Abstract
Background
Gastric adenocarcinoma of the fundic gland type is a new subtype of gastric adenocarcinoma. In 2019, the World Health Organization (WHO) listed gastric adenocarcinoma of the fundic gland type (GA-FG) as a new and rare gastric tumour with a low incidence due to the small number of cumulative cases worldwide. Twenty cases of GA-FG found in our centre were retrospectively analysed to improve the diagnostic ability of endoscopy and pathology in this disease.
Objective
To investigate the clinicopathological features of fundus-derived gastric tumours and to improve the understanding of and diagnostic accuracy of endoscopy for this disease.
Methods
The clinicopathological characteristics of 20 GA-FG cases between 2018 and 2022 were analysed using clinical and follow-up data and endoscopic, immunohistochemical, and pathological morphology characteristics.
Results
In all cases, GA-FG was found in the fundus and the body of the stomach. In total, there were 19 patients with 20 lesions, with most of the patients having a single lesion. One patient had multiple lesions, and another patient had complications from signet ring cell carcinoma (SRCC). All lesions occurred in non-atrophic areas, and 10 patients had gastric fundic gland polyps simultaneously. There were 14 cases of gastric fundus adenocarcinoma and 6 cases of acid-secreting adenoma. Fourteen lesions were treated with endoscopic submucosal dissection (ESD), without recurrence or metastasis during the follow up; 6 patients were followed up for observation, 2 of whom showed no lesions after the first biopsy by gastric endoscopy, and 4 of whom showed no significant changes.
Conclusions
The incidence rate for GA-FG lesions may be underestimated due to their benign course. ESD seems to be an adequate treatment for GA-FG.
Main Points
Gastric adenocarcinoma of the fundic gland type (GA-FG) is located in the fundus and body of the stomach. All lesions occur in non-atrophic areas, and almost one-half involve gastric fundus polyps simultaneously. GA-FG lesions typically follow a benign disease course. ESD seems to be an adequate treatment for GA-FG.
Introduction
Gastric epithelial neoplasm of fundic-gland mucosa lineage can be divided into three categories: acid-secreting adenoma, gastric adenocarcinoma of the fundic gland type (GA-FG) and gastric fundic type mucosa [Citation1]. In 2010, Ueyama first proposed a histological type of gastric cancer with differentiation into the fundic gland, named ‘gastric adenocarcinoma of the fundic gland type’ [Citation2]. In 2017, GA-FG was added to the Japanese classification of gastric cancer as a special cancer type [Citation3], and GA-FG has been included in the 2019 edition of the World Health Organization (WHO)’s list of digestive system-related cancers. Gastric adenomas without submucosal invasion are called acid-secreting adenomas by the WHO. Gastric fundus adenocarcinoma infiltrates only into the submucosa [Citation4]. The incidence of GA-FG, a well-differentiated gastric cancer entity originating from the fundic gland, is 0.98–1.6% [Citation2]. GA-FG is a well-differentiated type of gastric cancer occurring in the fundus gland area. No atrophic glands or intestinal metaplasia exist in the cancer region of GA-FG; however, whether clear cancer boundaries are observed varies according to the conclusions of different articles [Citation5,Citation6]. GA-FG can be divided into three histopathological subtypes: the chief cell-predominant type, oxyntic cell-predominant type and mixed type. The chief cell-predominant type accounts for approximately 99% of all GA-FG cases [Citation7]. The neoplastic cells are closely arranged to form so-called ‘endless glands’ such that cytological structural atypia can be observed, but cell atypia, characterized by an enlarged nuclei, is rare [Citation8]. The risk of malignancy and invasiveness of GA-FG is low, and lymphatic or venous invasion is rarely observed; however, most tumours invade the submucosa [Citation2,Citation9,Citation10]. Atsushi Uchida described a tumour that was classified as gastric adenocarcinoma of the fundic-gland mucosa type (GA-FGM). The cancer was located in the submucosal layer, and many heterotopic gastric glands (HGGs) were observed, suggesting that GA-FGM may arise from an HGG [Citation11]. Shigeo Manabe also encountered a case of GA-FG and suggested that cancer had invaded the submucosa by spreading to HGGs located in the proximal end of GA-FG [Citation5]. Yasuhiro Okumura described a case of GA-FG that was followed up for 10 years. With growth, the tumour developed a depressed area in the centre. Most tumour cells in the mucosa and superficial submucosa were similar to mucous neck cells. These tumour cells mostly formed isolated tubular glands that widely infiltrated the musculus propria and subserous layers. However, there was a gradual transition zone between the muscular and subserous layers in the tubular adenocarcinoma with poorly differentiated components [Citation12]. In addition to revealing the very rare growth and submucosal invasion pattern of GA-FG, these cases also confirmed that GA-FG may originate from atrophic gastric mucosa and develop into invasive carcinoma with lymph node (LN) metastasis, provided that gastric fundic glands remain [Citation13]. Therefore, more cases are needed to understand the clinical and physiological characteristics of GA-FG.
GA-FG is a tumour that mainly differentiates into chief cells [Citation9]. At present, its clinicopathological characteristics, including the mode of tumour growth, are not clear, and there are few clinical reports. However, with the normalization of Helicobacter pylori (HP) eradication therapy, the incidence of HP-negative gastric cancers will increase correspondingly [Citation14]. As one type of HP-negative gastric cancer, the incidence of fundus adenocarcinoma will also increase. Although the concept of GA-FG has been gradually popularized, many cases are still undiagnosed due to the difficulty of a correct diagnosis. Similar to traditional gastric cancer, GA-FG is considered to have a low risk of metastasis, so most patients undergo endoscopic submucosal dissection (ESD) [Citation5]. However, due to the unique biological characteristics of GA-FG, it tends to infiltrate into the submucosa. According to the Japanese guidelines for gastric cancer treatment, endoscopic resection is not in line with the treatment standard for submucosal infiltration lesions below a depth of 500 µm [Citation15]. Therefore, the question arises of whether GA-FG exceeding 500 µm should be treated according to the Gastric Cancer Treatment Guideline (GCTG) recommendations. Gastrectomy and local resection combined with LN dissection for common gastric cancers require more cases and studies to establish a treatment strategy suitable for GA-FG.
Materials and methods
Subjects
This study analysed data from 19 patients (20 lesions) diagnosed with GA-FG at XXX Hospital between 2018 and 2022. We prospectively and retrospectively analysed the clinicopathological features of these cases.
Methods
Statistical analyses were performed by the Statistical Package for Social Sciences (SPSS) version 23.0 software (IBM Corp.; Armonk, NY).
Endoscopic evaluation methods
The endoscopic morphology of tumours was evaluated according to the Paris classification criteria. We evaluated the following clinical characteristics: sex, age, lesion location and size, HP infection, atrophy, endoscopic characteristics (such as shape, colour and boundary, submucosal tumour (SMT) appearance, surface capillary expansion, and the presence of other fundic gland polyps, surface structure, structure of the capillaries of the gland (narrow-band imaging (NBI) amplification using the VS classification standard), histological and immunological features, and follow up after ESD. The HP detection method used was the 13 °C or 14 °C-urea breath test.
Pathological assessment methods
The submitted specimens were fixed with 4% neutral formaldehyde solution. All biopsy specimens were sampled at 2 mm intervals after ESD resection, followed by routine dehydration, paraffin embedding, tissue sectioning at a thickness of 4 μm and HE staining.
Immunohistochemical detection
An EnVision two-step method was used for immunohistochemical labelling. Primary antibodies against MUC5AC, MUC6, CDX2, KI-67, P53, HER2, and Ki-67 and secondary antibodies were purchased from Suzhou Baidao Medical Technology Co., Ltd. The primary anti-pepsinogen-1 (PEPsinogen-1) antibody and H+/K+-ATPase were both purchased from Abcam Company in the United States. Negative and positive controls were established for the above markers.
Results
Clinical features
There were 20 lesions in 19 patients, including 6 males and 14 females, all of whom had no family history of tumours. Four patients had concurrent reflux symptoms, while the remainder were asymptomatic, with the tumours being found during physical examination. The 20 lesions were all located in the upper body of the stomach and fundus of the stomach, with most of the patients having a single lesion. One patient had multiple lesions (2 fundus adenocarcinomas), and the other patient had signet ring cell carcinoma (SRCC) with an average size of 0.75 cm (0.4–1.7 cm). The age of the patients ranged from 47 to 81 years, and the average patient age was 62.5 years. The male-to-female ratio in this study was 6:14. HP infection and atrophy were observed in three patients (4 lesions) with background mucosa atrophic gastritis with an atrophy degree of C2-C3, active HP infection was found in two patients, and HP infection had been eradicated in three patients. All lesions occurred in non-atrophic areas, and 10 patients had gastric fundus polyps ().
Table 1. Clinical data and endoscopic characteristics of 19 patients with GA-FG.
Endoscopic features
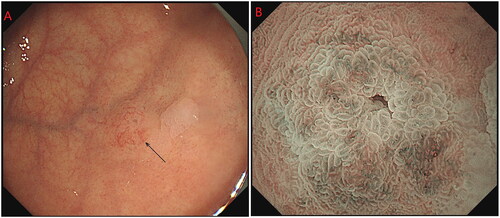
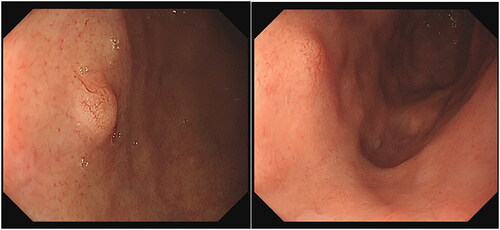
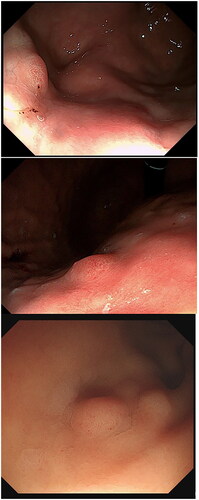
The tumours were all located in the body and fundus of the stomach, and most of them were single. One patient had multiple lesions [Citation2], and another patient had SRCC with an average size of 0.75 cm (0.4–1.7 cm). Nineteen patients had grade 0-IIA tumours, and 1 patient had a grade 0-IIB tumour. Due to the visible dilatation of capillaries on the surface, the faded-colour surface of the lesions showed filamentous redness changes. NBI magnification showed a boundary demarcation line (DL) between the lesion and the surrounding background mucosa, with DL(+) in 17 cases and DL(−) in 3 cases. Magnification and observation were performed in 8 cases, showing elongated and dilated fundic glands, surface microstructure (MSP): 7 cases were regular, and 1 case was irregular. The irregular case may have been attributed to magnification after biopsy. The surface microvascular (MVP) was regular in 7 cases and irregular in 1 case, which may have been related to magnification after biopsy (; ).
Pathological morphology
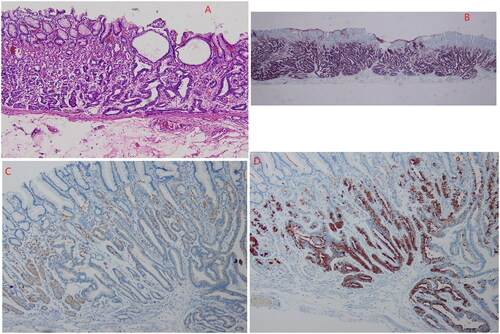
The tumour tissue was located in the lamina propria and was covered by normal concave epithelium. The glands were distorted, angulated, cystic dilated, and anastomosed in a so-called ‘endless gland’ pattern. Tumours have few atypic cells and are composed of two kinds of cells: one type is similar to the main cell (columnar in shape, with a slightly basophilic cytoplasm), accounting for the main component, and the other type is an ovoid or triangular parietal cell with eosinophils and a fine granular cytoplasm, constituting a minor component. In this study, all the cases were of the dominant cell type. All 14 GA-FG tumour cells broke through the mucosal muscle layer and entered the submucosa at 100 µm–600 µm (average 260 µm). Among these patients, one presented multiple GA-FG lesions located in the fundus and the gastric body, with the same pathological morphology. OGA tumour cells were confined to the mucosal layer in six cases, had infiltrated into the mucosal muscle layer in four cases, and were located in the mucosa in two cases. No atrophy, intestinal metaplasia, or lymphatic or vascular invasion was observed in any of the 20 cases. The tumour in one patient with GA-FG in the stomach was associated with differentiated adenocarcinoma of the fundus and SRCC.
Immunohistochemistry
Immunohistochemical examination showed that all tumours were diffusively positive for MUC6 and pepsinogen 1; H+/K+-ATPase was scattered positive; and MUC2, MUC5 and CDX2 were not expressed. Surface foveolar epithelial cells were positive for MUC5, but P53 was not overexpressed, and the Ki-67 marker index was 2–8%, with an average of 5.14% ().
Treatment and prognosis
Fourteen patients underwent ESD treatment, and six patients underwent biopsies. There was no recurrence or metastasis in the 14 patients treated with ESD by gastroscopy and enhanced abdominal CT. In the six patients who were followed up after the first biopsy, repeated endoscopic examination showed no lesions in two patients and no significant (one patient was followed up for 50 months) changes in the lesions of four patients.
Discussion
According to the WHO classification of gastric tumours in 2019, the age of onset of GA-FG is 60–70 years; however, Japanese reports state the mean age is 67.7 years (42–82 years) [Citation9]. This disease may have a sex bias, with a worldwide male-to-female ratio of 2.2:1.0. In Ga-FG cases in Japan, the male-to-female ratio is 1.4 [Citation5]. However, the male-to-female ratio in this study was 6:14, which may account for the small number of cases in this single-centre study.
GA-FG may have no or mild symptoms. However, in a report by Singhi et al., all 10 patients described had reflux symptoms, revealing high gastric acid secretion as there was no atrophy. In their study, enlarged gastric fundic glands with small concave cells, similar to gastric fundic gland polyps, were observed in most cases of GA-FG. Therefore, some speculate whether the development of fundic glandular gastric cancer is related to the use of proton pump inhibitors (PPIs) [Citation6]. However, studies on this subject are limited, and the relationship between the occurrence of GA-FG and the use of PPIs remains unclear. Nevertheless, most patients in this study had no history of persistent PPI use. Moreover, we found coexisting fundic gland polyps in approximately half of the cases in our centre. Among the ten patients complicated with fundic gland polyps, only two had a history of HP infection and had completed HP eradication treatment when the lesions were found, and 4 had taken PPIs and Chinese medicine repeatedly and discontinuously due to reflux symptoms. Half of the patients were complicated with gastric fundic gland polyps, and it seemed that cancer of the gastric fundic gland was related to gastric fundic gland polyps to a certain extent. However, most of the patients did not have a history of continuous PPI use, and most of the patients did not have a history of HP infection and eradication; thus, there seemed to be little correlation between cancer of the gastric fundic gland and HP eradication therapy or continuous PPI use. However, this study was performed at a single centre and involved few cases. Therefore, whether there is a correlation between GA-FG and the occurrence of fundic gland polyps needs to be confirmed by examining more cases. Most GA-FG lesions are single, while multiple lesions are rare. One patient may have two or three lesions. Most of the tumour diameters reported are generally less than 1 cm, though tumour diameters up to 8.5 cm have been reported [Citation16]. With the increase in diameter, the amount of submucosal invasion increases, which in turn increases the risk of GA-FG transformation and invasion. Leyama et al. reported that GA-FG can differentiate in several directions. For example, the fundic gland type can change to the foveolar type, undifferentiated type or other types during tumour progression, and these types have more malignant potential than GA-FGs that differentiate into chief cells [Citation14,Citation17]. In this study, one patient had coexisting SRCC, representing GA-FG differentiation to the undifferentiated type, which is not discussed herein. In our study, the average diameter was 0.75 cm, and the average patient age was 62.5 years, which was consistent with previous studies. Most patients had a single lesion, but one patient had two simultaneous lesions that occurred in a non-atrophic area of the HP active atrophic gastritis region. Therefore, when one lesion is discovered, we should also carefully examine other parts for similar tumours to avoid missing other lesions. If the diagnosis is uncertain, biopsy may be a good confirmation method.
GA-FG is a cancer entity originating from the fundic gland with a well-differentiated cell type and low malignant potential [Citation9]. According to previous reviews, in GA-FG cases, the proportion of tumours located in one-third of the stomach is as high as 98% [Citation8]. In all the cases in our study, the tumours were located in the upper part of the stomach and the fundus of the stomach. It has always been believed that differentiated adenocarcinoma originates from a background of intestinal metaplasia, including HP infection. Most FG-GA originates from the non-atrophic gastric fundic gland region [Citation9]. Takashi Chiba et al found that 15 of 20 patients were HP positive or had experienced HP eradication, and these lesions were mainly located in non-atrophic gastric mucosa [Citation14]. However, at present, the effect of HP on GA-FG, its progression rate and its degree of malignancy are not clear. HP infection status and mucosal atrophy may not be the key determinants in the occurrence of GA-FG [Citation18]. In the regulation of premalignant epithelial responses to HP, the activation of β-catenin signalling is a key factor [Citation19]. Ryosuke Nomura MD recently reported that with the activation of the Wnt/β-catenin pathway, approximately half of the GA-FG patients retained β-catenin and harboured at least 1 mutation in CTNNB1/AXINs/APC [Citation16]. Keitaro Takahashi et al. reported the first case of GA-FG conversion to intramucosal GA-FGM by endoscopic observation 15 years before and after HP eradication. In this study, the biopsy showed no morphological changes 15 years after endoscopy, including from the time 5 years prior when the first biopsy was taken to HP eradication. At the time of the 15-year biopsy, the tumour size was the same as it had been previously and without submucosal invasion. After HP treatment, the lesion changed from grade 0-IIA to 0-IIA + IIc and then to 0-I after HP eradication. The morphology was reported to change from an elevated to a depressed form after short-term eradication of HP [Citation20]. Furthermore, in GA-FG, HP eradication reduced the thickness of the tumour duct covering, similar to that of conventional gastric adenocarcinoma. This finding suggests that if the tumour size remains unchanged, the cancer maintains mild malignant potential, while HP eradication can change the endoscopic morphology [Citation21]. A previous study demonstrated the effect of HP infection on GA-FG: a comparison of the HP eradication group with the HP negative group showed a reduced number of gastric fundic glands in the HP eradication group, and the foveolar epithelium covering the tumour duct was thinner [Citation8]. Although some patients showed chronic atrophic gastritis or intestinal metaplasia under endoscopy, tumours surrounding the gastric mucosa were without pathological evidence of mucosal changes [Citation21]. In this study, there were four cases of atrophic gastritis of background mucosa with HP infection.
Most GA-FGs were identified as having an elevated shape under white light endoscopy, similar to submucosal tumour (SMT)-like shapes [Citation9]. In this study, 19 cases were of type 0-IIa, and 1 case was of type 0-IIb. Most of the tumours presented an SMT-like shape under white light endoscopy, with dilated branch vessels visible on the surface (). However, there were some non-SMT-like elevation changes, similar to the fundic gland polyps (), which showed the expansion of the gland with no dilated branch vessels. Therefore, the visible filament-like red changes on the surface of the discoloured lesions may be the key to the initial diagnosis of GA-FG; however, attention should also be given to elevation changes, with expansion of the gland in the upper portion of the stomach.
MUC6 is strongly expressed in GA-FG-CCP cells [Citation6]. In contrast, few MUC5AC-positive cells (differentiated into depressed epithelium) are found in GA-FG, and the Ki-67 index is low, approximately 3.6% on average, with no P53 expression, thereby demonstrating low proliferation activity and low invasiveness [Citation8]. In this study, 13 cases were of the main cell type, all tumours were diffusely positive for MUC6 and pepsinogen 1 expression, H+/K+-ATPase was scattered positive, and MUC2, MUC5 and CDX2 were not expressed, P53 was not overexpressed, and the Ki-67 marker index was 2–8%, with an average value of 5.14%.
OGA and GA-FG are located in the bottom of the mucosal layer, usually covered by a non-neoplastic mucous membrane and blocked by a thick mucous membrane [Citation14]. Therefore, the tumour components of GA-FG and OGA are located in the innermost layer of the stomach, and the changes occurring there cannot be explored. Therefore, magnification endoscopy can be used for evaluation assistance but not as a method of accurate diagnosis. In contrast, magnified NBI shows that some GA-FG MSP and MVP are normal. If irregular MSP and MVP are observed in the staining during magnification, these findings may indicate GA-FGM [Citation1]. Chiba et al. followed up 10 GA-FG lesions. None of the 10 lesions had any morphological changes 16 months later [Citation18]. Among our patients, 6 were diagnosed by biopsy and have been followed up for 6–50 months (median follow-up time, 16.3 months). Four patients have shown no morphological changes, and 2 have shown no lesions after repeated observation.
GA-FG tumours grow slowly, and their risk of malignancy and invasiveness is low. Even though most are benign, the submucosa is often involved, so we may not recommend careful follow-up alone. For common types of gastric cancer, the GCTG recommends local resection or gastrectomy with LN dissection [Citation12], where tumour size, histological type, and estimated depth are considered in the therapeutic decision [Citation12]. In recent years, most GA-FGs have been surgically removed via ESD. However, in view of the 2010 Japanese Guidelines for the treatment of gastric cancer (the third edition), endoscopic resection may be beyond the treatment indication for submucosal infiltration lesions at a depth below 500 µm [Citation1]. In this study, 14 patients were treated with ESD, and patients with HP infection were treated with HP eradication after ESD. These 14 patients were followed up for 5-18 months (median follow-up time, 12.4 months), and all patients survived. Additionally, no metastasis to the LNs or other sites was found by endoscopic review and enhanced abdominal CT. HP eradication did not affect the prognosis. GA-FG may not be a rare type of gastric cancer at our centre, thereby biasing our conclusions. Since this was a single-centre study with a small sample size, additional samples are warranted in future work.
Ethical approval
Ethical approval granted by The Third People’s Hospital of Chengdu Ethics Committees (2022-S-98).
Informed consent
This is a retrospective analysis article, informed consent is not required for this study.
Author contributions
Concept - M.Y., X.S; Design - M.Y., X.S; Supervision - M.Y., X.S; Materials - M.Y., YY.C., P.Y; Data Collection and/or Processing - M.Y., YY.C., P.Y; Analysis and/or Interpretation - M.Y; Literature Search - M.Y; Writing Manuscript - M.Y; Critical Review - X.S;
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Ueyama H, Yao T, Akazawa Y, et al. Gastric epithelial neoplasm of fundic-gland mucosa lineage: proposal for a new classification in association with gastric adenocarcinoma of fundic-gland type. J Gastroenterol. 2021;56(9):814–828.
- Miyazawa M, Matsuda M, Yano M, et al. Gastric adenocarcinoma of fundic gland type: five cases treated with endoscopic resection. World J Gastroenterol. 2015;21(26):8208–8214.
- Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma. 15th ed. Tokyo: Kanehara Shuppan; 2017.
- WHO Classification of Tumours Editorial Board. WHO classification of tumours. 5th ed. Vol. 1, Digestive system tumours. Lyon: IARC; 2019.
- Manabe S, Mukaisho KI, Yasuoka T, et al. Gastric adenocarcinoma of fundic gland type spreading to heterotopic gastric glands. World J Gastroenterol. 2017;23(38):7047–7053.
- Li C, Wu X, Yang S, et al. Gastric adenocarcinoma of the fundic gland type: clinicopathological features of eight patients treated with endoscopic submucosal dissection. Diagn Pathol. 2020;15(1):131.
- Benedict MA, Lauwers GY, Jain D. Gastric adenocarcinoma of the fundic gland type. Am J Clin Pathol. 2018;149(6):461–473.
- Xiang-Yu M, Yang G, et al. Gastric adenocarcinoma of the fundic gland: a review of clinicopathological characteristics, treatment and prognosis. Rare Tumors. 2021;13:1–7.
- Miyazawa M, Matsuda M, Yano M, et al. Gastric adenocarcinoma of the fundic gland (chief cell-predominant type): a review of endoscopic and clinicopathological features. World J Gastroenterol. 2016;22(48):10523–10531.
- Ueyama H, Matsumoto K, Nagahara A, et al. Gastric adenocarcinoma of the fundic gland type (chief cell predominant type). Endoscopy. 2014;46(2):153–157.
- Uchida A, Ozawa M, Ueda Y, et al. Gastric adenocarcinoma of fundic gland mucosa type localized in the submucosa. Medicine. 2018;97(37):e12341.
- Okumura Y, Takamatsu M, Ohashi M, et al. Gastric adenocarcinoma of fundic gland type with aggressive transformation and lymph node metastasis: a case report. J Gastric Cancer. 2018;18(4):409–416.
- Ueo T, Yonemasu H, Ishida T. Gastric adenocarcinoma of fundic gland type with unusual behavior. Dig Endosc. 2014;26(2):293–294.
- Ueyama H, Yao T, Nakashima Y, et al. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol. 2010;34(5):609–619.
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113–123.
- Nomura R, Saito T, Mitomi H, et al. GNAS mutation as an alternative mechanism of activation of the Wnt/β-catenin signaling pathway in gastric adenocarcinoma of the fundic gland type. Hum Pathol. 2014;45(12):2488–2496.
- Uozumi T, Seki H, Matsuzono E, et al. Gastric adenocarcinoma of fundic gland type arising from heterotopic gastric glands during a 19-year follow-up period. Clin J Gastroenterol. 2019;12(6):556–561.
- Chiba T, Kato K, Masuda T, et al. Clinicopathological features of gastric adenocarcinoma of the fundic gland (chief cell predominant type) by retrospective and prospective analyses of endoscopic findings. Dig Endosc. 2016;28(7):722–730.
- Kino H, Nakano M, Kanamori A, et al. Gastric adenocarcinoma of the fundic gland type after endoscopic therapy for metachronous gastric cancer. Intern Med. 2018;57(6):795–800.
- Takahashi K, Ueno N, Sasaki T, et al. Long-term observation of gastric adenocarcinoma of fundic gland mucosa type before and after Helicobacter pylori eradication: a case report. J Gastric Can. 2021;21(1):103–109.
- Ishibashi F, Fukushima K, Ito T, et al. Influence of Helicobacter pylori infection on endoscopic findings of gastric adenocarcinoma of the fundic gland type. J Gastric Cancer. 2019;19(2):225–233.