Abstract
Background
Paraconduit hernia is a relatively common long-term complication after esophagectomy which has the potential to cause great morbidity and even mortality. The aim of this study is to examine the risk factors and incidence of paraconduit hernia after minimally invasive esophagectomy (MIE) in esophageal adenocarcinoma patients who have received neoadjuvant treatment.
Methods
Minimally invasive esophagectomies done for patients with neoadjuvant-treated esophageal or esophagogastric junction adenocarcinoma at our institution between 2008 and 2018 were included in this study. All patients with symptomatic or incidentally found paraconduit hernias on computed tomography scans were identified. Patient demographics were analyzed using logistic and Cox regression.
Results
The incidence of paraconduit hernia was 14 out of 171 patients (8.2%). The hernia was surgically repaired in 10 (71.4%) of patients. Laparoscopic approach was used in 90% of the repairs, with one (11.1%) conversion to laparotomy. Emergency operations accounted for three (30%) of the operations. The complication rate was 10% (n = 1) and 90-day mortality was 10% (n = 1). Neither sarcopenia nor muscle mass loss was not associated with paraconduit hernia development, whereas preoperative radiotherapy (OR = 8.57, CI = 1.98–33.8, p = .002) was a strong risk factor for paraconduit hernia. Higher BMI had a protective effect (OR = 0.83 per point, 95% CI = 0.69–0.97, p = .027).
Conclusions
Paraconduit hernia is a relatively common complication after MIE for neoadjuvant-treated adenocarcinoma patients. Preoperative radiotherapy was associated with a higher risk of paraconduit hernia. Minimally invasive repair of paraconduit hernia after esophagectomy is efficient and has a low complication rate both in elective and emergency cases.
Introduction
The incidence of esophageal adenocarcinoma is increasing in many Western countries [Citation1,Citation2]. Esophagectomy is the only curative treatment in advanced disease, but it is associated with a high rate of perioperative complications [Citation3–5]. With current multimodality treatments and advanced staging methods, the 5-year survival of surgically treated esophageal adenocarcinoma has improved significantly [Citation3,Citation6]. Minimally invasive esophagectomy (MIE) has been established as a safe approach to treat esophageal cancer and is associated with less pulmonary complications and postoperative pain than open esophagectomy (OE) [Citation3].
Paraconduit hernia after esophagectomy is a rare but notable late-onset postoperative complication. It occurs when intraperitoneal contents, such as small or large bowel, herniate through the hiatal orifice and accounts for considerable morbidity due to life threatening emergencies with incarceration, strangulation and perforation of the bowel [Citation7–9]. The incidence of paraconduit hernia after MIE has been higher than after OE [Citation3,Citation7,Citation10–13]. With prolonged life expectancy, an increasing number of patients suffering from paraconduit hernia will potentially need treatment in the future. A recent review found the rate of paraconduit hernia after MIE to be 4.5% and 1.0% after OE, incidence rates ranging between 0 and 26% after MIE and 0 and 10% after OE [Citation14]. Treating paraconduit hernias by minimally invasive approach has been gaining traction and seems to be safe [Citation8,Citation10,Citation15].
The aim of this study was to explore the incidence of and risk factors related to paraconduit hernia development. As the link between muscle loss during neoadjuvant treatments and survival has been previously established, we postulated that the same effect could also be seen in paraconduit hernia development. To avoid unnecessary heterogeneity related to different neoadjuvant treatments and tumor location, only patients with adenocarcinoma of the esophagus or esophagogastric junction were examined. This population, according to our speculation, has a high risk of paraconduit hernia development due to tumor location and preoperative treatments received.
Methods
This is a retrospective consecutive case-series of paraconduit hernia after MIE from 2008 to 2018. Only patients with esophageal or esophagogastric junction adenocarcinoma were included. This was done to minimize the confounding brought by esophageal squamous cell cancer patients whose comorbidities and neoadjuvant treatments are different, and who have less hiatal hernias and gastroesophageal junction tumors compared to adenocarcinoma. As per previous reports, the incidence of paraconduit hernia in this population is low due to these factors. The primary endpoint was the development of paraconduit hernia.
Data acquisition
The data were collected from the electronic medical records, after a minimum of two-year follow up after MIE. Information collected included patient age, sex, BMI, tumor characteristics, neoadjuvant- and adjuvant treatment details, co-morbidities, and symptoms. Presence of paraconduit hernias was analyzed from follow-up CT scans operative notes. The operation reports of both the primary esophagectomy and hernia repair were studied for technical details. Follow-up reports and surveillance imaging were investigated for treatment outcomes including complications, mortality, readmissions, reoperations and hernia recurrence. The complications were graded according to the Clavien-Dindo classification [Citation16].
Neoadjuvant treatment
In our institution, patients with cT2–4 and N0–2 esophageal adenocarcinoma are considered for neoadjuvant therapy in a multidisciplinary meeting. The neoadjuvant regimen used during the study period was EOX (epirubicin, oxaliplatin and capecitabine), XELOX (oxaliplatin and capecitabine) or FLOT (docetaxel, oxaliplatin, fluorouracil and leucovorin); however, other regimens can be recommended based on patient factors [Citation17].
Diagnosis
Paraconduit hernia can be suspected based on the patient’s symptoms, such as recurrent episodes of abdominal and/or thoracic pain, fullness, early satiety, vomiting, respiratory or cardiac problems. Diagnosis is made by computed tomography, which delineates the involved anatomy well [Citation18].
Surgical treatment
Indications for surgery in our institution are based on individual patient characteristics. Incarcerated, strangulated or perforates paraconduit hernia is an absolute indication for emergent surgery. For elective repairs, the presence of paraconduit hernia with symptoms is an indication unless the patient is a poor candidate regarding anesthesia and/or surgical risk. Asymptomatic patients are evaluated for surgery and based on the overall fitness of the patient, expected survival is either followed or offered elective surgery. Our indications closely follow previously published reports [Citation18].
Minimally invasive and open Ivor Lewis and McKeown esophagectomies are performed in our center [Citation19–21]. In Ivor Lewis reconstruction, we use a 4 cm wide gastric conduit pulled up to the thoracic cavity and anastomosed approximately at the level of the azygos vein. In general, at the end of the laparoscopic procedure the conduit is fixed with sutures to the diaphragmatic crura. If the hiatal opening appears wide, it is loosely closed after the conduit is placed in the thoracic cavity during the laparoscopic portion of the operation.
Our approach to paraconduit hernia repair is a 4–5 port laparoscopic repair, in which, the hernia contents are mobilized completely and reduced back to abdomen. The possible hernia sac is excised, and the hiatus is closed with non-absorbable sutures and if needed, reinforced with mesh. The conduit is then re-fixed to the crura with sutures. Laparotomy and/or thoracotomy incisions are used if minimally invasive repair is not feasible. A mesh (GORE® DUALMESH®) repair is used when the hiatus cannot be repaired without tension without it. We use mesh by making a relaxing incision on the diaphragm and reapproximating the crura with sutures and then repairing the new diaphragmatic defect with biologic mesh.
Follow-up
Patients undergo a surveillance program within our institution for up to five years postoperatively. The follow-up for esophageal cancer includes outpatient appointments at 1, 3, 6, 12 and 18 months and then annually from 2 to 5 years, including CT scans at 6 months, 18 months, and 2, 3, 4 and 5 years postoperatively. A gastroscopy is performed at 3 months, 1 year, 2 years and 4 years post operatively.
Measurement of muscle parameters and sarcopenia definition
Esophageal cancer patients undergo a full body CT scan before the start of neoadjuvant therapy, after completion of neoadjuvant therapy and at 6 months after surgery. These scans were used to measure muscle mass and define sarcopenia before neoadjuvant treatments, before surgery and at 6 months of follow-up respectively. Scans were coded in order to blind the researchers from outcome. Images were imported to Osirix® Version 12.0 (32-bit Pixmeo, Sarl, Bernex, Switzerland). Abdominal musculature was delineated by use of a semi-automatic selection of region of interest tool from the level of L3. Psoas, quadratus lumborum, paraspinal, transverse abdominal, external oblique, internal oblique and rectus abdominis muscles were included. The Hounsfield Unit threshold range for skeletal muscle was −29 to +150. The images were manually corrected, if needed, by the propulsion and brush tools in Osirix©. Sarcopenia is defined as the progressive loss of muscle related to aging or disease [Citation22]. The cross-sectional total muscle area at the level of L3, skeletal muscle area (SMA; unit: cm2) was divided by the square of height (m2), which produced the skeletal muscle index (SMI). This method is suggested as the preferred method of measuring the muscle mass of cancer patients [Citation23]. SMI limit for sarcopenia was <52.4 cm2/m2 for men and <38.5 cm2/m2 for women, based on a previous study by Prado et al. [Citation24].
Statistics
The data were analyzed with R project (R Core Team (2020), R: A language and environment for statistical computing, R Foundation for Statistical Computing, Vienna, Austria, URL https://www.R-project.org/). The risk factors for hernia were calculated using binary logistic regression. Correlation to complications with categorical variables was done by χ2, or Fisher’s exact test and with numerical variables with binary logistic regression.
The study was approved by the Helsinki University Institutional Review Board (IRB) as part of the rare hernias study (HUS/60/2019).
Results
Patients
A total of 466 esophagectomies for esophageal cancer were performed in our center between 2008 and 2018. Of these, 335 cases were operated with MIE and 131 OE approach. Of these patients, 171 had adenocarcinoma of the esophagus or gastroesophageal junction, underwent neoadjuvant treatment and were operated on with MIE. Of these patients, 5.9% (n = 10) went through chemoradiotherapy with paclitaxel and carboplatin, 93.6% chemotherapy alone (n = 160) and 0.6% (n = 1) radiotherapy alone. The most used regimen for chemotherapy was three rounds of EOX (n = 116, 67.8%). Other regimens included XELOX (4.1%, n = 7), FLOT (2.9%, n = 5), SOX (S-1 and oxaliplatin; 0.6%, n = 1) and FOLFOX (folinic acid, fluorouracil and oxaliplatin; 0.6%, N = 1). The used regimen was not specified in 17.5% (n = 30).
Risk factors of paraconduit hernia
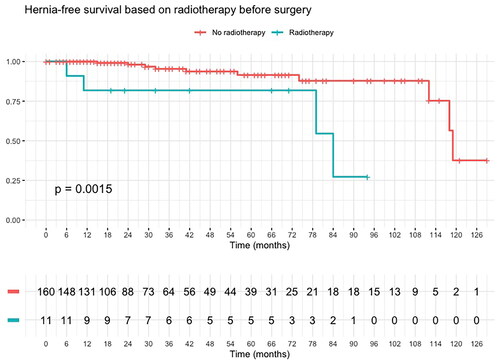
Paraconduit hernia was found in 14 (8.2%) patients after MIE for esophageal adenocarcinoma, the median time from operation to the diagnosis of paraconduit hernia was 17 months (range 0–113 months) (). At 3 years, the incidence of paraconduit hernia in the surviving patients was 11.4% and at 5 years 13.6%. The cumulative hazard of paraconduit hernia after MIE for esophageal and esophagogastric junction adenocarcinoma is shown in .
Figure 1. Cumulative hazard of paraconduit hernia after minimally invasive esophagectomy for esophageal and esophagogastric junction adenocarcinoma.
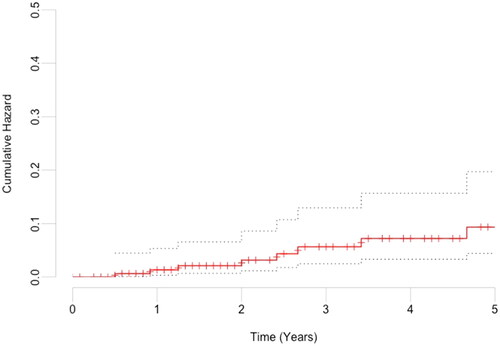
Table 1. Demographics of patients who underwent MIE after neoadjuvant treatment for esophageal and esophagogastric junction adenocarcinoma.
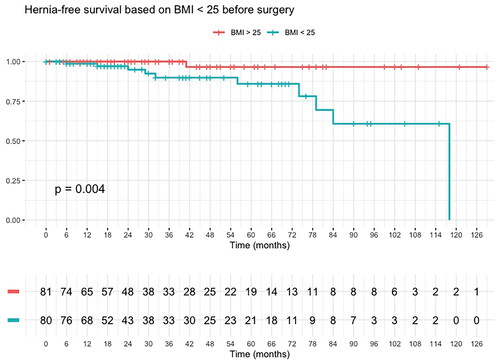
Univariate binomial logistic regression showed preoperative radiotherapy (OR = 8.57, CI = 1.98–33.8, p = .002) was a strong risk factor for paraconduit hernia. On the other hand, higher BMI had a protective effect (OR = 0.83, 95% CI = 0.69–0.97, p = .027). Using multivariate binomial logistic regression model, only preoperative radiotherapy (OR = 16.0, 95% CI = 2.11–14.4, p = .008) remained significant. These analyses are shown in . Kaplan–Meier’s curves of radiotherapy (p = .0015) and BMI under 25 (p = .004) can be appreciated in and , respectively.
Table 2. Risk factors for paraconduit hernia development.
A total of 120 (70.2%) patients in the group were sarcopenic before start of neoadjuvant treatments, 119 (70%) after neoadjuvant treatments and 127 (74.7%) at 6 months of follow-up. None of the skeletal muscle parameters were statistically significantly different between the groups.
Paraconduit hernia repair
The paraconduit hernia was operatively repaired in 71% of cases (n = 10). The overall rate of paraconduit hernia requiring operative repair in the study population was 5.8%. The median time from esophagectomy to hernia repair was 14 months (range 0–113 months). Laparoscopic approach was used in 90% (n = 9) of the hernia repairs, and 30% (n = 3) were operated on in urgent or emergent basis. The median operating time was 100 min (SD = 52 min). The details related to paraconduit repairs can be appreciated in . There was one (10%) mortality after repair of paraconduit hernia. The patient in question had peritoneal tumor implants and presented after seven days of symptoms from acute small bowel obstruction. Laparotomy was performed in a life-threatening situation with vital indication. The patient suffered from multiple organ failure and died on postoperative day 8.
Table 3. Characteristics of paraconduit hernia repair operations.
Discussion
Paraconduit hernia is a relatively common long-term complication after esophagectomy for distal esophageal and gastroesophageal junction adenocarcinoma with an incidence of 8.1%. It can be repaired safely with laparoscopic approach in experienced centers, with a complication rate of 10% in our series. Preoperative radiotherapy and a BMI of <25 were linked to higher risk of paraconduit hernia.
Preoperative radiotherapy was linked to a higher risk of paraconduit hernia, contrary to previous reports [Citation13]. Lung et al. surmised from their findings that radiation potentially provides an inflammatory and fibrosing effect to the hiatus that promotes formation of adhesions; however, regarding our opposite finding we postulate that radiation could weaken the tissues around the hiatus, making crural repairs ineffective [Citation13]. A BMI under 25 was also associated with increased risk of paraconduit hernia. Previous reports have found a similar effect regarding low body weight and paraconduit hernia risk [Citation25]. It is possible that malnutrition plays a role in paraconduit hernia risk, even though our series could not find correlation between sarcopenia and/or muscle loss and paraconduit hernia. On the other hand, a high BMI could protect against a paraconduit hernia as more fat in the mesenterium could lessen the mobility of small intestine and colon, and a high amount of fat along the greater curvature in the gastric tube could help fill the hiatus. Other factors, including hiatal hernia at the time of esophagectomy or stenting before esophagectomy were not associated with paraconduit hernia.
Patients with or without paraconduit hernia did not have differing skeletal muscle measurements before neoadjuvant treatments, preoperatively or after 6 months of follow-up. Kaplan–Meier’s survival curves did not differ between the groups based on degree of muscle loss between preneoadjuvant state and 6 months follow-up. These results indicate that sarcopenia or progressing muscle loss are not related to paraconduit hernia formation. To our knowledge, this study is the first to examine this relationship. Previous studies have, however established a strong link with loss of muscle mass during neoadjuvant treatments, sarcopenia in general, and worsened overall survival [Citation26–28].
In our study, the incidence of paraconduit hernia (8.1%) is in line with previous reports [Citation3,Citation10]. Rate of paraconduit hernia repair after MIE for esophageal or esophagogastric junction adenocarcinoma was 5.8%, which is also similar to earlier reports [Citation3,Citation8,Citation10]. Laparoscopic repair was used in 90% of paraconduit hernia repair. Only one conversion to laparotomy was necessary, and this patient had peritoneal carcinosis and was in extremis from small bowel obstruction. Apart from the single patient whose prognosis was very grim related to the carcinomatosis of the peritoneal space, there were no mortality or complications related to the hernia repairs. In previous review by Oor et al., the pooled morbidity rate was 25% (range: 0–60%) [Citation14].
The majority (71.4%, N = 10) of the paraconduit hernias in this study were diagnosed more than one year after the esophagectomy, and only one case within 3 months. To prevent early paraconduit hernia in our institution, the graft is sutured to the crura. If the hiatal opening is loose, we perform hiatoplasty at the posterior or anterior hiatus, depending on the anatomy. We also leave most of the omentum to the gastric conduit, which fills up the crura. Some authors greatly advocate for hiatoplasty during esophagectomy, especially anterior hiatoplasty. We believe that these methods could prevent paraconduit hernia formation in the immediate postoperative period. However, no comparative data on the effect of these maneuvers exist. In previous studies, the hernias that appeared early after esophagectomy were associated with high morbidity [Citation29]. To the authors’ knowledge, no data on prevention of late paraconduit hernia have been published, but we believe that prevention of malnutrition, weight loss and cancer recurrence are the most significant factors.
The strength of this study was that even though it is a retrospective study, there was good follow-up data available due to standard follow-up protocols after esophagectomy for esophageal cancer. Additionally, Finland has a centralized archive for medical records, so that data on complications can be achieved even if they are treated at another institution.
However, there are some limitations. There is a chance for selection and information bias. As esophageal cancer is a disease with high mortality, there is considerable mortality in follow-up. We included only patients with adenocarcinoma of the distal esophagus and gastroesophageal junction, who had received neoadjuvant therapy in order to maximize homogeneity. This means the results can not automatically be generalized to all esophagectomy patients. Also, mortality is a competing outcome with paraconduit hernia, and same factors could be affecting both.
According to our study, paraconduit hernia is a relatively common complication in patients undergoing MIE for esophageal- or esophagogastric junction cancer after neoadjuvant treatments. Preoperative radiotherapy was associated with paraconduit hernia development, as was a low preoperative BMI. Minimally invasive repair seems to be safe in experienced centers.
| Abbreviations | ||
| BMI | = | Body mass index |
| CI | = | Confidence interval |
| CT | = | Computed tomography |
| ECOG | = | Eastern Cooperative Oncology Group |
| EOX | = | epirubicin, oxaliplatin and capecitabine |
| FLOT | = | docetaxel, oxaliplatin, fluorouracil and leucovorin |
| FOLFOX | = | folinic acid, fluorouracil and oxaliplatin |
| MIE | = | Minimally invasive esophagectomy |
| OE | = | Open esophagectomy |
| OR | = | Odds ratio |
| SMA | = | Skeletal muscle area |
| SD | = | Standard deviation |
| SMI | = | Skeletal muscle index |
| SOX | = | S-1 and oxaliplatin |
| XELOX | = | oxaliplatin and capecitabine |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381–387.
- Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49(6):1374–1403.
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379(9829):1887–1892.
- Scheepers JJ, Mulder CJ, Van Der Peet DL, et al. Minimally invasive oesophageal resection for distal oesophageal cancer: a review of the literature. Scand J Gastroenterol Suppl. 2006;41(Suppl. 243):123–134.
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349(23):2241–2252.
- Shapiro J, van Lanschot JJB, Hulshof M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol. 2015;16(9):1090–1098.
- Matthews J, Bhanderi S, Mitchell H, et al. Diaphragmatic herniation following esophagogastric resectional surgery: an increasing problem with minimally invasive techniques?: Post-operative diaphragmatic hernias. Surg Endosc. 2016;30(12):5419–5427.
- Ulloa Severino B, Fuks D, Christidis C, et al. Laparoscopic repair of hiatal hernia after minimally invasive esophagectomy. Surg Endosc. 2016;30(3):1068–1072.
- Price TN, Allen MS, Nichols FC, et al. Hiatal hernia after esophagectomy: analysis of 2,182 esophagectomies from a single institution. Ann Thorac Surg. 2011;92(6):2041–2045.
- Gooszen JAH, Slaman AE, van Dieren S, et al. Incidence and treatment of symptomatic diaphragmatic hernia after esophagectomy for cancer. Ann Thorac Surg. 2018;106(1):199–206.
- Bronson NW, Luna RA, Hunter JG, et al. The incidence of hiatal hernia after minimally invasive esophagectomy. J Gastrointest Surg. 2014;18(5):889–893.
- Crespin OM, Farjah F, Cuevas C, et al. Hiatal herniation after transhiatal esophagectomy: an underreported complication. J Gastrointest Surg. 2016;20(2):231–236.
- Lung K, Carroll PA, Rogalla P, et al. Paraconduit hernia in the era of minimally invasive esophagectomy: underdiagnosed? Ann Thorac Surg. 2021;111(6):1812–1819.
- Oor JE, Wiezer MJ, Hazebroek EJ. Hiatal hernia after open versus minimally invasive esophagectomy: a systematic review and meta-analysis. Ann Surg Oncol. 2016;23(8):2690–2698.
- Lowe C, Subar D, Hall C, et al. Hiatal hernias presenting as a late complication of laparoscopic-assisted cardio-oesophagectomy. Hernia. 2010;14(2):211–213.
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196.
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
- Hölscher AH, Fetzner UK. Paraconduit hiatal hernia after esophagectomy. Prevention-indication for surgery-surgical technique. Dis Esophagus. 2021;34(9):doab025.
- Lewis I. The surgical treatment of carcinoma of the oesophagus; with special reference to a new operation for growths of the middle third. Br J Surg. 1946;34:18–31.
- McKeown KC. Total three-stage oesophagectomy for cancer of the oesophagus. Br J Surg. 1976;63(4):259–262.
- Orringer MB, Sloan H. Esophagectomy without thoracotomy. J Thorac Cardiovasc Surg. 1978;76(5):643–654.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European Consensus on Definition and Diagnosis: report of the European Working Group on Sarcopenia in older people. Age Ageing. 2010;39(4):412–423.
- Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495.
- Prado CMM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–635.
- Ganeshan DM, Correa AM, Bhosale P, et al. Diaphragmatic hernia after esophagectomy in 440 patients with long-term follow-up. Ann Thorac Surg. 2013;96(4):1138–1145.
- Paireder M, Asari R, Kristo I, et al. Impact of sarcopenia on outcome in patients with esophageal resection following neoadjuvant chemotherapy for esophageal cancer. Eur J Surg Oncol. 2017;43(2):478–484.
- Tamandl D, Paireder M, Asari R, et al. Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. Eur Radiol. 2016;26(5):1359–1367.
- Jarvinen T, Ilonen I, Kauppi J, et al. Loss of skeletal muscle mass during neoadjuvant treatments correlates with worse prognosis in esophageal cancer: a retrospective cohort study. World J Surg Oncol. 2018;16(1):27.
- Sutherland J, Banerji N, Morphew J, et al. Postoperative incidence of incarcerated hiatal hernia and its prevention after robotic transhiatal esophagectomy. Surg Endosc. 2011;25(5):1526–1530.