Abstract
Background and Aims
The clinical course of patients with liver cirrhosis and adherence to hepatocellular carcinoma (HCC) screening guidelines are not well studied in the Netherlands. We investigated this and potential risk factors for decompensation and transplant-free survival (TFS) in a large regional cohort.
Methods
We performed a retrospective cohort study of patients with confirmed liver cirrhosis in Amsterdam, the Netherlands. Clinical parameters, decompensation events, development of HCC, and medication use were extracted from medical records.
Results
In total, 681 hospitalized and outpatients were included. Mortality risk was increased by: age (aHR 1.07, p < 0.01), smoking (aHR 1.83, p < 0.01), decompensated initial presentation (aHR 1.43, p = 0.04) and increased MELD (aHR 1.07, p < 0.01). PPI use tended to increase mortality risk (aHR 1.35, p = 0.05). The risk of future decompensation was increased with increased age (aHR 1.02, p < 0.01), decompensated initial presentation (aHR 1.37, p = 0.03) and alcohol misuse as etiology (aHR 1.34, p = 0.04). Adequately screened patients for HCC had a longer TFS compared to patients who were not (48 vs 22 months), p < 0.01).
Conclusions
In patients with cirrhosis, decompensation at initial presentation was associated with an increased risk of future decompensation and mortality. Alcoholic cirrhosis was associated with an increased risk of future decompensation. Adequate HCC surveillance was associated with markedly better survival.
Introduction
Liver cirrhosis is a pathophysiologic entity resulting from chronic liver injury. The development of cirrhosis is characterized by chronic inflammation leading to fibrosis in the liver [Citation1]. As fibrosis progresses, increased structural and functional hepatic vascular resistance is observed. As a result, cirrhosis is associated with impaired liver function and hyperdynamic circulation and splanchnic vasodilation. Subsequently increased inflow in the portal vein leads to portal hypertension. Furthermore, the risk of development of hepatocellular carcinoma (HCC) is increased [Citation2]. Chronic alcohol misuse and chronic hepatitis C (CHC) are still the main underlying causes of liver cirrhosis in developed countries [Citation2]. But due to effective therapeutic options in CHC on the one hand and the obesity epidemic on the other hand, the incidence of cirrhosis due to non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) is rising alarmingly [Citation3].
Cirrhotic patients in a clinically compensated state are often asymptomatic. Whilst the disease progresses, complications such as ascites, spontaneous bacterial peritonitis (SBP), variceal bleeding, or hepatic encephalopathy (HE) occur. The presence of any of these complications marks the decompensated state, which is observed at annual rates of 5–7% [Citation4,Citation5]. Overall, median survival in patients with compensated liver cirrhosis is approximately 12 years but decreases to approximately 2 years in the decompensated state [Citation4]. Decompensation is often triggered by an event like infection or gastrointestinal bleeding, or ongoing alcohol misuse [Citation6]. Nevertheless, in the majority of patients, a trigger of decompensation cannot be identified [Citation6].
Recently, proton pump inhibitors (PPIs) and, lipophilic statins became of interest due to their possible influence on the course of the disease. PPIs were reported to increase the risk of hepatic encephalopathy in patients with liver cirrhosis [Citation7–9]. In contrast, lipophilic statins (like simvastatin and atorvastatin) appeared to reduce the risk of hepatic decompensation and might improve overall survival [Citation10]. In addition to reducing serum lipid levels, statin use is associated with reduced hepatic inflammation and collagen production [Citation11,Citation12]. A multicentre randomized controlled trial including 59 patients with liver cirrhosis, showed a decrease (8.3%) in portal pressure in patients treated with simvastatin compared to placebo [Citation13].
HCC has a marked impact on survival and a rising incidence. From 1990 to 2016, incidence rates of HCC increased by 4.3% in the Netherlands [Citation14], higher than in any other 195 countries reported [Citation14]. The Netherlands was followed by the United Kingdom (4.1%) and the USA (3.3%) [Citation14].
Currently, data regarding the incidence, etiology, natural course, and transplant-free survival (TFS) of patients with liver cirrhosis in the Netherlands are scarce. Moreover, insight into adherence to HCC screening protocols is lacking. Therefore, we investigated the clinical course, potential factors associated with decompensation and TFS, and adherence to HCC screening protocols in a large cohort of cirrhotic patients from the Amsterdam metropolitan area.
Methods
This is a retrospective observational cohort study of patients diagnosed with liver cirrhosis in two large hospitals in Amsterdam, one university hospital (Amsterdam University Medical Centres, location AMC) and one large community hospital (Onze Lieve Vrouwe Gasthuis, OLVG). Patients were identified by ICD-10 codes in the electronic health record (EHR) systems (2015–2020 OLVG; 2014–2018 AMC). Retrospectively the date of diagnosis of cirrhosis was identified, as a starting point for data collection. Data was extracted from EHRs by two investigators and reviewed by an independent third investigator.
Inclusion and exclusion criteria
Patients were included when there was a confirmed diagnosis of cirrhosis by standard clinical criteria, radiologic features and/or histology, independent of etiology and patients were 18 years or older at the time of diagnosis. This could be either hospitalized patients or patients from the outpatient clinic.
Patients were excluded when they were lost-to-follow-up within the first year of presentation or when there were fewer than two visits to the outpatient clinic. However, patients who died within one year because of liver-related disease were included. Uncertainty about the diagnosis or medical information was discussed with a hepatologist (RBT).
Recruitment and consent
All identified living patients from the OLVG were informed by mail and requested to give informed consent (opt-in). In case of no response, a repetitive effort was made to make contact by telephone four weeks after sending the letters. Living patients that met inclusion criteria from Amsterdam University Medical Centers, location AMC, were sent a letter and given the chance to object by an opt-out letter before collecting the data from their health records (opt-out).
Outcome variables
The primary outcome measure of this study was transplant-free survival (TFS). Secondary outcomes were the occurrence of decompensation, the number of episodes of decompensation, and the occurrence of other liver-related complications (SBP, variceal bleeding, HE, and development of HCC). Study parameters were gender, age, etiology of cirrhosis, type of initial presentation of liver cirrhosis (compensated or decompensated), comorbidity at baseline, concomitant use of PPI and/or statins, tobacco and/or current alcohol use at the time of diagnosis, Model for End-stage Liver Disease (MELD) score, Child-Pugh (CP) score, participation in a screening program for HCC, liver transplantation and cause of death. Chronic drug use (PPI, statins) was defined as the use for at least six months. A composite variable of relevant high-risk comorbidity related to NAFLD/NASH was composed of cardiovascular disease, diabetes mellitus, and peripheral artery disease. Patients were categorized into the following groups of etiologies; alcoholic, viral hepatitis C/B/B + D, alcoholic and viral hepatitis, cholestatic, autoimmune, NAFLD/NASH, cryptogenic or other. If multiple causes of liver cirrhosis were documented in the medical record, all were registered. When the cause was unclear, this was registered as cryptogenic cirrhosis. Cases were categorized as “other” if the cause was known but uncommon, for example, alpha-1-antitrypsin deficiency.
Decompensated cirrhosis was defined by the occurrence of any of the following events: variceal bleeding, (refractory) ascites, hepatic hydrothorax, HE grades 3–4 and/or hepatorenal syndrome. Variceal bleeding was registered if the bleeding was confirmed by endoscopy. The classification of ascites was purely based on clinical examination and categorized into three groups; absent, slight/moderate (grade 2), including ascites suppressed/under control by diuretics, and severe when visible with physical examination (grade 3). Classification of ascites was only based on clinical examination, not on radiological examination. HE was registered if stated clearly in the medical record. The classification was according to the West Haven (or Conn) criteria, which was divided into absent, grade 1–2, and grade 3–4.
CP and MELD scores were calculated at baseline using the blood results in combination with clinical findings (if available). If only prothrombin time (PT) was registered and INR was unknown, the normal limits of the PT were used to calculate a derived INR.
The EASL guideline recommends that HCC surveillance using abdominal ultrasound should be performed every 6 months [Citation15]. In some cases, there were limiting factors to surveillance by ultrasound, for example, a patient's habitus. Therefore, screening with any radiologic modality was included.
Transplant-free survival was measured as the time until death, liver transplantation or end of follow-up; here defined as the assessment date of the patient file. Death, including date and cause of death, were registered if available.
Statistical analysis
Continuous variables were expressed as medians with ranges or 95% confidence interval (CI) or mean and standard deviation (SD), and categorical variables as absolute and relative frequencies. Pearson’s Chi-square or Fisher’s exact test was used, where appropriate, to compare categorical data in the compensated and decompensated groups. Continuous data were compared using the Student’s t-test or Mann–Whitney U-test. The Kaplan–Meier method and log-rank tests were used to estimate and compare transplant-free survival. Cox regression analysis was used to calculate hazard ratios (HRs) and assess the association of baseline variables, with TFS, HE and the development of future decompensation events. All variables with a two-sided p-value < 0.05 in univariate analysis were included in a subsequent multivariable model using conditional backward selection. A two-sided p-value < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS statistics (version 26.0; IBM Corp., Armonk, NY).
Ethics
The study protocol was approved by the Medical Ethics Review Committee of the Academic Medical Centre in Amsterdam (W19_031#19.050) and the local scientific advising committee of the OLVG, Amsterdam (WO 19.131) and was executed in line with the principles of the Declaration of Helsinki and current regulations.
Results
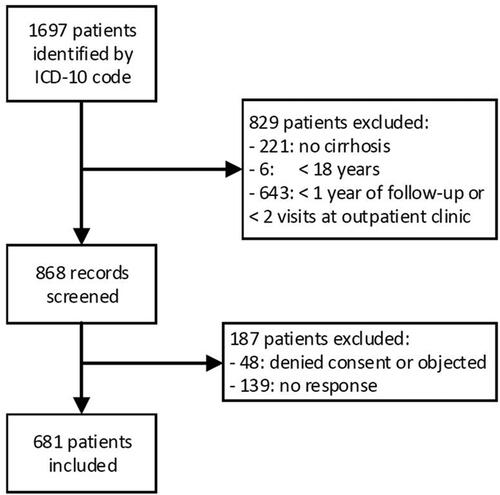
A total of 1697 patients were identified by ICD-10 code. 829 patients did not meet the in-/exclusion criteria and 187 patients were excluded because of no consent, no response or opt-out of the use of data. In total 681 hospitalized and outpatients were included ().
Figure 1. Flowchart of included patients. From the 1697 identified patients, 868 records were screened and 681 patients were included in analyses.
The mean age at diagnosis was 57 years (± 12 years) and the majority was male (67%). The main etiologies were alcoholic cirrhosis (40%), chronic hepatitis C (15%) and NAFLD/NASH (10%). Thirty-eight percent of the patients were decompensated at the initial presentation. One-third of the patients had a MELD score ≤9, 34% had a MELD score of 10-19, 8% of 20-29, 2% of 30-39 and 1 patient had a MELD of 40. From 24% of the patients, no MELD score could be calculated, mainly due to missing haemostatic parameters. The CP classification could be determined in 70% of the patients at baseline. Thirty-eight per cent had CP-A, 22% had CP-B and 11% CP-C cirrhosis at baseline.
In characteristics of compensated and decompensated patients are shown. Compared to patients with compensated cirrhosis, patients presenting with decompensated cirrhosis were more often alcoholic (71% vs 34%; p < 0.01) and fewer had underlying viral hepatitis or NAFLD/NASH (12% vs 35%; p < 0.01, 9% vs 18%; p < 0.01 respectively). Patients presenting with decompensated liver cirrhosis had ascites in 62% of the cases, followed by variceal bleeding (15%), ascites and variceal bleeding (4%) and HE (3%). After the initial presentation, either compensated or decompensated, patients with decompensated cirrhosis had more episodes of decompensation (2.73 vs 1.61; p < 0.01) during follow-up.
Table 1. Patients presenting at diagnosis of cirrhosis: comparing compensated and decompensated patients.
Transplant-free survival
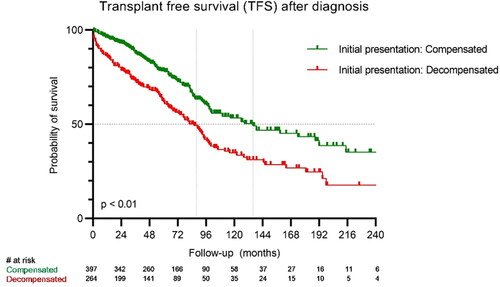
Overall, the median TFS was 100 months (95% CI 86–114 months). Thirty-two (5%) patients underwent liver transplantation. Patients who presented with decompensated liver cirrhosis at the time of diagnosis had a lower TFS compared to patients who presented with a compensated liver cirrhosis at the time of diagnosis (86 months (95% CI 73–99 months) versus 136 months (95% CI 92–180 months); p < 0.01). () Considering Child-Pugh (CP) scores, of the patients with a CP-A score 50% were still alive at the end of follow-up (median 189 months). CP-B patients had a median survival of 90 months, which is higher compared to CP-C patients: 56 months (p < 0.01) using the Log Rank test.
Mortality
Risk factors of mortality in univariate analysis were age (HR 1.06 per year, p < 0.01), alcoholic cirrhosis (HR 1. 77, p < 0.01), smoking (HR 1.53, p < 0.01), high-risk comorbidity related to NAFLD/NASH (HR 1.55, p < 0.01), initial presentation with decompensated liver cirrhosis (HR 1.85, p < 0.01), MELD score (HR 1.06 per point increase, p < 0.01), PPI use (HR 1.74, p < 0.01) and statin us (HR 1.53, p < 0.01). In multivariate analysis, higher age (aHR 1.07 per year, p < 0.01), smoking (aHR 1.83, p < 0.01), initial presentation with decompensated liver cirrhosis (aHR 1.43, p = 0.04) and MELD score (aHR 1.07, p < 0.01) were identified as independent risk factors for mortality (). PPI use showed a clear trend (aHR 1.35, p = 0.05) as a risk factor for mortality.
Table 2. Risk factors, univariate and multivariate for mortality in cirrhotic patients.
First decompensation events of initially compensated patients
Patients who were initially compensated (n = 403) and during follow-up decompensated (44%) mainly had as a first decompensating event: ascites 99 (56%), variceal bleeding 35 (20%), HE 43 (25%) or combinations of those events.
Future decompensation
Univariate analysis showed that risk factors for future development of new episodes of decompensation were age (HR 1.02, p < 0.01), alcoholic liver cirrhosis (HR 1.44, p < 0.01), initial presentation with decompensated liver cirrhosis (HR 1.40, p < 0.01), a higher MELD score (HR 1.04 per point increase, p < 0.01) and PPI use (HR 1.25, p < 0.05). In multivariate analysis, age (aHR 1.02, p < 0.01), alcoholic liver cirrhosis (aHR 1.34, p = 0.04) and initial presentation with decompensated liver cirrhosis (aHR 1.37, p = 0.03) were associated with future decompensation ().
Table 3. Risk factors, univariate and multivariate for future decompensation in cirrhotic patients.
Hepatic encephalopathy
Two hundred-one patients (29%) experienced at least one episode of admission for HE during follow-up, possibly in combination with another event. Admissions for HE as further decompensation event occurred in 115 patients. Univariate analysis showed that risk factors for HE was age (HR 1.03, p < 0.01), high-risk comorbidity related to NAFLD/NASH (HR 1.59, p = 0.01), initial presentation with decompensated liver cirrhosis (HR 1.55, p = 0.02), higher MELD score (HR 1.05 per point increase, p < 0.01). In multivariate analysis increased age and MELD were associated with an increased risk for developing HE (aHR 1.03, p < 0.01 and 1.05, p < 0.02 respectively).
PPI use
Since chronic use of PPI was very common (81%), we further explored the indications. Of 54% of the patients using PPI, there was no clear indication. Thirteen per cent used it for documented peptic reflux disease, 13% for co-medication and 10% after documented peptic ulcers. PPI use was more common in patients with diabetes (34% vs 23%), chronic kidney disease (8% vs 5%), heart failure (7% vs 3%), peripheral artery disease (6% vs 2%) and a history of acute myocardial infarction (8% vs 3%) compared to non-PPI users. We did not find a correlation between the development of HE and the use of PPI.
HCC screening
Six monthly screenings for HCC occurred in 46% of the patients. Reasons to deviate from the guidelines were mainly the decision of the physician (30%) or incompliance (21%), but mostly it was unknown (35%).
HCC survival
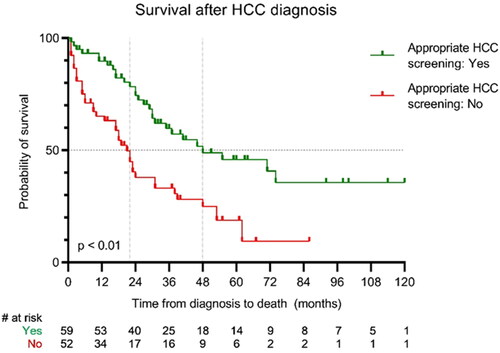
One hundred twenty-seven patients (19%) were diagnosed with HCC over a median period of 36 months after diagnosis of cirrhosis (IQR 8.5–78.5). Once diagnosed they had a median TFS of 31 months. There was a difference in TFS between patients who were adequately screened for HCC according to the screenings guidelines compared to patients who were not screened according to guidelines (48 months (95% CI 18–78 months) versus 22 months (95% CI 17–27 months); p < 0.01) (). The distribution of CP-scores over the groups was equal: 94% CP-A/B in the group of patients with adequate screening vs. 91% CP-A/B in the group who were not screened according to guidelines.
Discussion
In this study, a cohort of patients with liver cirrhosis in a metropolitan area in the Netherlands was studied. Patients who presented with decompensated liver cirrhosis had a lower TFS compared to patients who presented with compensated liver cirrhosis. After smoking, decompensation was the strongest independent risk factor for both the future development of a new episode of decompensated liver cirrhosis and for mortality. Other risk factors for mortality were higher age and higher MELD scores. For chronic PPI use, there was a clear trend towards increased mortality risk. The main risk factor for the development of future episodes of decompensation, next to the presentation with decompensated cirrhosis at baseline, was alcoholic cirrhosis. Patients with HCC who participated in a screening program for HCC had a more than double life expectancy compared to patients who did not.
Overall TFS of compensated cirrhotic patients in our cohort was comparable to previously reported survival in literature (189 months compared to previously reported > 144 months) [Citation4]. Remarkable for us, decompensated cirrhotic patients in our cohort had a better median survival (86 months compared to previously reported 24–36 months) [Citation4]. A critical appraisal of the survival curves () shows short-term increased mortality in patients who present with decompensated cirrhosis. Thereafter is a phase of comparable survival. However, after approximately 86 months there is a long decline in the curve of patients with decompensated cirrhosis compared to compensated cirrhosis, despite maximum care. Although selection bias cannot be excluded due to the nature of the study, improved survival in decompensated patients might be due to the fact that countries with diverse healthcare systems and public health standards were included in the previous report. There are also differences in etiologies of cirrhosis and progress in therapeutic interventions [Citation16].
Our study confirmed alcohol use as a risk factor for liver-related mortality. Our cohort is similar to other Western cohorts, with a high percentage of alcohol use as etiology of cirrhosis [Citation17]. It didn’t surprise us that patients who initially presented with decompensated cirrhosis more often have alcohol use as etiological factor. Alcohol use remains a major challenge for healthcare workers and society as a whole, as it is a major cause of cirrhosis and decompensation [Citation2,Citation6]. Even low alcohol consumption negatively influences outcomes in alcohol-related cirrhosis [Citation18]. In society, alcohol misuse results in extraordinary costs, which might be partly avoidable [Citation19]. To decrease the burden of cirrhosis, decompensation, mortality and related costs, public policymakers try to reduce alcohol misuse. Unfortunately, progress in tackling problematic alcohol use is slow [Citation19].
In our study, the use of statins was not associated with improved survival or a decreased risk of decompensation. The beneficial effects of statins on portal pressure in cirrhotic patients and HE in particular have been described before [Citation13,Citation20–22]. Mechanistically, statins lower the portal pressure by reducing systemic and intrahepatic inflammation and with that the risk of decompensation. Administering a low dose (20 mg/day) of simvastatin to cirrhotic patients is safe and could have an impact on prognosis [Citation23]. However, most data come from retrospective cohort studies. Currently, a prospective study (LIVERHOPE_EFFICACY trial) is underway to evaluate the efficacy of this low-dose simvastatin combined with rifaximin in patients with (advanced) liver cirrhosis (NCT03780673).
There was a clear trend for chronic PPI use as a risk factor for mortality (aHR 1.35; p = 0.05). This phenomenon has been discussed extensively before and remains a topic of ongoing debate [Citation9,Citation24]. In our analyses, we aimed to correct confounding factors like NAFLD/NASH and related comorbidities such as patients with a cardiovascular risk profile and type 2 diabetes mellitus by adding a composite variable. Both are associated with liver-related mortality and morbidity [Citation25]. Strikingly, in more than half of the cases, it was unknown why patients used PPIs. There is a need for awareness among physicians of the potential risk associated with unnecessary chronic use of PPIs and discontinuation should be considered in every patient with liver cirrhosis [Citation9,Citation26]. Recently the American Gastroenterological Association published a Clinical Practice Update on the de-prescribing of PPIs [Citation26]. Appropriate PPI use with a proper indication and a dose as low as possible should be the treating principle, which should be evaluated regularly.
There is increasing awareness of the benefit of disease prevention and adequate screening. Unfortunately, in the United States a recent analysis of HCC screening showed only 9% adherence to the guidelines [Citation27]. In this cohort adherence to screening, guidelines were better (46%). This number is also higher than the 27% that was described before in a national Dutch HCC cohort [Citation28]. Patients who were adequately screened had better survival, mainly because tumours were first detected at a smaller size and were more accessible to curative treatment options like surgery and ablation [Citation28]. Since this study confirmed this difference in survival between patients who are adequately screened versus those patients who are not, both physicians and patients need to be aware of the importance of HCC screening. Since CP-scores were equally divided over the screened and non-screened groups, we do not expect a bias towards milder disease in the screened group.
This study has strengths and limitations. The strength of this study is that the data comes from real-time practice. However, due to the retrospective nature, possible selection bias and missing data could have influenced the outcomes along the baseline differences in the compensated and decompensated patient groups. This should be taken into consideration whilst interpreting the outcomes. For example, when INR values were missing but PT values were available, a derived INR value was calculated. This might cause slightly altered MELD and Child-Pugh scores. Confounding factors such as alcohol use were only scored at baseline, if available. No follow-up data were available. The percentage of alcohol misuse was higher in the group of patients with decompensated cirrhosis, compared to the patients with compensated cirrhosis. We acknowledge that this is an important factor with a heavy weight on outcomes in patients with cirrhosis. Also, the determination of HE grade is difficult to quantify in retrospective studies. We attempted to overcome the influence of different factors by applying multivariate analysis and included a composite variable of relevant high-risk comorbidities. Nevertheless, residual confounding cannot be fully excluded. Due to privacy regulations, we were not able to validate the living status of all patients. Some might have died without our knowledge. Furthermore, there was a possible selection bias, because patients who did not reply to our invitation to participate and were still alive according to available information in our systems could be included in one hospital, but not in the other due to different local regulations (opt-in vs. opt-out). We attempted to lower the number of patients who did not respond to an opt-in invitation by phone call. Despite these efforts, there was still a considerable amount of patients excluded due to non-response. Consequently, the true mortality rate of the total study population could possibly be different. However, the lower mortality rates among decompensated patients found in our study fit with European data showing that the Netherlands is one of the countries with the lowest age-standardized mortality rates due to cirrhosis [Citation17]. A solid prospective cohort study is needed to confirm the findings of this study.
In conclusion, this study showed that the survival of patients with liver cirrhosis in a metropolitan area in the Netherlands is better than previously described. Still, there are several opportunities to improve care for patients with liver cirrhosis like the improvement and awareness of the importance of HCC screening and avoidance of unnecessary PPI use to possibly reduce the risk of mortality and prevention of decompensation to reduce future decompensation episodes.
| Abbreviations | ||
| HCC | = | hepatocellular carcinoma |
| TFS | = | transplant-free survival |
| HE | = | hepatic encephalopathy |
| PPI | = | proton pump inhibitors |
| CHC | = | chronic hepatitis C |
| NAFLD | = | non-alcoholic fatty liver disease |
| NASH | = | non-alcoholic steatohepatitis |
| SBP | = | spontaneous bacterial peritonitis |
| EHR | = | electronic health record |
| MELD | = | Model for End-stage Liver Disease |
| CP | = | Child-Pugh |
| PT | = | prothrombin time |
Disclosure statement
KdW, TK, KvdP, LCB, UB and RBT declare no conflicts of interest in relation to this manuscript.
Additional information
Funding
References
- Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383(9930):1749–1761.
- Detlef S, Nezam HA. Liver cirrhosis. Lancet. 2008;371:838–851.
- Younossi Z, Tacke F, Arrese M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69(6):2672–2682.
- D'Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol. 2006;44(1):217–231.
- D'Amico G, Pasta L, Morabito A, et al. Competing risks and prognostic stages of cirrhosis: a 25-year inception cohort study of 494 patients. Aliment Pharmacol Ther. 2014;39(10):1180–1193.
- Trebicka J, Fernandez J, Papp M, et al. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol. 2021;74(5):1097–1108.
- Tsai CF, Chen MH, Wang YP, et al. Proton pump inhibitors increase risk for hepatic encephalopathy in patients with cirrhosis in a population study. Gastroenterology. 2017;152(1):134–141.
- Janka T, Tornai T, Borbély B, et al. Deleterious effect of proton pump inhibitors on the disease course of cirrhosis. Eur J Gastroenterol Hepatol. 2020;32(2):257–264.
- Mahmud N, Serper M, Taddei TH, et al. The association between proton pump inhibitor exposure and key liver-related outcomes in patients with cirrhosis: a veterans affairs cohort study. Gastroenterology. 2022;163(1):257–269.e6.
- Kim RG, Loomba R, Prokop LJ, et al. Statin use and risk of cirrhosis and related complications in patients with chronic liver diseases: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2017;15(10):1521–1530.e8.
- Schierwagen R, Uschner FE, Magdaleno F, et al. Rationale for the use of statins in liver disease. Am J Physiol Gastrointest Liver Physiol. 2017;312(5):G407–G412.
- Vargas JI, Arrese M, Shah VH, et al. Use of statins in patients with chronic liver disease and cirrhosis: current views and prospects. Curr Gastroenterol Rep. 2017;19(9):43.
- Abraldes JG, Albillos A, Bañares R, et al. Simvastatin lowers portal pressure in patients With cirrhosis and portal hypertension: a randomized controlled trial. Gastroenterology. 2009;136(5):1651–1658.
- Liu Z, Jiang Y, Yuan H, et al. The trends in incidence of primary liver cancer caused by specific etiologies: results from the global burden of disease study 2016 and implications for liver cancer prevention. J Hepatol. 2019;70(4):674–683.
- Galle PR, Forner A, Llovet JM, et al. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
- Asrani SK, Devarbhavi H, Eaton J, et al. Burden of liver diseases in the world. J Hepatol. 2019;70(1):151–171.
- Pimpin L, Cortez-Pinto H, Negro F, et al. Burden of liver disease in Europe: epidemiology and analysis of risk factors to identify prevention policies. J Hepatol. 2018;69(3):718–735.
- Louvet A, Bourcier V, Archambeaud I, et al. Low alcohol consumption influences outcomes in individuals with alcohol-related compensated cirrhosis in a french multicenter cohort. J Hepatol. 2022;S0168-8278(22)03304-9 (online ahead of print).
- European Commission. Alcohol-Related Harm in Europe – Key Data. 2006(October):10–13 Europe.
- Tapper EB, Zhao L, Nikirk S, et al. Incidence and bedside predictors of the first episode of overt hepatic encephalopathy in patients with cirrhosis. Am J Gastroenterol. 2020;115(12):2017–2025.
- Tapper EB, Parikh ND, Sengupta N, et al. A risk score to predict the development of hepatic encephalopathy in a population-based cohort of patients with cirrhosis. Hepatology. 2018;68(4):1498–1507.
- Gu Y, Yang X, Liang H, et al. Comprehensive evaluation of effects and safety of statin on the progression of liver cirrhosis: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19(1):231.
- Pose E, Napoleone L, Amin A, et al. Safety of two different doses of simvastatin plus rifaximin in decompensated cirrhosis (LIVERHOPE-SAFETY): a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Gastroenterol Hepatol. 2020;5(1):31–41.
- Wu X, Zhang D, Yu Y, et al. Proton pump inhibitor use and mortality in patients with cirrhosis: a meta-analysis of cohort studies. Biosci Rep. 2020;40(6):BSR20193890.
- Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69(9):1691–1705.
- Targownik LE, Fisher DA, Saini SD. AGA clinical practice update on de-prescribing of proton pump inhibitors: expert review. Gastroenterology. 2022;162(4):1334–1342.
- Yeo YH, Hwang J, Jeong D, et al. Surveillance of patients with cirrhosis remains suboptimal in the United States. J Hepatol. 2021;75(4):856–864.
- Van Meer S, De Man RA, Coenraad MJ, et al. Surveillance for hepatocellular carcinoma is associated with increased survival: results from a large cohort in The Netherlands. J Hepatol. 2015;63(5):1156–1163.