Abstract
Objective
The aim of the study was to characterize the drug utilization and switch patterns of biological treatment of ulcerative colitis (UC) and Crohn’s disease (CD).
Methods
Using Danish national registries, this nationwide study included individuals diagnosed with UC or CD, bio-naïve at the initiation of treatment with infliximab, adalimumab, vedolizumab, golimumab, or ustekinumab in 2015–2020. Hazard ratios of discontinuing the first treatment or switching to another biological treatment were explored using Cox regression.
Results
Among 2995 UC patients and 3028 CD patients, infliximab was used as a first-line biologic treatment in 89% of UC patients and 85% of CD patients, followed by adalimumab with 6%, vedolizumab with 3%, and golimumab with 1% for UC, and adalimumab with 12%, vedolizumab with 2%, and ustekinumab with 0.4% for CD.
When comparing adalimumab as the first treatment series to infliximab, there was a higher risk of treatment discontinuation (excluding switch) among UC patients (hazard ratio: 2.02 [95% confidence interval: 1.57; 2.60]) and CD patients (1.85 [1.52; 2.24]). When comparing vedolizumab to infliximab, there was a lower risk of discontinuation for UC patients (0.51 [0.29–0.89]), and for CD patients, although not significantly (0.58 [0.32–1.03]). We observed no significant difference in the risk of switching to another biologic treatment for any of the biologics.
Conclusion
More than 85% of UC and CD patients initiating biologic therapy had infliximab as their first-line biologic treatment, in accordance with official treatment guidelines. Future studies should explore the higher incidence of treatment discontinuation of adalimumab as the first treatment series.
Several biologic therapies are available in the treatment of ulcerative colitis and Crohn’s disease.
Clinical guidelines stipulate that infliximab should be the first-line biologic therapy.
Drug utilization studies comparing biologic therapies head-to-head are sparse.
In Denmark, during 2015–2020 infliximab remained the most widely used biologic treatment, with adalimumab being second.
One in four patients experienced more than one biologic during the study period.
The risk of discontinuation of biologic treatment (and not starting a new biologic) was higher for initiators of adalimumab.
Clinical and social background factors available from the registers could not account for the observed risk difference in discontinuation.
Key summary
Introduction
Inflammatory bowel diseases (IBD) are chronic, immune-mediated inflammatory disorders manifested by inflammation and tissue damage of the gastrointestinal tract. IBD comprises two main subtypes known as Crohn’s disease (CD), which can affect the entire digestive system, and ulcerative colitis (UC), which mainly affects the large intestine. As a lifelong chronic condition, the majority of patients with IBD experience cycles of remission and relapse with the flaring of the disease [Citation1]. For patients not adequately responding to conventional therapies such as aminosalicylates (5-ASAs), corticosteroids, and immunosuppressants, and for those resistant or intolerant to conventional therapies, several biologic treatments have become available, including infliximab (IFX), adalimumab (ADA), and vedolizumab (VDZ) and ustekinumab (UST) for both CD and UC, and golimumab (GOL) for UC [Citation2,Citation3].
While biologics for CD and UC have demonstrated efficacy in clinical trials, treatment is also associated with side effects, intolerance, and treatment failure. An understanding of the utilization of biologic therapy and the treatment patterns can help optimize treatment with biologics. The present study aimed to describe the utilization of biologic therapy including switch patterns for the treatment of CD and UC in Denmark in 2015–2020 using national registers.
Materials and methods
The study population was defined by the following a) diagnosis-related criteria and b) treatment-related criteria:
Adult (≥18 years) patients with Danish residence identified from the Danish National Patient Registry [Citation4] from 2005 through 2020 with at least two hospital contacts (inpatient hospitalizations, outpatient visits, or emergency room visits) with Crohn’s disease (ICD-10: K50) or ulcerative colitis (ICD-10: K51) of which at least one contact was a primary diagnosis [Citation5,Citation6]. The date of the defining second contact was considered the CD or UC entry date, respectively. Some patients may change diagnosis from UC to CD, or vice versa, during the course of the disease as a consequence of initial misdiagnosis or the progression of the disease. Consequently, patients initially diagnosed with UC changed to the CD group on the date of their second hospital contact with CD diagnosis after an initial UC entry. In this case, the patient was censored from the UC analysis by the CD entry date; and vice versa for patients initially diagnosed with CD who later shifted to the UC diagnosis. Upon a second shift in CD/UC diagnosis, the patient was censored from both the UC and CD analyses.
Patients assigned to either the UC or the CD category initiating first-line biologic therapy of one of the study drugs between 01 January 2015 and 31 December 2020. The biologic treatment was identified using SKS codes (clinical procedure codes) from the Danish National Patient Registry or ATC codes from the National Prescription Registry, considering the following biological therapies: infliximab (IFX; SKS code: BOHJ18A1, ATC code: L04AB02); adalimumab (ADA; SKS code: BOHJ18A3, ATC code: L04AB04); vedolizumab (VDZ; SKS code: BOHJ19H4, ATC code: L04AA33); and strictly for the UC cohort: golimumab (GOL; SKS code: BOHJ18A4, ATC code: L04AB06); and strictly for the CD cohort: ustekinumab (UST; SKS code: BOHJ18B3, ATC code: L04AC05). The analyses did not differentiate between tradenames or biosimilars of the same biologic treatment. UC patients who had a colectomy (SKS procedure codes KJFH) prior to the start of biologic therapy were excluded. UC patients starting UST and CD patients starting GOL were censored.
Exposure
Biologic therapy administrations were linked in a continuous treatment series defined as starting on the date of the first administration (index date) and ending on the date of the last administration plus the therapeutic duration and 90 days of the grace period. The therapeutic duration was set to 8 weeks for IFX and VDZ, and 12 weeks for ADA, GOL, and UST based on clinical practice and frequency of visits at the hospital for infusions or hand-out of medication.
Outcomes
Since the exact date of treatment cessation is not available through the Danish registries, which include dates of administration and type of biologic, a treatment series was considered stopped at the earliest of either: 1) Switch in therapy, defined as the administration of another biologic before the end of the current treatment period. 2) Discontinuation, defined as no other biologic administered before the end of the treatment series, as defined above. 3) Censoring upon death, migration, initiation of off-label treatment, full colectomy (for UC patients only), end of the study, or double administration of biologics. National clinical guidelines do not recommend the combination of biologic therapies, therefore, in the current analysis, patients could only be exposed to one biologic at a time. In the case of the administration of more biologics on the same date, the treatment series was censored from analysis from the date of administration of the concomitant biologic.
Covariates
Data on sex, date of birth, date of death, and date of migration were retrieved from the Danish Civil Registration System [Citation7]. Information on tax-reported household income was registered by Statistics Denmark in the Income Statistics Register [Citation8], and calculated as an age- (18–24; 25–34; 35–44; 45–64, ≥65 years) and year-indexed average annual income reported by quartiles. Educational level [Citation9] was defined as the highest attained education categorized as primary/lower secondary; upper secondary/short cycle; Bachelor/Master/Doctoral; missing/not classified.
Data of diagnoses acquired at hospital contacts (primary, secondary, or additional diagnoses) and medical procedures were retrieved from the Danish National Patient Registry [Citation4]. IBD-related surgical procedures were defined by procedure codes (Table S1). Data of administration of biologics were identified as procedure codes from the Danish National Patient Registry [Citation4], or as a redeemed prescription from community pharmacies, retrieved from the National Prescription Registry [Citation10]. Information on comorbidity was included using the Charlson Comorbidity Index (CCI) [Citation11]. The CCI categorization was based on diagnosis (primary and secondary) assigned within ten years prior to the index date considering the diagnoses listed in Table S2 and the weighing suggested by Quan et al. 2011 [Citation12].
Concomitant treatment (within 120 days from treatment start) with 5-aminosalicylic acid, immunosuppressants (azathioprine, mercaptopurine, methotrexate, or corticosteroids was retrieved from the Danish National Patient Registry [Citation4] as procedure codes or as prescriptions redeemed from community pharmacies found in the National Prescription Registry [Citation10], supplementary table S3. As a covariate, comedication status was updated at the starting date of each biologic treatment series, considering prescriptions or administrations within 120 days prior to treatment start.
Statistical analyses
The descriptive characteristics were presented as medians with interquartile ranges (IQRs) for continuous non-normally distributed variables, and as frequencies with percentages for categorical variables. For the baseline characteristics, the distribution by biologic was analyzed using Kruskal–Wallis test for continuous variables and Fisher’s exact test for categorical variables, excluding GOL and UST due to few numbers.
Treatment patterns were presented separately for UC and CD as sunburst plots in which re-initiation of previous therapies and treatment breaks were allowed. A break implicated a new treatment line, irrespective of whether the following biologic was the same as the former or of another type (i.e. switch).
Drug persistence was evaluated at 6 months, 1, and 2 years for patients with full observation time throughout the given intervals, i.e. death, migration, end of study (31 december 2020) or other censoring factors not occurring before the end of the given interval.
Cumulative incidence plots of time to discontinuation and switch for the first and second treatment series separately were generated using cmprsk in R with death, migration, and the alternative to switch/discontinuation (or colectomy for UC patients only) as competing events.
For the endpoints discontinuation and switch, hazard ratios (HR) for first-time events were analyzed using Cox proportional hazard regression with time since the index as the underlying time scale and IFX as index drug, restricted to only first-line treatment. For second-line treatments, restricted to patients who had IFX as first-line treatment, VDZ, GOL and UST were compared to ADA. P-values were calculated using the Wald test. The estimates were presented as crude and adjusted for age (continuous); sex; education level; administrative health region; CCI (0; 1; >1); time since UC/CD diagnosis (continuous); previous IBD-related surgery (yes/no); and concurrent medical treatment of 5-ASA, immunosuppressants, or corticosteroids (yes/no for either of the comedication group). The analysis was censored at death, migration, change in IBD diagnosis, or end of the observation period (31 December 2020), and for UC patients also colectomy, whichever came first. For discontinuation as outcome, a switch was included as a competing risk.
The assumption of linearity was checked for all continuous variables for the survival analysis. Disease history was modelled as a polynomial to meet the assumption of linearity. The assumption of proportional hazards was checked using the assess statement in proc phreg [Citation13]. The proportionality assumption was violated as a high number of discontinuations following only one biologic treatment. Stratifying the follow-up period into before and after 174 days (12 weeks + 90 days) showed an improvement in the proportional hazards and no difference in risk estimates for CD. For UC, the risk estimates were significantly higher in the early period compared to the latter, however, the risk estimates were increased in the second period as well, and the final model included the entire time period.
In the survival analysis, an alternative definition of treatment length of 180 days (instead of 90 days).
Data management, statistical analyses, and graphics were performed in SAS Enterprise Guide 9.3 (SAS institute Inc), except the sunburst plots and the cumulative incidence plots generated in R using the packages sunburstR and cmprsk, respectively. The level of significance was 0.05, and estimates (incidence rates, HRs) were reported with 95% confidence intervals (CIs), where applicable.
Results
Study population
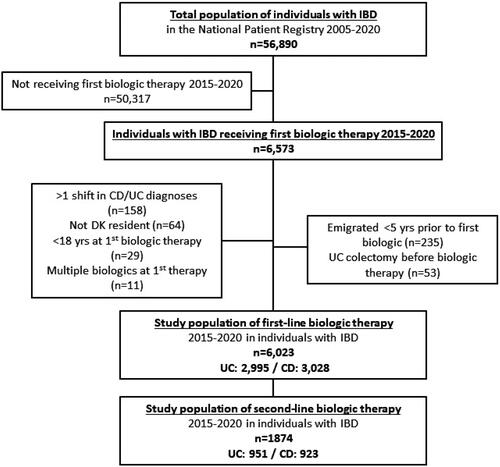
In the present study, 2,995 UC patients who received a first-time biologic therapy in 2015–2020 were identified (). The median age at treatment initiation was 40 years (IQR: 28–54), 49% were women and the median time from diagnosis to initiation of biologic therapy was 2 years (IQR: 0–8). Correspondingly, 3028 CD patients who received a first-time biologic therapy in the years 2015–2020 were identified. The median age at treatment initiation was 37 years (IQR: 25–51), 54% were women and the median time from diagnosis to initiation of biologic therapy was 1 year (IQR: 0–6).
Treatment pattern - UC
The majority of UC patients initiated biologic treatment with IFX (88%, n = 2677) ( and ). ADA was the second most used biologic therapy for first-line biologic treatment (6%, n = 178), followed by VDZ (3%, n = 98), and GOL (1%, n = 42).
Figure 2. Sunburst plots of biologic treatment patterns among UC (A) and CD (B) patients, starting at first-line biologic treatments only, 2015-2020.
IFX: infliximab; ADA: adalimumab; VDZ: vedolizumab; UST: ustekinumab; GOL: golimumab.
The inner ring represents the first biologic treatment (index biologic treatment) that the patient ever received (first-line treatments only) in 2015–2020. The second and following rings represent the next biologic treatment series either defined as a biologic therapy different from the former or separated from the former by an intermittent break. Note that treatment breaks and re-initiation of previous therapies are allowed. Treatment series are censured on migration, the second shift in IBD diagnosis (UC vs CD), reception of GOL in CD or UST in UC, simultaneous reception of more than one biologic. A patient may not appear in both the UC and CD cohorts.
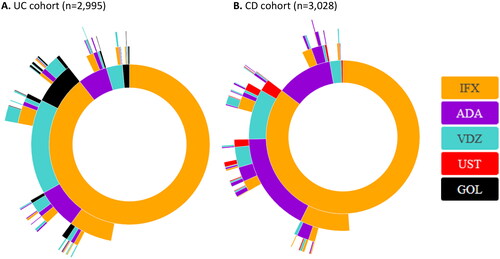
Table 1. Population baseline characteristics by IBD phenotype and type of first-line biologic treatment.
Among UC patients initiating biologic therapy, 35% (n = 1047) experienced more than one type of biologic therapy during the observation period, and 9% (n = 273) experienced ≥3 different therapies.
Overall, VDZ was the most used biologic as the second treatment among UC patients (49%, n = 423), followed by GOL (23%, n = 197), ADA (21%, n = 182), and IFX (6%, n = 54). Among UC patients initially treated with IFX, 29% (n = 767) switched to another biologic, mainly to VDZ (15%, n = 407). For UC patients with ADA as the first treatment series, 28% (n = 49) switched therapy, mainly to IFX (17%, n = 31). For UC patients starting on VDZ, 22% (n = 22) switched to another biologic during the study period, mainly to IFX, 16% (n = 16).
displays the treatment series of biologics for up to 4 treatment lines following the patients until the censoring or end of the study but allowing for treatment discontinuation and re-initiation of previous therapies (non-naïve) or new therapies (naïve). When restricting the cohort to patients with at least 1 year of follow-up, the proportion of patients not continuing beyond first-line treatment was reduced and especially for IFX, however, the treatment patterns were very similar for the restricted and the full cohort (Figure S1A). Restricting the cohort to patients with at least 2 years of follow-up made only a negligible difference (Figure S1C).
Treatment pattern - CD
Most CD patients initiated biologic treatment with IFX (85%, n = 2585) ( and ). ADA was the second most common initial biologic treatment (12%, n = 359), followed by VDZ (2%, n = 73) and lastly UST (0.4%, n = 11).
Among CD patients initiating biologic therapy, 34% (n = 1032) experienced more than one type of biologic therapy during the observation period, and 10% (n = 291) experienced ≥3 different therapies.
Overall, ADA was the most used biologic in the second treatment series among CD patients (56%, n = 419), followed by VDZ (29%, n = 215), UST (8%, n = 56), and IFX (7%, n = 52). In CD patients initially treated with IFX, 26% (n = 664) switched to another biologic, mainly to ADA (16%, n = 416). For CD patients initially treated with ADA, the frequency of switches was 16% (n = 59), mainly to IFX (11%, n = 39). For CD patients starting on VDZ, 23% (n = 17) switched to another biologic during the study period, mainly to IFX, 16% (n = 12).
We observed no substantial differences in treatment pattern when reducing the cohort to patients with at least 1 or 2 years of follow-up, respectively (Figure S1B and Figure S1D).
Patient characteristics by first-line treatment
Compared to IFX, UC patients starting on ADA as their first biologic treatment were generally older (p = 0.0002) when starting biologic treatment and at first IBD diagnosis (p = 0.04), they had a longer history with the UC diagnosis (p < 0.0001), a higher CCI (p < 0.0001) and were less frequent users of corticosteroids (p = 0.01) and 5-ASA (p = 0.01) before the index ().
To and even higher extent, UC patients starting VDZ were older at IBD diagnosis (p < 0.0001) and at biologics initiation (p < 0.0001), with a longer history of IBD diagnosis (p < 0.0001), and a higher CCI (p < 0.0001). Compared to IFX, more patients had a history of previous IBD-related surgery (p < 0.0001). For both ADA and VDZ, the majority were included in the last years of the study period. The number of UC patients starting on GOL was too low for comparison of patient characteristics.
CD patients starting on ADA as their first biologic treatment had a longer history with CD (p = 0.0002), a higher level of education (p = 0.02), more often a history of IBD surgery (p = 0.002), and less frequent users of immunosuppressants (p = 0.003) and corticosteroids (p = 0.006) before index compared to IFX. CD patients starting VDZ, compared to IFX, were older at diagnosis (p < 0.0001) and biologics initiation (p < 0.0001) and had a longer history with CD (p < 0.0001). Furthermore, they more often had a history with previous IBD-related surgery (p < 0.0001), a higher CCI (p < 0.0001), and less frequent use of immunosuppressants at index (p = 0.002). The number of CD patients starting on UST was too low for comparison of patient characteristics.
Drug persistence and risk of discontinuation or switch
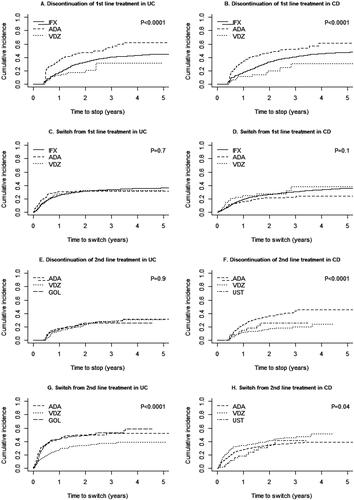
Among UC patients, ADA had the lowest drug persistence at all evaluated time points, with a particularly high discontinuation rate early after treatment initiation. Drug persistence at 6 months was the lowest for ADA with 55% vs ≥70% for the remaining biologics (). The same pattern was seen at 1 year (ADA: 29%, GOL: 37%, VDZ: 46%, IFX: 50%) and 2 years as well (with 6% for ADA vs >20% for the remaining biologics).
Table 2. Drug persistence in numbers and percentages of patients with Crohn’s disease or ulcerative colitis at 6 months, 1 year, and 2 years after start of first biologic treatment series, respectively.
Among CD patients, the drug persistence at 6 months was ≥60% for all biologics (). The highest level was for IFX (84%) and the lowest for ADA (73%). At 1 year the persistence was again the lowest for ADA (45% and the highest for IFX (61%). At 2 years, VDZ had the highest persistence (36%) followed by IFX (34%) and ADA at only 17% (). Numbers of UST users were too small to give an accurate estimate.
Separating the causes of non-persistence into discontinuation without switch to another biologic and switch to another biologic, we saw a higher risk of discontinuation of treatment comparing ADA to IFX among UC patients (adjusted HR: 2.02 [95% confidence interval: 1.57–2.60]) and CD patients (1.85 [1.52–2.24]), whereas no significant differences in the risk of switch to another biologic treatment were observed (). For both the UC and CD cohorts, ADA had a high discontinuation rate that started early after treatment initiation (). In contrast, there was a reduced risk of discontinuation of treatment in VDZ compared to IFX among UC patients (0.51 [0.29–0.89]), and a numerically reduced risk of discontinuation for CD patients (0.58 [0.32–1.03]). In both CD and UC patients, analyses of GOL, and UST as first-line treatments were impaired due to few events. Unadjusted analyses are presented in Figure S2.
Figure 3. Hazard ratios for discontinuation of treatment and switch to another biologic treatment among CD and UC patients, respectively. Estimates are presented with 95% confidence intervals (CI) and p-value. N: number of individuals in the analysis (N for exposure biologic [ADA or VDZ] / N for IFX). All HR estimates are relative to IFX (reference) and adjusted for age, sex, education, administrative health region, year of starting the biologic, time since diagnosis, previous IBD-related surgery, Charlson Comorbidity Index, and concurrent treatment with ASA-5, corticosteroids, and/or immunosuppressants.
![Figure 3. Hazard ratios for discontinuation of treatment and switch to another biologic treatment among CD and UC patients, respectively. Estimates are presented with 95% confidence intervals (CI) and p-value. N: number of individuals in the analysis (N for exposure biologic [ADA or VDZ] / N for IFX). All HR estimates are relative to IFX (reference) and adjusted for age, sex, education, administrative health region, year of starting the biologic, time since diagnosis, previous IBD-related surgery, Charlson Comorbidity Index, and concurrent treatment with ASA-5, corticosteroids, and/or immunosuppressants.](/cms/asset/316373eb-6510-4308-8c8d-f61bdf455754/igas_a_2173988_f0003_b.jpg)
While there was no difference in risk of discontinuation for VDZ vs ADA as second-line treatment (1.04 [0.73–1.47]) among UC patients with IFX as first-line treatment, the HR for switch from second-line treatment comparing VDZ to ADA was 0.66 (0.51–0.86) (Figure S3). The corresponding estimates for GOL were 1.30 [0.86–1.95]) for discontinuation and 1.03 [0.77–1.37] for switch (Figure S3). In the CD cohort, both VDZ and UST showed reduced risk of discontinuation (VDZ: (0.43 [0.29–0.62]), UST: (0.48 [0.25–0.93])) when compared to ADA. None of the analyses with switch as outcome showed any significant association (VDZ: (1.24 [0.95–1.61]), UST: (0.94 [0.54–1.63])).
Sensitivity analysis
The exposure algorithm was tested using an alternative grace period of 180 days instead of the originally defined 90 days. The sensitivity analyses showed no meaningful change in estimates (data not shown).
Discussion
The present study of biologic drug utilization in UC and CD patients in Denmark yielded five major findings. First, for both UC and CD patients, IFX remained the first-line biologic therapy for almost 9 in 10, as is also the official recommendation in Denmark [Citation14]. Second, patients initiating biologic therapy with ADA or VDZ differed from IFX initiators on a range of clinical and socio-demographic parameters in both UC and CD patients. Third, the most frequently initiated second-line treatment was VDZ for UC patients and ADA for CD patients. Fourth, one-year drug persistence was highest for initiators of IFX in the first treatment series, closely followed by VDZ, for both the UC and CD cohorts, whereas less than a third of ADA initiators remained on treatment in the UC cohort. Fifth, the time from diagnosis to initiation of biologic therapy was relatively short, with a median time of 2 and 1 years, in UC or CD, respectively.
The choice of a first-line biologic alternative to standard-of-care is most likely founded in reasonable clinical or personal circumstances that would lead to confounding by indication when analyzing the effect or adverse events of the different biologics. The risk of discontinuation of first-line biologic treatment was higher for ADA than for IFX for both CD and UC patients, while the risk of discontinuation was lower for VDZ than for IFX for UC but not for CD. Adjustment for predefined confounding variables did not substantially change the risk estimates, which raises the concern that the included covariables might not be sufficient to account for the suspected confounding by indication mentioned above. Application of propensity score matching might have helped eliminate the potential confounding of the risk estimates, however, the number of patients during the period 2015–2020 in Denmark treated with biologics other than IFX was too low to apply this method.
The persistence of biologic therapy might be influenced by co-medication. The use of 5-ASA, immunosuppressants, and corticosteroids at baseline was included in analyses of risk of discontinuation and switch, whereas co-medication after therapy start was not. It is unlikely that the inclusion of co-medication as a time-variable co-variate would have changed the estimates substantially, as the baseline use was already adjusted for. Future studies could investigate the effects of concomitant use of co-medication on the persistence of biologic therapies.
The median time span of 2 and 1 years from first diagnosis with UC or CD, respectively, to initiation of biologic treatment, was comparable to previous findings from the pan-European IBD cohort studies [Citation15,Citation16] and from the recently published Danish register-based study including patients with IBD during the period 2011 to 2018 [Citation17].
The present study observed the lowest drug persistence for ADA in both UC and CD patients, with a remarkably large proportion of discontinuation early after treatment initiation. In a Canadian population-based study, drug persistence at 1 year in CD patients with at least 3 years of history was 64.2% and 56.5% for initiators of ADA and IFX, respectively [Citation18], in line with a US study finding that first-line ADA initiators had 1-year drug persistence of 50.9%, followed by IFX with 47.6% [Citation19]. These estimates for IFX and ADA are comparable to the present findings. An earlier US-based study of 2330 CD patients found no difference in 6-months’ drug persistence between first-line IFX and ADA [Citation20], whereas in a French cohort study, the mean first-line drug persistence time was longer for IFX than ADA treatments in 587 CD patients [Citation21]. For UC patients, similarly to the present study, a US-based study of 1400 patients found higher 1-year drug persistence in IFX vs ADA [Citation22], corroborated recently in a US-based observational study of larger sample size showing higher persistence rates in both bio-naïve and bio-experienced UC patients [Citation23]. In a Brazilian observational study of UC patients, remission rates at 6 and 12 months were higher for IFX compared to ADA, although important clinical baseline characteristics also differed between the two groups including increased Mayo score and reduced frequency of immunomodulatory co-medication in ADA users [Citation24]. Similar to the present cohort, UC patients starting ADA had a longer disease history before initiating biologics than IFX users. Also in this cohort, persistence of IFX was higher than ADA [Citation25].
The increased likelihood of drug discontinuation of ADA users was also reported in the aforementioned Canadian study of CD patients who reached maintenance phase, resulting in an HR of 1.65 (95% CI: 1.15–2.37) compared to IFX [Citation18]. Real-life evidence of drug utilization of VDZ remains limited. Compared to anti-TNF biologics (IFX, ADA, and certolizumab together), a study of 1266 CD patients not conditioning on biologic history found lower 1-year drug persistence in VDZ users [Citation26]. Unfortunately, the combination of IFX and ADA in a single group, which in our study were not alike, impairs the comparability of our study. An Australian observational propensity score matched study found that first-line VDZ in UC had significantly longer persistence than first-line IFX (>50.2 versus 22.2 months, p = 0.001) [Citation27], which our study did not corroborate.
The present study included patients during the period 2015 to 2020 of which the latter two years were based on information from the new Danish National Patient Registry data model [Citation28]. The increasing trend in biologic use was similar across the period, and there were no indications of data breaches following the introduction of the new register. Further, the proportion of patients included was the highest in the latter period because of the increasing trend in the use of biologics seen throughout the study period 2015–2020. The entire study period was combined in the analyses. A Danish register-based study investigated the demographic profile of IBD patients initiating biologics according to year of initiation and found no difference, suggesting that pooling of the study years was permissible [Citation17].
The strength of the present study is relatively little socio-economic selection in the provision of healthcare due to the Danish national health insurance providing universal and tax-paid medical care including biologic treatment. Moreover, because no or negligible amounts of prescription drugs are consumed outside the reimbursement system or hospital settings these registrations have a high degree of completeness [Citation10,Citation29].
As the Danish National Patient Registry does not contain end dates of therapy, these were calculated by the pre-specified drug-dependent expected treatment duration for each administration, with an added grace period of 90 days. Comparable models have been employed by Brady et al. 2018 [Citation30] and Targownik et al. 2017 [Citation18]. To control for a potential bias in the algorithm failing to account for an excess in dose dispensation, an alternative analysis using a 180-day period was used. Another inherent limitation of the lack of dosing information is that dose escalation or reduction could not be calculated.
Further, the health registries did not contain any information on the reason for switching or discontinuing the biologic therapy. This impaired the interpretation of the analysis of discontinuation in particular, which may be due to clinical remission, lack or loss of clinical response, or adverse events [Citation31].
Conclusion
In conclusion, the present study showed that more than 85% of CD and UC patients initiating biologic therapy had IFX as their first-line biologic treatment, which is recommended by the official treatment guidelines. The reason for deviation from treatment guidelines and the higher incidence of treatment discontinuation for ADA as first-line treatment should be further explored in future studies.
Ethical approval
Ethical permission is not required for anonymized register studies according to Danish law. This study was approved by the Danish Data Protection Agency (ref. no. P-2020-39). The study was registered with ENCePP (EUPAS34845).
Author contributions
KJ and CB contributed equally to this work, share first authorship, and take responsibility for the integrity of the work as a whole, from inception to published article. KJ, CB, JP, CW designed the study. KJ, CB analyzed the data. JP supervised the statistical analyses. All authors contributed to the final version of the manuscript. All authors approved the final version of the manuscript.
Supplemental Material
Download MS Word (367.3 KB)Acknowledgments
We thank Mikkel Zöllner Ankarfeldt, Espen Jimenez Solem, Kristine Allin, and Kari Stougaard Jacobsen for invaluable input to the study design. The authors would further like to thank Kasper Vadstrup and Karen Jytte Summer former employees of Janssen Cilag A/S, for valuable input during the study progression.
Disclosure statement
No potential conflict of interest was reported by the author(s). CW is employee of Janssen Cilag. The sponsor and its employee had no access to the data at any time or any role in the data management and data analysis of the study.
Data availability statement
Data analyzed for the present study is in the domain of Statistics Denmark (www.dst.dk) and cannot be transferred to third party. Access to data can be applied for through Statistics Denmark and the Danish Health Data Authority, respectively.
Additional information
Funding
References
- Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. The Lancet. 2017;390:2769–2778.
- Kornbluth A, Sachar DB, Ulcerative colitis practice guidelines in adults: American College of gastroenterology. Am J Gastroenterol. 2010;105(3):501–523; quiz 524.
- Dubinsky MC. Reviewing treatments and outcomes in the evolving landscape of ulcerative colitis. Postgrad Med. 2017;129(5):538–553.
- Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490.
- Lo B, Vind I, Vester-Andersen MK, et al. Validation of ulcerative colitis and Crohn’s disease and their phenotypes in the Danish national patient registry using a population-based cohort. Scand J Gastroenterol. 2020;55(10):1171–1175.
- Lophaven SN, Lynge E, Burisch J. The incidence of inflammatory bowel disease in Denmark 1980–2013: a nationwide cohort study. Aliment Pharmacol Ther. 2017;45(7):961–972.
- Schmidt M, Pedersen L, Sørensen HT. The Danish civil registration system as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541–549.
- Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health. 2011;39(7 Suppl):103–105.
- Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health. 2011;39(7 Suppl):91–94.
- Pottegård A, Schmidt SAJ, Wallach-Kildemoes H, et al. Data resource profile: the danish national prescription registry. Int J Epidemiol. 2017;46(3):798–798f.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- Quan H, Li B, Couris CM, et al. Updating and validating the charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682.
- Borgan Ø, Zhang Y. Using cumulative sums of martingale residuals for model checking in nested case-control studies. Biometrics. 2015;71(3):696–703.
- medicinraadet.dk. Lægemiddelrekommandation med dyre lægemidler til behandling af kroniske inflammatoriske tarmsygdomme (IBD) - version 3.6. Available: https://medicinraadet.dk/arkiv/laegemiddelrekommandationer/mave-og-tarmsygdomme/kroniske-inflammatoriske-tarmsygdomme/kroniske-inflammatoriske-tarmsygdomme-3-6-rads-arkiv.
- Burisch J, Katsanos KH, Christodoulou DK, et al. Natural disease course of ulcerative colitis during the first five years of follow-up in a European population-based inception cohort-an Epi-IBD study. J Crohns Colitis. 2019;13(2):198–208.
- Burisch J, Kiudelis G, Kupcinskas L, et al. Natural disease course of Crohn’s disease during the first 5 years after diagnosis in a European population-based inception cohort: an Epi-IBD study. Gut. 2019;68(3):423–433.
- Zhao M, Sall Jensen M, Knudsen T, et al. Trends in the use of biologicals and their treatment outcomes among patients with inflammatory bowel diseases – a Danish nationwide cohort study. Aliment Pharmacol Ther. 2022;55(5):541–557.
- Targownik LE, Tennakoon A, Leung S, et al. Factors associated with discontinuation of anti-TNF inhibitors among persons with IBD: a population-based analysis. Inflamm Bowel Dis. 2017;23(3):409–420.
- Chen C, Hartzema AG, Xiao H, et al. Real-world pattern of biologic use in patients with inflammatory bowel disease: treatment persistence, switching, and importance of concurrent immunosuppressive therapy. Inflamm Bowel Dis. 2019;25(8):1417–1427.
- Osterman MT, Haynes K, Delzell E, et al. Comparative effectiveness of infliximab and adalimumab for Crohn’s disease. Clin Gastroenterol Hepatol. 2014;12(5):811–817.e3.
- Olivera P, Thiriet L, Luc A, et al. Treatment persistence for infliximab versus adalimumab in Crohnʼs disease: a 14-year single-center experience. Inflamm Bowel Dis. 2017;23(6):976–985.
- Comparative effectiveness and safety of infliximab and adalimumab in patients with ulcerative colitis - Singh - 2016. - Alimentary Pharmacology & Therapeutics - Wiley Online Library. [cited 7 Jul 2021]. Available: https://onlinelibrary.wiley.com/doi/full/10.1111/apt.13580.
- Sah J, Teeple A, Muser E, et al. Treatment persistence and maintenance dose titration among ulcerative colitis patients on biologics: a pooled study of three United States claim databases. Curr Med Res Opin. 2022;38(7):1093–1101.
- Sassaki LY, Magro DO, Saad-Hossne R, et al. Anti-TNF therapy for ulcerative colitis in Brazil: a comparative real-world national retrospective multicentric study from the Brazilian study group of IBD (GEDIIB). BMC Gastroenterol. 2022;22(1):268.
- Bargo D, Tritton T, Cappelleri JC, et al. Living with ulcerative colitis in Japan: biologic persistence and health-care resource use. Inflamm Intest Dis. 2021;6(4):186–198.
- Bohm M, Xu R, Zhang Y, et al. Comparative safety and effectiveness of vedolizumab to tumour necrosis factor antagonist therapy for Crohn’s disease. Aliment Pharmacol Ther. 2020;52(4):669–681.
- Pudipeddi A, Ko Y, Paramsothy S, et al. Vedolizumab has longer persistence than infliximab as a first-line biological agent but not as a second-line biological agent in moderate-to-severe ulcerative colitis: real-world registry data from the persistence Australian national IBD cohort (PANIC) study. Therap Adv Gastroenterol. 2022;15:175628482210807.
- vejledning-til-lpr3_f.pdf. Available: https://sundhedsdatastyrelsen.dk/-/media/sds/filer/forskerservice/vejledning-til-lpr3_f.pdf?la=da.
- Broe MO, Jensen PB, Mattsson TO, et al. Validity of antineoplastic procedure codes in the danish national patient registry: the case of colorectal cancer. Epidemiology. 2020;31(4):599–603.
- Brady JE, Stott-Miller M, Mu G, et al. Treatment patterns and sequencing in patients with inflammatory bowel disease. Clin Ther. 2018;40(9):1509–1521.
- Larsen L, Drewes AM, Broberg MCH, et al. Changing infliximab prescription patterns in inflammatory bowel disease: a population-based cohort study, 1999-2014. Inflamm Bowel Dis. 2018;24(2):433–439.