Abstract
Objective
Vedolizumab (VDZ) for subcutaneous (SC) administration has recently become available. We aimed to assess feasibility, safety and clinical outcome when switching from intravenous (IV) to SC VDZ maintenance treatment in a real world cohort of patients with inflammatory bowel disease (IBD) followed by therapeutic drug monitoring (TDM).
Methods
Eligible IBD patients were switched from IV to SC treatment and assessed six months prior to switch, at baseline and six, twelve and twenty-six weeks after switch. Primary outcome was proportion of patients on SC treatment after 26 weeks. Secondary outcomes included adverse events (AEs), clinical disease activity, biochemical markers, treatment interval, serum-VDZ (s-VDZ), preferred route of administration and health-related quality of life.
Results
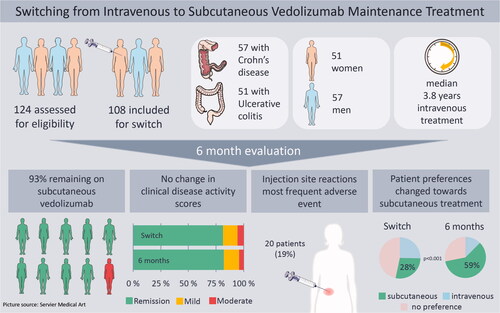
In total, 108 patients were switched. After 26 weeks, 100 patients (92.6%) were still on SC treatment and median s-VDZ was 47.6 mg/L (IQR 41.3 − 54.6). The most frequent AE was injection site reaction (ISR), reported by 20 patients (18.5%). There were no clinically significant changes in disease activity, biochemical markers and quality of life. The proportion of patients preferring SC administration increased from 28.0% before switch to 59.4% after 26 weeks (p < 0.001).
Conclusions
Nine out of ten patients still received SC treatment after 26 weeks. No change in disease activity occurred, and levels of serum VDZ increased. Although almost one fifth of patients experienced ISRs, a higher proportion favored SC administration at 26 weeks. This study demonstrates that SC maintenance treatment is a safe and feasible alternative to IV treatment.
Introduction
Inflammatory bowel diseases (IBD), Crohn’s disease (CD) and ulcerative colitis (UC), are chronic diseases of the gastrointestinal tract. IBD reduces quality of life, and is associated with accompanying diseases, such as depression, anxiety and symptoms like fatigue [Citation1]. A way to ameliorate the physical and psychological burden is shared decision making between the physician and patient concerning choice of treatment, and route of administration is then relevant [Citation2]. Vedolizumab (VDZ) is a monoclonal antibody targeting the gut-specific α4β7 integrin. It is effective in inducing and maintaining remission in patients with moderate to severe CD and UC [Citation3–6]. The drug has previously only been available for intravenous (IV) treatment but has recently become available as a subcutaneous (SC) formulation [Citation7,Citation8].
In general, but also for IBD patients, studies have demonstrated that patients prefer SC treatment when both administration routes are available [Citation9–11], and SC administration also reduces the need for hospital visits. Thus, SC administration may be beneficial for the patient, health care capacity and direct as well as indirect costs.
The VISIBLE 1 and 2 trials examined SC VDZ as treatment for moderate to severe UC and CD, respectively, after an IV induction period. Both studies concluded that SC and IV treatments have comparable efficacy and safety profiles [Citation7,Citation8]. However, patients on VDZ maintenance treatment, including patients on non-standardized dosing, were not included.
Studies have demonstrated an exposure-efficacy relationship for VDZ, suggesting that serum concentrations may have a role in clinical management [Citation12]. Recent studies support the use of therapeutic drug monitoring (TDM) in maintenance treatment with anti-TNF alpha drugs [Citation13,Citation14], but there are currently no RCTs on the use of TDM in VDZ treatment. In Norway, serum VDZ concentration measurements are available at a low cost, and thus widely used in clinical practice despite the ongoing discussion about this strategy. Hence, real world data are needed to elucidate both feasibility and safety when switching from IV to SC maintenance treatment, including changes in VDZ serum concentrations.
The aims of this study were to assess the feasibility, safety and clinical outcome when switching from IV to SC VDZ maintenance treatment in a real world cohort of patients with IBD followed by TDM.
Materials and methods
The study was conducted as a prospective single center study at the department of Gastroenterology at Oslo University hospital in Norway. All adult patients (>18 years) with IBD receiving maintenance IV VDZ were assessed for eligibility for switch to SC treatment between 15 February 2021 and 3 June 2021. All patients had signed a broad consent to be included in the locally approved IBD register at the department (PVO 19/02408), where data from hospital visits are included prospectively as part of our clinical routine. The project protocol was submitted to and approved by the data protection officer at the hospital (PVO 21/00119).
Non-eligibility was defined as IV treatment shorter than six months, planned surgery within three months, planned change of drug treatment for their IBD, current investigation for other significant diseases or relapse in need of corticosteroids. In addition, we had to exclude patients who were switched later than planned due to COVID restrictions. All other patients established on VDZ maintenance treatment were switched, regardless of clinical disease activity scores.
Patients were followed prospectively from baseline (switch). Follow-up visits were set to the time point of the fourth injection (median 6 weeks, range 3–9 weeks depending on individual treatment interval), 12 weeks (range 11–25) and 26 weeks (range 19–34) after switching. This follow-up regimen corresponds with the department’s standard visit schedule for patients on SC IBD treatment.
Prospectively recorded data available in the structured electronic patient journal and/or local IBD register were retrospectively collected for a period of 26 (range 20–36) weeks prior to the switch. At baseline, defined as the time point of first injection with SC VDZ, characteristics of the IBD patients including demographic data, phenotype according to the Montreal classification [Citation15], previous medical treatment and previous surgery were recorded. In addition, the patients were asked to fill out two questionnaires: the EQ-5D-5L VAS score [Citation16] and a simple questionnaire recording the patients’ preferred administrative route of VDZ before the first injection and at every follow-up visit. The EQ-5D-5L VAS questionnaire scores overall health from zero to one hundred, with higher numbers indicating higher health-related quality of life. For the questionnaire estimating the preferred route of administration, patients were asked to report whether they preferred SC treatment, IV treatment or had no preference.
Data collection at follow-up included time interval of injections, self-reported compliance assessed by number of missed injections, laboratory data including hemoglobin (Hgb), C-reactive protein (CRP), fecal calprotectin (FC), serum-VDZ (s-VDZ), disease activity (Harvey Bradshaw Index [HBI] for CD, Partial Mayo Score without physician’s assessment (PMS) for UC) and the patient reported questionnaires. All adverse events (AEs) reported by patients at follow-up visits or in between visits were recorded consecutively.
Data from six months prior to switch included the VDZ IV dose and infusion interval, Hgb, CRP, ferritin, FC, s-VDZ, disease activity index and serious AEs (SAE).
Algorithm for SC dosing
Patients on IV VDZ treatment at our department were followed with measurement of s-VDZ concentration at each infusion aiming at a through level of >20 mg/L. This approach results in individual intervals for infusion, and therefore the necessity to convert to individual intervals for SC treatment to maintain treatment intensity. Conversion from IV to SC dosage was based on a locally, arithmetically derived algorithm with standard IV dosage of 300 mg every eighth week [Citation6,Citation17] equaling the standard SC dosage of 108 mg SC every second week [Citation7,Citation8]. The resulting SC dosing intervals based on the algorithm are given in . To prevent a decrease in s-VDZ at switch, the first SC injection was given halfway into the individual interval for IV treatment. Dose adjustments were not routinely planned during the first six months of follow-up after switch.
Table 1. Conversion table for intervals of intravenous (IV) to subcutaneous (SC) administration.
Disease activity
Clinical remission was defined as HBI ≤ 4 and PMS ≤ 1 [Citation18,Citation19]. Biochemical remission was defined as CRP < 5 mg/L, FC < 250 mg/kg and Hgb within the reference area according to the definitions of anemia from the World Health Organization (WHO) [Citation20,Citation21].
Hgb and CRP were collected at each visit, FC was included if the date was ± 2 months for the date at six months prior to switching, or ± 1 month for all other visits.
Study outcomes
The primary outcome was the proportion of patients remaining on SC formulation six months after switching. Secondary outcomes included AEs, changes in disease activity, Hgb, CRP, FC, s-VDZ, VDZ treatment interval, preferred route of administration and health-related quality of life.
Measurement of s-VDZ and antibodies
VDZ-serum concentrations were measured using validated 3-step time-resolved immunofluorometric assay automated on the AutoDELFIA (PerkinElmer, Waltham, MA) platform. The assay was performed in streptavidin-coated 96-well microplates using two anti-VDZ murine monoclonal antibodies (D130, D136.2) developed in-house. Biotinylated F(ab’)2 fragments of D130 were used as solid phase reagent, and europium-labeled D136 as tracer antibody (manuscript in preparation). Antibodies were only measured at low serum concentrations of VDZ.
Statistical analysis
Continuous data are presented as mean (standard deviation [SD]) when normally distributed or median (range or interquartile range [IQR]) for variables with skewed distribution. Categorical data are presented as counts and percentages. Pairs of categorical data were compared using the Chi-squared test or Fisher’s exact test when appropriate. Continuous variable measured at two time points were compared using paired samples t-test. Possible crude changes in repeatedly measured categorical variables were assessed using 3 × 3 tables and marginal homogeneity tests (Stuart– Maxwell test). Changes over time after drug switch were analyzed using linear mixed models for repeated measures. The effect of time was estimated as a fixed effect, and gender and age were included in the regression model as possible confounders. All overall effects were analyzed using the F-test. The number of patients eligible for switch at the department determined the sample size. The results are presented as estimated marginal means with 95% confidence intervals. As the study was considered exploratory, p values < 0.05 were considered statistically significant and no correction for multiple testing was performed. All analyses were conducted using SPSS version 26 (SPSS Inc., Chicago, IL) or Stata version 17 (StataCorp LLC, College Station, TX).
Results
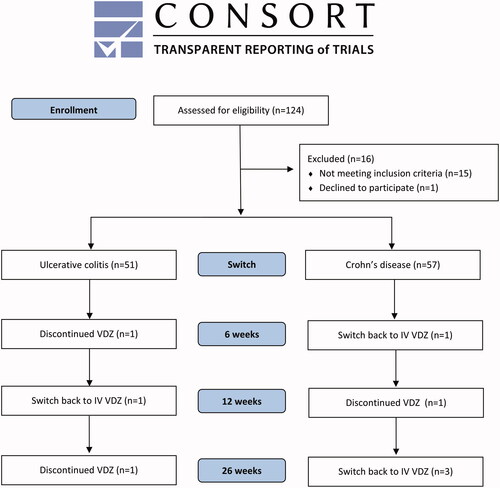
A total of 124 patients receiving IV VDZ were identified, of which 108 patients were defined as eligible based on our predefined criteria and switched to SC VDZ. The process of inclusion and follow-up is illustrated in a Consolidated Standards of Reporting Trials (CONSORT) diagram ().
Figure 1. CONSORT flow diagram of the patient cohort at the time of switch, at 6 weeks, 12 weeks and 26 weeks follow-up. VDZ: Vedolizumab; SC: subcutaneous; IV: intravenous.
The study population consisted of 57 CD patients (53%) and 51 UC patients (47%). The majority (n = 103, 95%) of patients had received one or more biologic drugs before starting treatment on VDZ, and 12 patients (11%) were receiving two different biologic drugs at the time of switching. All 12 patients received an anti-TNF-alpha drug as second biologic drug, either because of combination treatment for IBD or concurrent rheumatic disease. The median time from first IV dose of VDZ to first SC injection was 3.8 years (range 0.6 − 10.3), and the shortest treatment duration on IV VDZ before switching was seven months ().
Table 2. Baseline characteristics of included patients.
Treatment persistence and changes in clinical disease activity score
At 26 weeks follow-up, 100 patients (92.6%, 95% CI [85.9–96.7]) were still receiving SC treatment. Three patients had switched back to IV treatment due to injection site reactions (ISRs) and two patients due to administration route preference; two patients had changed treatment to ustekinumab due to loss of response to VDZ and one patient had stopped treatment due to a recurrent infection with Clostridium difficile.
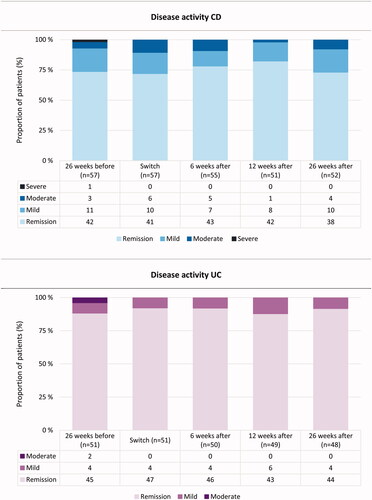
There were no statistically significant changes in disease activity scores from the time of switching and throughout follow-up in CD and UC patients (). Proportion of CD patients in clinical remission at switch was 72% (58–83) versus 82% (69–92) at three months and 73% (59–84) at six months. For UC patients, the percentages in clinical remission at switch, three months and six months were 92% (81–98), 88% (75–95) and 92% (80–98), respectively.
Figure 2. Disease activity scores 26 weeks before, at switch, 6, 12 and 26 weeks after switching from intravenous to subcutaneous vedolizumab (VDZ). For Crohns’s disease (CD), each column represents proportions of patients classified according to the Harvey-Bradshaw Index (HBI): remission (HBI 0–4), mild disease (HBI 5–7), moderate disease (HBI 8–15), severe disease (HBI > 16). For ulcerative colitis (UC), each column represents proportion of patients classified according to the Partial Mayo Score without physician’s assessment (PMS): remission (PMS 0–1), mild disease (PMS 2–4) and moderate disease (PMS 5–6).
Serum VDZ and treatment interval
Before switching, the individual interval of IV administration ranged from four to 12 weeks, with a median of seven weeks. In total, 56% of the patients had a shorter interval than eight weeks, and 27% had a longer interval than eight weeks. Details on treatment intervals and s-VDZ are listed in .
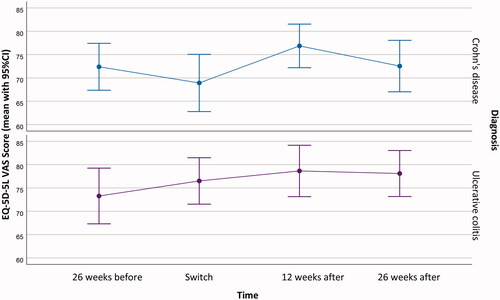
The first injection was administered halfway in the individual interval, ranging from two weeks to five weeks after last infusion; the median s-VDZ at first injection was 38.9 mg/L (IQR 33.7 − 42.3). After six weeks, the median s-VDZ was 40.7 mg/L (IQR 36.8 − 46.3), and after 12 and 26 weeks the median s-VDZ was 44.5 mg/L (IQR 38.0 − 51.8) and 47.6 mg/L (IQR 41.3 − 54.6), respectively. Profiles for s-VDZ for CD and UC separately are depicted in .
Figure 3. Serum-Vedolizumab (s-VDZ) concentration profile (mean, 95% CI) at the different time-points during the study.
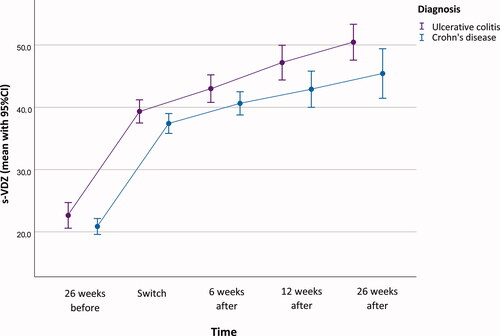
Twenty-six weeks after switching, the median interval for all patients was 13 d (range 7–21) between injections, whereas CD patients had a median interval of 12 d (range 7–18) and UC patients 14 d (range 7–21). Data on self-reported patient compliance revealed that two patients had missed one single injection during the first six months.
Biochemical markers of inflammation
No significant changes in biochemical markers for inflammation were detected. Proportions of patients with CRP < 5 mg/L, FC < 250 mg/L and anemia at different time points of the study are shown in . There were no statistically significant changes in CRP, FC and ferritin over time, neither before nor after the switch. In CD patients we observed a statistically significant decrease in Hbg from 14.5 mg/dL at switch to 13.9 mg/dL at 26 weeks (p = 0.021), whereas no change was observed for the total observation period of 52 weeks (p = 0.297) ().
Figure 4. Inflammatory markers before and after switch. The columns represent the proportion of patients (%) with (i) C-reactive protein (CRP) below or above/equal to 5 mg/L, (ii) fecal calprotectin (FC) below or above/equal to 250 mg/kg, and (iii) anemia estimated by Hemoglobin (Hgb) according to the definitions from the World Health Organization.
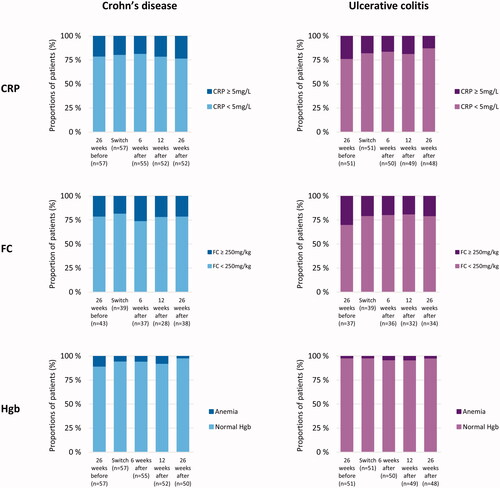
Table 3. Mixed models for repeated measure analyses.
Safety
The most common AEs were injection site reactions (ISRs) with swelling, pruritus, erythema and rubor. At 26 weeks follow-up, 20 patients (18.5%) had experienced one or more ISRs, and 15 of these (75%) had three or more consecutive reactions. In three cases, patients were switched back to IV treatment, the first two patients were switched back after their sixth injection, approximately eight weeks after their first injection, due to severe and persistent ISR, the third patient was switched back due to mild ISR combined with general pruritus at 12 weeks follow-up. The majority (55%) of ISRs appeared after the third or fourth injection. In addition to ISRs, 39 patients reported 52 AEs and seven patients experienced more than one AE (). The most common AEs after ISR were symptom worsening and abdominal pain. Four CD patients presented with abscesses or new fistulas, which in two cases led to discontinuation of VDZ. One case of Clostridium difficile occurring directly after switching was classified as a recurrent infection and led to discontinuation of VDZ. In four cases, the AEs led to hospitalization, and thus classified as SAEs.
Table 4. Adverse events reported during the follow-up period of six months (n = 108).
Patient preference and self-reported health
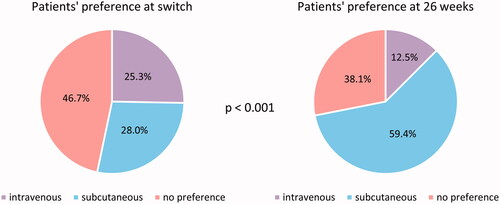
A higher percentage of patients preferred SC at 26 weeks (59.4%) compared with baseline (28.0%), and the proportion of patients preferring IV decreased from 25.3% (baseline) to 12.5% (, p < 0.001). Half of the patients who reported a preference toward IV at 26 weeks also reported a preference for IV before switching.
Figure 5. Patients’ preference when asked before administration of first subcutaneous injection (n = 107) and at 26 weeks follow-up (n = 96), p < 0.001.
There was no change in EQ-5D-5L VAS scores between baseline and 26 weeks with mean scores of 72.8 (SD 19.7) and 75.2 (SD 17.8), respectively (p = 0.45) ().
Discussion
This study demonstrates that more than 90% of the patients remained on SC VDZ treatment after 26 weeks, and that switching was safe and feasible, also in patients who had received IV maintenance treatment for up to 10 years. No changes in objective disease variables or self-estimation of disease activity were observed, and serum levels of VDZ increased throughout the follow-up period. Furthermore, an increasing proportion of patients preferred SC treatment.
To date, three articles on switching from IV VDZ maintenance treatment to SC treatment have been published [Citation22–24]. Bergqvist et al. published their results from a six months follow-up in 89 IBD patients who were switched to standard SC treatment with injections every two weeks. Ventress et al. reported 12 weeks observations including disease activity scores, safety and persistence, and persistence 6 months after switch from IV to a standard dose of SC VDZ treatment in 99 IBD patients on established VDZ treatment in a real world setting [Citation22]. Furthermore, Volkers et al. reported data after switching 135 IBD patients at 10 different medical centers to standard dose of SC VDZ with a median follow-up of 27 weeks [Citation24]. Our results are in line with these studies showing that switching is safe and does not have any clinically significant impact on disease activity evaluated by clinical scores and biochemical markers. However, in the three available studies, patients were switched to a standard SC dosage. The treatment approach at our department includes frequent serum drug concentration measurements with subsequent individual dosing for all IBD patients receiving biologic drugs. Thus, our study does not only strengthen the evidence of safety of switching, but also contributes to new knowledge in a group of patients switched to individual doses of SC VDZ. Furthermore, novel insight on serum drug profiles after switch to SC treatment is gained.
At 26 weeks follow-up, 93% of our patients were still on SC treatment, which is comparable to the finding of 92% drug persistence reported by Ventress et al. at twelve weeks follow-up and 87% after six months [Citation22], to the 95.5% reported by Bergqvist et al. and 88.1% reported by Volkers et al. In addition, we did not find any difference in treatment persistence between CD and UC, and to the best of our knowledge there is no such data available in the published literature. The high persistence is most likely due to the selected group of patients, where all had established clinical effect of VDZ treatment before switching.
Almost one out of five patients experienced one or more ISRs, and this percentage was higher in our study compared with numbers available from randomized clinical trials [Citation7,Citation8]. The proportion of patients treated with concomitant immunomodulators and/or corticosteroids may explain the difference, as more than 40% of UC patients were treated with concomitant corticosteroids at week zero in VISIBLE 1 and more than 50% of CD patients were treated with either immunomodulators, corticosteroids or a combination of both in VISIBLE 2 [Citation7,Citation8]. In contrast, only 9% of CD patients and 4% of UC patients were treated with concomitant oral corticosteroids in our study. Moreover, no CD patients were treated with immunomodulators and only 4% of UC patients received methotrexate due to arthritis. Noteworthy, none of our patients treated with corticosteroids or immunomodulators experienced any ISRs. In addition, both VISIBLE trials included patients according to a research protocol in contrast to our real world approach. Following ISRs, the most common AEs reported were symptoms of disease worsening and abdominal pain, whilst only 25% of these patients had an increase in FC. Use of biological treatment in Norway follows a national tender, where VDZ is being prescribed as a second or third line biologic, resulting in a selected patient group were 95% had failed treatment on infliximab/adalimumab. Our study population therefore represents IBD patients with complicated disease. Considering all the AEs reported, the findings are consistent with previous studies showing a favorable safety and tolerability profile for SC VDZ [Citation7,Citation8,Citation22].
Bergqvist et al. found a significant decrease in FC in CD patients, from 64 to 49 mg/L. Even though this finding was statistically significant, the absolute levels are low and clinical significance is questionable. In our study, switching from IV to SC formulation did not result in significant clinical changes in disease activity, and the biochemical markers CRP, FC and Hgb were similar during the 52 weeks observation period (from 26 weeks prior to switching until 26 weeks after). These observations strengthen our conclusion that it is safe and feasible to switch patients on maintenance treatment.
In the present study, patients received an optimized IV dosing interval based on trough levels of s-VDZ before switch. In order to maintain the optimized dosing regimen, we had to convert the IV interval (in weeks) to a SC interval (in days) intending to ensure a comparable treatment intensity. We observed an increase in s-VDZ from IV trough values to serum concentration after switch to SC, which is consistent with previous studies [Citation7,Citation8,Citation22]. Noteworthy, the levels were numerically higher at 26 weeks compared to 12 weeks. At present, there is no available data on optimal serum concentration for patients treated with SC VDZ, and studies on VDZ trough level monitoring on IV treatment vary in their recommendations for trough levels [Citation4,Citation25,Citation26]. Observations on VDZ concentration indicate that VDZ follows an exposure-efficacy trajectory, and even though the receptor saturation is nearly complete at low doses, clinical trials suggest that these concentrations are sub therapeutic [Citation27]. The safety of VDZ is regarded as high, but higher VDZ concentrations may still correspond with a higher risk of unwanted events [Citation28]. In VISIBLE 1, patients treated with IV VDZ had a significant increase in clinical remission rates with a trough level ≥ 10.5 mg/L. This exposure-efficacy relationship was not as clear in the SC treatment group. Hence, a median s-VDZ of 47.6 mg/L (IQR 41.3 − 54.6) at 26 weeks observed in our study suggests that the interval can be increased in our cohort, especially for the patients in clinical remission.
All our patients eligible for switching were asked for consent to perform the switch to SC treatment. They were informed that the switch was not mandatory and that they could switch back to IV treatment. Asnong et al. evaluated the willingness of patients to switch to SC VDZ treatment, and reported that 51% showed willingness whilst 12% were unwilling to switch [Citation29]. During the 26 weeks follow-up, only two of our patients were switched back to IV due to preference. We found that twelve patients (12.5%) still were reluctant to SC treatment at 26 weeks, comparable to the numbers reported by Asnong et al. Nevertheless, a majority of patients (60%) preferred SC treatment at 26 weeks follow-up. These findings are consistent with other previous studies regarding preferred route of administration [Citation10,Citation11,Citation27]. Additionally, we observed a significant change in attitude toward route of administration. We also conducted a sensitivity analysis to account for patients leaving the study before the 26 weeks follow-up. Missing data for patients’ preference at 26 weeks was then set to IV, and the results were still statistically significant. This demonstrates that it is favorable to give patients the opportunity to try SC treatment, as their attitude toward SC treatment may change with own experience.
This study includes a 26 weeks prospective follow-up after switch to SC in a real world population with complicated disease and long-term VDZ IV use before switch. The patients acted as their own control group, as we additionally included data from 26 weeks before switching. The close follow-up with frequent visits represent a strength of the study. Another strength is the tight follow-up by one dedicated doctor and one nurse, which reduces bias in reporting and data collection at the different time-points for follow-up. The selected population in this single-center study represents a group of patients followed by TDM with complicated disease as almost all received VDZ as their second or third line treatment. The findings of this study may therefore be more relevant in countries that follow the same treatment strategy. In addition, the gold standard for evaluation of disease activity in IBD is endoscopy, combined with imaging and biochemical markers [Citation30]. Endoscopy data would have been optimal; nevertheless, as this was a real world study and we had a high rate of adherence according to surrogate markers of inflammation, we do not think that inclusion of endoscopy would have changed our findings. A longer follow-up period would also have been fortunate.
In conclusion, we have demonstrated that switching from IV to SC VDZ maintenance treatment in a real-world IBD cohort on various doses of VDZ is feasible, safe and preferred by the patients. The optimal interval and s-VDZ in patients have yet to be determined, and further studies are needed to evaluate the exposure-efficacy relationship in patients on SC treatment. Furthermore, the present results may have implications for a large group of patients currently on IV VDZ treatment where SC treatment is a relevant option, and the possibility to perform a safe switch may increase the level of shared decision-making and patient satisfaction.
Ethical approval
The study was approved by the local data protection officer (PVO 21/00119), based on a written broad informed consent given by the participants. Except for the questionnaires, only data from standard clinical follow-up were included in the study database.
Author contribution
THW was involved in study design, statistical analysis, data interpretation, drafting and revising the manuscript. AWM, MLH and BM were involved in study design, data interpretation, drafting and revising the manuscript. DJW and NB were involved in data interpretation and revising the manuscript. MCS was involved in study design, statistical analysis, data interpretation and revising the manuscript. All authors approved the final document.
| Abbreviations | ||
| AE | = | adverse event |
| CI | = | confidence interval |
| CD | = | Crohn’s disease |
| CRP | = | C-reactive protein |
| FC | = | fecal calprotectin |
| HBI | = | Harvey Bradshaw Index |
| Hgb | = | haemoglobin |
| IBD | = | Inflammatory bowel disease |
| IV | = | intravenous |
| IQR | = | interquartile range |
| P MS | = | Partial Mayo Score |
| RCT | = | randomized clinical trial |
| SAE | = | serious adverse event |
| SC | = | subcutaneous |
| SD | = | standard deviation |
| TDM | = | therapeutic drug management |
| UC | = | ulcerative colitis |
| VDZ | = | Vedolizumab |
Acknowledgments
The authors thank Maren Sjåmo for contributing to data collection.
Disclosure statement
THW and KA report consultant fees from Takeda outside the submitted work. AWM reports unrestricted research grant from Takeda, NB, DJW and MCS report no conflicts of interest. BM reports consultant fees from Takeda, Janssen, AbbVie, Pfizer; advisory board Takeda, Janssen, AbbVie, Pfizer, Sandoz, Pharma Cosmos; speaker fees from Takeda, Janssen, AbbVie, Sandoz, Orion Pharma. MLH reports speaker fees, consultant fees and serving as an advisory member for or receiving research funding from MSD, AbbVie, Pfizer, Takeda, Janssen, Gilead/Galapagos, Ferring and Tillots Pharma.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Cohen BL, Zoëga H, Shah SA, et al. Fatigue is highly associated with poor health-related quality of life, disability and depression in newly-diagnosed patients with inflammatory bowel disease, independent of disease activity. Aliment Pharmacol Ther. 2014;39(8):811–822.
- De Mits S, Lenaerts J, Vander Cruyssen B, et al. A nationwide survey on patient’s versus physician’s evaluation of biological therapy in rheumatoid arthritis in relation to disease activity and route of administration: the be-raise study. PLoS One. 2016;11(11):e0166607-e.
- Amiot A, Serrero M, Peyrin-Biroulet L, et al. Three-year effectiveness and safety of vedolizumab therapy for inflammatory bowel disease: a prospective multi-Centre cohort study. Aliment Pharmacol Ther. 2019;50(1):40–53.
- Buer LCT, Moum BA, Cvancarova M, et al. Real world data on effectiveness, safety and therapeutic drug monitoring of vedolizumab in patients with inflammatory bowel disease. A single center cohort. Scand J Gastroenterol. 2019;54(1):41–48.
- Feagan BG, Rubin DT, Danese S, et al. Efficacy of vedolizumab induction and maintenance therapy in patients With ulcerative colitis, regardless of prior exposure to tumor necrosis factor antagonists. Clin Gastroenterol Hepatol. 2017;15(2):229–239.e5.
- Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for crohn’s disease. N Engl J Med. 2013;369(8):711–721.
- Sandborn WJ, Baert F, Danese S, et al. Efficacy and safety of vedolizumab subcutaneous formulation in a randomized trial of patients With ulcerative colitis. Gastroenterology. 2020;158(3):562–572.e12.
- Vermeire S, D'Haens G, Baert F, et al. Efficacy and safety of subcutaneous vedolizumab in patients With moderately to severely active Crohn’s disease: results From the VISIBLE 2 randomised trial. J Crohns Colitis. 2022;16(1):27–38.
- Gingele S, Koch M, Saparilla AC, et al. Switch from intravenous to subcutaneous immunoglobulin IgPro20 in CIDP patients: a prospective observational study under real-world conditions. Ther Adv Neurol Disord. 2021;14:17562864211009100.
- Van Assche G, Vermeire S, Ballet V, et al. Switch to adalimumab in patients with Crohn’s disease controlled by maintenance infliximab: prospective randomised SWITCH trial. Gut. 2012;61(2):229–234.
- Stoner KL, Harder H, Fallowfield LJ, et al. Intravenous versus subcutaneous drug administration. Which do patients prefer? A systematic review. Patient. 2015;8(2):145–153.
- Alsoud D, Vermeire S, Verstockt B. Monitoring vedolizumab and ustekinumab drug levels in patients with inflammatory bowel disease: hype or hope? Curr Opin Pharmacol. 2020;55:17–30.
- Syversen SW, Goll GL, Jørgensen KK, et al. Effect of therapeutic drug monitoring vs standard therapy during infliximab induction on disease remission in patients with chronic immune-mediated inflammatory diseases: a randomized clinical trial. JAMA. 2021;325(17):1744–1754.
- Syversen SW, Jørgensen KK, Goll GL, et al. Effect of therapeutic drug monitoring vs standard therapy during maintenance infliximab therapy on disease control in patients with immune-mediated inflammatory diseases: a randomized clinical trial. JAMA. 2021;325:2375–2384.
- Satsangi J, Silverberg MS, Vermeire S, et al. The montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut. 2006;55(6):749–753.
- Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–1108.
- Feagan BG, Rutgeerts P, Sands BE, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710.
- Travis SPL, Higgins PDR, Orchard T, et al. Review article: defining remission in ulcerative colitis. Aliment Pharmacol Ther. 2011;34(2):113–124.
- Vermeire S, Schreiber S, Sandborn WJ, et al. Correlation between the Crohn’s disease activity and Harvey–Bradshaw indices in assessing Crohn’s disease severity. Clin Gastroenterol Hepatol. 2010;8(4):357–363.
- Buer LCT, Moum BA, Cvancarova M, et al. Switching from remicade® to remsima® is well tolerated and feasible: a prospective, open-label study. J Crohns Colitis. 2017;11(3):297–304.
- Zittan E, Kelly OB, Kirsch R, et al. Low fecal calprotectin correlates with histological remission and mucosal healing in ulcerative colitis and colonic Crohn’s disease. Inflamm Bowel Dis. 2015;22:623–630.
- Ventress E, Young D, Rahmany S, et al. Transitioning from intravenous to subcutaneous vedolizumab in patients with inflammatory bowel disease (TRAVELESS). J Crohns Colitis. 2022;16(6):911–921.
- Bergqvist V, Holmgren J, Klintman D, et al. Real-world data on switching from intravenous to subcutaneous vedolizumab treatment in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2022;19:1389–1401.
- Volkers A, Straatmijer T, Duijvestein M, et al. Real-world experience of switching from intravenous to subcutaneous vedolizumab maintenance treatment for inflammatory bowel diseases. Aliment Pharmacol Ther. 2022;56(6):1044–1054.
- Williet N, Boschetti G, Fovet M, et al. Association between low trough levels of vedolizumab during induction therapy for inflammatory bowel diseases and need for additional doses Within 6 months. Clin Gastroenterol Hepatol. 2017;15(11):1750–1757.e3.
- Rosario M, French JL, Dirks NL, et al. Exposure-efficacy relationships for vedolizumab induction therapy in patients with ulcerative colitis or Crohn’s disease. J Crohns Colitis. 2017;11(8):921–929.
- Chilton F, Collett RA. Treatment choices, preferences and decision-making by patients with rheumatoid arthritis. Musculoskeletal Care. 2008;6(1):1–14.
- Loftus EV, Feagan BG, Panaccione R, et al. Long-term safety of vedolizumab for inflammatory bowel disease. Aliment Pharmacol Ther. 2020;52(8):1353–1365.
- Asnong K, Pouillon L, Bossuyt P. Is also the patient ready for switching from intravenous to subcutaneous biologics? J Crohns Colitis. 2022;16(3):515–516.
- Maaser C, Sturm A, Vavricka SR, et al. ECCO-ESGAR guideline for diagnostic assessment in IBD part 1: initial diagnosis, monitoring of known IBD, detection of complications. J Crohns Colitis. 2019;13(2):144–164.