 0.001), respectively (, ). However, the average ages of two groups were 51.90 ± 18.14 and 44.47 ± 14.05 years, respectively, with no statistically significant differences and the rest parameters between the two groups were also not statistically significant differences, including sex, the diameter and number of stones, clinical symptoms, AST, ALT, and TB ().
Figure 2. Morphology of gallbladder under MRI and HE staining. A ∼ B Cross-section view of gallbladder in high group and normal group under MRI. C ∼ D Gallbladder mucosa morphology of high group and normal group under HE staining (Scale bars = 50 μm). E Gallbladder wall thickness in the high group and normal group (Measured by Carestream on MRI). A and C, B and D are the same patients, respectively. P-value is determined by t-test. *** p < 0.001.
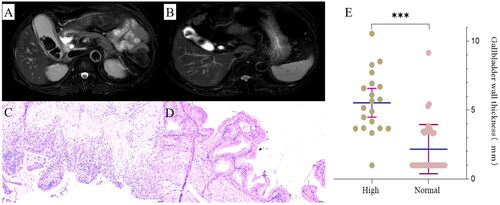
Table 1. Clinical characteristics of 60 patients in the high group and normal group.
The degree of gallbladder inflammation is reflected by gallbladder mucosal damage, and the HE staining results showed that 70% of the gallbladder mucosa has been exfoliated in the high group, and the rest also have significant damage. However, the gallbladder mucosa structure was relatively intact in the normal group ().
High serum CA19-9 returned to normal after CCY
To confirm that high serum CA19-9 is indeed caused by calculous cholecystitis, all patients in the high group underwent CCY and were followed up, and the results showed that serum CA19-9 of all patients returned to normal after CCY (). Among them, 4 patients had detailed monitoring records of serum CA19-9, which decreased significantly within one week after CCY and gradually over time, eventually returned to normal within one month ().
Figure 3. The change of serum CA19-9 before and after CCY in the high group. (A) Pre-operative serum CA19-9 level in the high group and normal group. (B) Changes of serum CA19-9 before and after CCY in the high group (median follow-up time: 9 months). (C) Dynamic changes of serum CA19-9 in 4 patients within one month after CCY. Serum CA19-9 level of three patients with serum CA19-9 exceeding 1000 was marked as 1000 because laboratory only reported more than 1000 (upper limit of detection) instead of an accurate value. P-value is determined by t-test. *** p < 0.001.
Ca19-9 was positive for mucosal epithelial cells, vascular endothelial cells, and intercellular substances of gallbladder in the high group
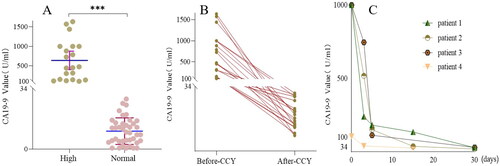
To explore the origin of high serum CA19-9 in calculous cholecystitis patients, gallbladder specimens from both groups were stained by IHC, and the results showed that gallbladder mucosal epithelial cells were positive for CA19-9 in both groups, but the intensity was higher in the high group. In addition, vascular endothelial cells, and intercellular substances in the high group were positive, but negative in the normal group (, ).
Figure 4. Expression of CA19-9 in different gallbladder regions in the high group. CA19-9 expression of mucosal epithelial cells, vascular endothelial cells, and intercellular substances or tumors in the negative control group(A ∼ C), normal group(D ∼ F), high group(G ∼ I), and positive control(J ∼ L). Scale bars = 50 μm.
Table 2. Expression of CA19-9 in different gallbladder regions in all patients.
Discussion
CA19-9 is an antigen of the Lewis A blood group determinant [Citation1], mainly distributed in stomach, pancreas, and biliary epithelium [Citation10–13]. Since it was discovered, serum CA19-9 is most commonly used as a tumor marker to help diagnosis of hepatobiliary and pancreatic malignancies [Citation14,Citation15]. A large number of studies have shown that the sensitivity and specificity of serum CA19-9 are 69-93% and 46–98% in pancreatic cancer and 28.7–88% and 67–94.5% in biliary tract cancer, respectively [Citation16–20]. In addition, another study based on 819 patients found that the higher CA19-9 level, the greater the probability of malignant disease, which was 87.2% when serum CA19-9 was between 37 U/ml and 1000 U/ml, and as high as 93.5% when serum CA19-9 was greater than 1000 U/ml [Citation21]. Nevertheless, high serum CA19-9 level caused by benign diseases is still worthy of attention, including pancreatitis and biliary obstruction [Citation21]. Furthermore, there have also been some case reports about high serum CA19-9 in cholecystitis. Moshref et al. reported a cholecystitis patient with high serum CA19-9, but ignored the patient was accompanied by Mirizzi’s syndrome that leads to biliary obstruction [Citation22]. Haring et al. reported a xanthogranulomatous cholecystitis patient with high serum CA19-9, but ignored the patient also had obstructive choledocholithiasis [Citation23]. Akimoto et al. reported a calculous cholecystitis patient with high serum CA19-9, but ignored the patient also had a liver mass detected by PET-CT, which could be the reason of high serum CA19-9 [Citation24]. Mehmet et al. reported a calculous cholecystitis patient whose serum CA19-9 returned to normal after CCY, but no further IHC analysis was performed on gallbladder specimens [Citation25]. None of these case reports can define the causality between calculous cholecystitis and serum CA19-9. Therefore, in this research, the causality between calculous cholecystitis and serum CA19-9 was studied excluding pancreatitis and biliary obstruction which were reported to be associated with high serum CA19-9, and gallbladder specimens were also analyzed by IHC.
Gallbladder wall thickening has been regarded as the diagnostic standard of cholecystitis, and it can also reflect the degree of inflammation [Citation26,Citation27]. This study found that the thickness of gallbladder wall and the degree of gallbladder mucosa damage in the high group were significantly higher than those in the normal group, so that it can be considered that high serum CA19-9 is usually related to severe inflammation. Based on latest research that CA19-9 can promote the development of pancreatitis and pancreatic cancer [Citation28] and considering CA19-9 is expressed in epithelial cells of normal biliary-pancreatic system [Citation11,Citation12], it is reasonable to consider whether CA19-9 can aggravate gallbladder inflammation or promote the transformation from cholecystitis to gallbladder carcinoma, which deserves further study.
In our study cohort, serum CA19-9 level returned to normal after CCY in 20 patients, 11 of whom were first reexamined postoperatively between 6 to 12 months and 5 between 1 to 3 years. To avoid possible influence of other factors on CA19-9 over long interval between time of CCY and reexamination, we followed up 4 latest patients dynamically, and whose serum CA19-9 decreased significantly within one week and returned to normal within one month after CCY, which confirmed that inflammatory gallbladder is the only source of high serum CA19-9, and also ruled out the possibility of malignancies. A case needs to be mentioned specially that a patient was diagnosed with calculous cholecystitis without malignant evidence on routine imaging examination (BUS and MRI) and his serum CA19-9 was 1483 U/ml at admission. However, serum CA19-9 increased to 3865 U/ml after CCY rather than returned to normal and pancreatic cancer was eventually detected after 6 months. The case gives clinicians a warning that if high serum CA19-9 does not return to normal after CCY, it is necessary to have a more detailed examination for patients to avoid missing malignancies or other diseases related to high serum CA19-9.
To confirm that high serum CA19-9 was derived from the inflammatory gallbladder, we performed IHC on gallbladder specimens, and the results showed that CA19-9 was positive in mucosal epithelial cells of the negative control group and the normal group, which was consistent with the previous studies [Citation11]. It was also found that the expression intensity of CA19-9 in mucosal epithelial cells of the high group was higher than the normal group and the negative control group, which may be caused by the fact that mucosal epithelial cells secreted more CA19-9 under severe inflammation. In addition, in the positive control group and the high group, CA19-9 was also found to be positive in vascular endothelial cells, which was attributed to the existence of endogenous receptor (E- selectin) binding to CA19-9 in vascular endothelial cells [Citation29,Citation30]. Finally, gallbladder intercellular substances were widely positive for CA19-9 in the high group, which needs further study to explain this phenomenon. The above results provided solid evidence that high serum CA19-9 originated from the inflammatory gallbladder.
In summary, this study demonstrated that high serum CA19-9 has direct relationship with calculous cholecystitis and preoperative high serum CA19-9 in this part of population can be normalized after CCY, so that the reasons for high serum CA19-9 should include calculous cholecystitis in the future clinical practice besides other CA19-9 related diseases. Admittedly, there are still some shortcomings in this study. On the one hand, the sample size is small and needs to be expanded. On the other hand, this study did not completely clarify the detailed mechanism of high serum CA19-9 in calculous cholecystitis and could not contribute to differential diagnosis of benign and malignant diseases with CA19-9 elevation, which need detailed research in the future.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Koprowski H, Steplewski Z, Mitchell K, et al. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5(6):957–971.
- Li Y, Li D-J, Chen J, et al. Application of joint detection of AFP, CA19-9, CA125 and CEA in identification and diagnosis of cholangiocarcinoma. Asian Pac J Cancer Prev. 2015;16(8):3451–3455.
- Rao H, Wu H, Huang Q, et al. Clinical value of serum CEA, CA24-2 and CA19-9 in patients with colorectal cancer. Clin Lab. 2021;67(4). DOI:10.7754/Clin.Lab.2020.200828
- Zhang J, Wang Y, Zhao T, et al. Evaluation of serum MUC5AC in combination with CA19-9 for the diagnosis of pancreatic cancer. World J Surg Oncol. 2020;18(1):31.
- Kim HJ, Kim MH, Myung SJ, et al. A new strategy for the application of CA19-9 in the differentiation of pancreaticobiliary cancer: analysis using a receiver operating characteristic curve. Am J Gastroenterol. 1999;94(7):1941–1946.
- Bind MK, Mishra RR, Kumar V, et al. Serum CA 19-9 and CA 125 as a diagnostic marker in carcinoma of gallbladder. Indian J Pathol Microbiol. 2021;64(1):65–68.
- Ge L, Pan B, Song F, et al. Comparing the diagnostic accuracy of five common tumour biomarkers and CA19-9 for pancreatic cancer: a protocol for a network meta-analysis of diagnostic test accuracy. BMJ Open. 2017;7(12):e018175.
- Jing R, Cui M, Ju S, et al. The changes and clinical significance of preoperative and postoperative serum CEA and CA19-9 in gastric cancer. Clin Lab. 2020;66(4). DOI:10.7754/Clin.Lab.2019.190732
- Kambara Y, Miyake H, Nagai H, et al. CA19-9 is a significant prognostic marker of patients with stage III gastric cancer. Eur J Surg Oncol. 2020;46(10 Pt A):1918–1924.
- Atkinson BF, Ernst CS, Herlyn M, et al. Gastrointestinal cancer-associated antigen in immunoperoxidase assay. Cancer Res. 1982;42(11):4820–4823.
- Agrawal V, Goel A, Krishnani N, et al. p53, carcinoembryonic antigen and carbohydrate antigen 19.9 expression in gall bladder cancer, precursor epithelial lesions and xanthogranulomatous cholecystitis. J Postgrad Med. 2010;56(4):262–266.
- Shimizu M, Saitoh Y, Ohyanagi H, et al. Immunohistochemical staining of pancreatic cancer with CA19-9, KM01, unabsorbed CEA, and absorbed CEA. A comparison with normal pancreas and chronic pancreatitis. Arch Pathol Lab Med. 1990;114:195–200.
- Eskelinen M, Haglund U. Developments in serologic detection of human pancreatic adenocarcinoma. Scand J Gastroenterol. 1999;34(9):833–844.
- Ma S, Duan J, Li W, et al. Exploration of the value of MRCP combined with tumor marker CA19-9 in the diagnosis of pancreatic cancer. Artif Cells Nanomed Biotechnol. 2016;44(2):717–721.
- Qin XL, Wang ZR, Shi JS, et al. Utility of serum CA19-9 in diagnosis of cholangiocarcinoma: in comparison with CEA. World J Gastroenterol. 2004;10(3):427–432.
- Haglund C, Roberts PJ, Jalanko H, et al. Tumour markers CA 19-9 and CA 50 in digestive tract malignancies. Scand J Gastroenterol. 1992;27(3):169–174.
- Kang JS, Hong SY, Han Y, et al. Limits of serum carcinoembryonic antigen and carbohydrate antigen 19-9 as the diagnosis of gallbladder cancer. Ann Surg Treat Res. 2021;101(5):266–273.
- Benini L, Cavallini G, Zordan D, et al. A clinical evaluation of monoclonal (CA19-9, CA50, CA12-5) and polyclonal (CEA, TPA) antibody-defined antigens for the diagnosis of pancreatic cancer. Pancreas. 1988;3:61–66.
- Yoshikawa T, Nishida K, Tanigawa M, et al. Carbohydrate antigenic determinant (CA 19-9) and other tumor markers in gastrointestinal malignancies. Digestion. 1985;31(2-3):67–76.
- Steinberg WM, Gelfand R, Anderson KK, et al. Comparison of the sensitivity and specificity of the CA19-9 and carcinoembryonic antigen assays in detecting cancer of the pancreas. Gastroenterology. 1986;90(2):343–349.
- Ong SL, Sachdeva A, Garcea G, et al. Elevation of carbohydrate antigen 19.9 in benign hepatobiliary conditions and its correlation with serum bilirubin concentration. Dig Dis Sci. 2008;53(12):3213–3217.
- Moshref LH, Mandili RA, Almaghrabi M, et al. Elevation of CA 19-9 in mirizzi syndrome in the absence of malignancy: a case report. Am J Case Rep. 2021;22:e931819.
- Haring MPD, de Cort BA, Nieuwenhuijs VB. [Elevated CA19-9 levels; not always cancer]. Ned Tijdschr Geneeskd. 2021;164:D4048.
- Akimoto S, Banshodani M, Nishihara M, et al. Acute cholecystitis with significantly elevated levels of serum carbohydrate antigen 19-9. Case Rep Gastroenterol. 2016;10(2):410–416.
- Şahin M, Cüre E, İşler M, et al. Elevated Ca 19-9 Levels in Patient With Cholecystitis. 2007.
- Yokoe M, Hata J, Takada T, et al. Tokyo guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25(1):41–54.
- Domeyer PJ, Sergentanis TN, Zagouri F, et al. Chronic cholecystitis in elderly patients. Correlation of the severity of inflammation with the number and size of the stones. In Vivo. 2008;22:269–272.
- Engle DD, Tiriac H, Rivera KD, et al. The glycan CA19-9 promotes pancreatitis and pancreatic cancer in mice. Science. 2019;364(6446):1156–1162.
- Takada A, Ohmori K, Yoneda T, et al. Contribution of carbohydrate antigens sialyl lewis a and sialyl lewis X to adhesion of human cancer cells to vascular endothelium. Cancer Res. 1993;53(2):354–361.
- Takada A, Ohmori K, Takahashi N, et al. Adhesion of human cancer cells to vascular endothelium mediated by a carbohydrate antigen, sialyl lewis A. Biochem Biophys Res Commun. 1991;179(2):713–719.