Abstract
Objectives
Immune responses following SARS-CoV-2 vaccination in patients with inflammatory bowel disease (IBD) are not well characterized. The aims of this study were to explore the serological response associated with IBD, and immunosuppressive medications including serum concentrations of biologics and thiopurine metabolites.
Materials and methods
This prospective, observational study included adult patients with ulcerative colitis (UC) and Crohn’s disease (CD), and healthy controls. Antibodies to the receptor-binding domain of SARS-CoV-2 spike proteins, and serum concentrations of ongoing biologic and immunomodulatory medications were assessed prior to, and 2-5 weeks after the second vaccine dose. Serologic response was defined as anti-Spike antibodies ≥70 AU/ml.
Results
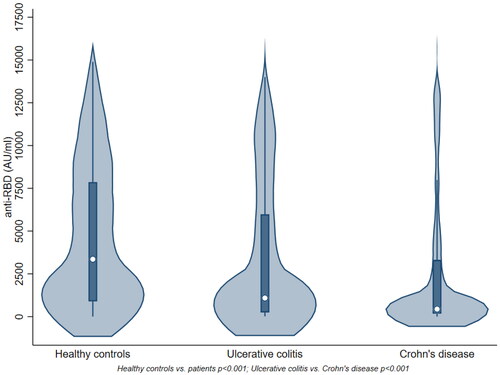
In 958 IBD patients (380 UC, 578 CD) and 323 healthy controls, the median (Q1; Q3) anti-Spike antibody level (AU/ml) was lower in patients (618 (192; 4370)) compared to controls (3355 (896; 7849)) (p < 0.001). The antibody levels were lower in CD (439 (174; 3304)) compared to UC (1088 (251; 5975)) (p < 0.001). No associations were demonstrated between antibody levels and serum drug concentrations for TNF inhibitor (TNFi), vedolizumab and ustekinumab. Patients receiving TNFi + thiopurines with a subtherapeutic 6-thioguanine nucleotide (6-TGN) level had higher response rate (93%) compared to patients with 6-TGN within the therapeutic range (53%) (p = 0.003). A diagnosis of UC, mRNA-1273 vaccine, and other treatments than TNFi + thiopurines were associated with humoral response.
Conclusions
Patients with CD had an attenuated humoral response to SARS-COV-2 vaccination as compared to patients with UC. The lack of association between serum levels of biologics and serologic response indicates vaccination regardless of proximity to drug administration.
Introduction
Inflammatory bowel disease (IBD), comprising Crohn’s disease (CD) and ulcerative colitis (UC), is characterised by chronic inflammation arising from an abnormal host immune response to environmental factors and microbial antigens, influencing both innate and adaptive immunity in genetically susceptible patients. The primary goal of IBD management is to control this inflammation with immunosuppressive medication including immunomodulators, tumor necrosis factor inhibitors (TNFi) and other biologics, and targeted small molecules [Citation1].
Vaccines against SARS-CoV-2 have been shown to be efficacious and safe in the general population. As patients with immune-mediated inflammatory diseases using immunosuppressive medication were excluded from clinical phase III vaccine trials, the immune responses following SARS-CoV-2 vaccination in patients with IBD are less well described. Recently, the vaccine response in immuncompromised patients has been a research priority, where the impact of immunmodulatory and biologic treatment in IBD has been a topic of great interest [Citation2–9]. Current position statements have recommended SARS-CoV-2 vaccination regardless of immunosuppressive therapies [Citation10,Citation11]. They also advice against tapering immunosuppressive medications or adapting timing of vaccination within dosing intervals of biologicals in relation to vaccination in IBD, although data exploring associations between serum concentrations of biological drugs and vaccine response in IBD are limited [Citation12,Citation13]. Thus, larger studies evaluating effectiveness of SARS-CoV-2 vaccine in IBD patients receiving different immunosuppressive medications with a variety of serum concentrations are needed to support these recommendations. Likewise, further studies exploring possible associations between characteristics of the underlying bowel disease and vaccine response are warranted [Citation2,Citation8]. Identifying factors that have an impact on the vaccine response may provide guidance for further monitoring of vaccine responses and scheduling further booster vaccination in IBD patients.
The aims of this prospective, observational study were to assess the humoral response after two-dose SARS-CoV-2 vaccination in a large, well-characterised cohort of IBD patients receiving immunosuppressive treatment, and to explore the associations between the vaccine response and characteristics of the underlying bowel disease, ongoing immunosuppressive medication, and serum concentrations of biologics and thiopurine metabolites.
Materials and methods
Study population
IBD patients were recruited into the study from the two largest referral centres for IBD in Norway: Akershus University Hospital (AHUS) and Oslo University Hospital (OUH), Ullevål. At AHUS, patients enrolled in the prospective observational Nor-vaC study (Norwegian study of vaccine response to COVID-19 vaccines) were included (Clinialtrials.gov NCT04798625) [Citation7,Citation14]. At OUH, the patients were recruited from the OUH study on SARS-CoV-2 vaccine response and clinical data were collected from a local IBD registry (PVO 2014/7822). Eligibility criteria are presented in the Supplementary Appendix. Adult patients (aged ≥18 years) with a diagnosis of UC and CD treated with immunosuppressive medications were consecutively included in the study prior to the onset of the national vaccination program in February 2021. The healthy controls consisted of health care workers from AHUS, Diakonhjemmet Hospital, and OUH. The study was approved by an independent ethics committee (Regional Committees for Medical and Health Research Ethics South-East, reference numbers 235424, 135924, 204104, 233704) and by appropriate institutional review boards. All patients and controls provided written informed consent.
Study procedures and data collection
All patients and controls received SARS-CoV-2 vaccines according to the Norwegian national vaccination program, with three SARS-CoV-2 vaccine types available: BNT162b2, mRNA-1273, and ChAdOx1. The two mRNA vaccines were given with a dosing interval of 3-6 weeks. The ChAdOx1 vaccine was withdrawn from the Norwegian vaccination programme in March 2021, and all persons who had received one dose of this vaccine received one of the mRNA vaccines as the second dose after an interval of 9-12 weeks. The vaccines were administered to the patients according to availability and following a priority list determined by the Norwegian Institute of Public Health.
Demographic data including diagnosis, disease characteristics, age, gender, body mass index (BMI), and smoking and snuffing (AHUS only) habits were recorded at baseline. Type and dosage of medications, serum drug concentrations for TNFi, vedolizumab and ustekinumab, and serum thiopurine metabolites (AHUS only), disease activity indices (partial Mayo score (pMS) for UC, Harvey-Bradshaw Index (HBI) for CD), faecal calprotectin, and standard laboratory measurements were collected before vaccination and during follow-up. Due to the low frequency of covid-19 infection at the time of data registration, this patient cohort were not analysed separately. Information regarding the participants’ vaccination dates and type of vaccines were obtained from the Norwegian Immunisation Registry, SYSVAK [Citation15]. Data were collected using an electronic case report form (Viedoc, version 4, Sweden) at AHUS, and Medinsight database (version 2.17.90, Norway) at OUH, respectively. For healthy controls, age, gender, and date and type of vaccines received were collected.
Assessments
Patients and controls were asked to provide serum samples prior to the first vaccine dose and 2-5 weeks after the second vaccine dose. IgG antibodies to the receptor- binding domain (RBD) at the SARS-CoV-2 Spike protein (anti-Spike antibodies) were assessed and reported in standardised units (AU/mL) by using an in house bead-based method validated against a micro-neutralization assay at the Department of Immunology at OUS [Citation16]. Serologic response was defined as an anti-Spike antibody level ≥70 AU/ml. Serum concentrations of biologic drugs were measured using validated in house 3-step fluorometric assays fully automated on the AutoDELFIA (PerkinElmer, Waltham, MA) immunoassay platform (assay format previously described for our belatacept assay [Citation17]. Vedolizumab was measured using two murine monoclonal antibodies (developed in house) specific to vedolizumab, biotinylated D130 F(ab’)2 as solid phase antibody, and europium-labelled D136 IgG as tracer antibody. The assay for ustekinumab utilized a biotinylated F(ab’)2 murine monoclonal antibody Å21 (developed in house, specific to ustekinumab) as solid phase antibody, and europium-labelled murine monoclonal antibody K13 (developed in house, anti-human kappa light chain) F(ab’)2 as tracer antibody. TNFi were measured using biotinylated rhTNF (produced in house) as solid phase protein and europium-labelled recombinant protein A (Aaston, Wellesley, MA) as tracer protein.
Statistical analyses
Demographic data and serologic response according to medication group were summarised using descriptive statistics. Comparison of anti-Spike antibody levels between patients and healthy controls was carried out using χ2-test for categorical and median test for continuous characteristics. Associations between response and pre-chosen patient characteristics (diagnosis, smoking, HBI/pMS, TNFi mono- and combination therapy, BMI, faecal calprotectin, vaccine type, gender, and age) were assessed by bivariate and multiple logistic regression models. Regression models included only cases with no missing values on characteristics, thus estimated on smaller sample of patients. All tests were two-sided and the results with p-values below 0.05 were considered statistically significant. The statistical analyses were performed in SPSS v27 and STATA v16.
Data availability
The datasets underlying the research results described in this article are available upon request to the corresponding author.
Results
General characteristics
A total of 958 IBD patients (380 (40%) UC, 578 (60%) CD), median age 40 (Q1; Q3 29; 52), 409 (43%) women, and 323 healthy controls, median age 44 (Q1; Q3 33; 56), 241 (75%) women underwent serological testing before and after two doses of SARS-CoV-2 vaccine between February 2, 2021, and November 22, 2021, and were included in the present analyses. Antibody results were obtained median 21 (Q1; Q3 15; 34) days after the second vaccination, 22 (15; 34) days in UC and 21 (15; 34) days in CD (p = 0.289). Overall, the patients presented with low disease activity (C-reactive protein (CRP), faecal calprotectin, HBI/pMS) at baseline, and the frequency and type of ongoing immunosuppressive therapies were comparable in UC and CD (). Seventy percent of patients and 50% of controls received the BNT162b2 vaccine for both doses (). Baseline characteristics for patients and controls are presented in .
Table 1. Characteristics of patients and healthy controls.
Humoral immune response to two-dose SARS-CoV-2 vaccination according to disease
After two SARS-CoV2 vaccine doses, 773 (93.6%) patients and 321 (99.4%) healthy controls demonstrated serologic response (anti-Spike antibodies ≥70 AU/ml) (p < 0.001). The median anti-Spike antibody level was lower in patients (618 AU/ml, Q1; Q3 192; 4370) compared to controls (3355 AU/ml, Q1; Q3 896; 7849) (p < 0.001) (). Among patients, the percentage of responders was lower in CD compared to UC (91.9% vs. 96.1%, p = 0.016). Likewise, the median level of anti-Spike antibodies was lower in CD compared to UC (439 AU/ml (Q1; Q3 174; 3304) vs. 1088 (251; 5975)) (p < 0.001) ().
Impact of medication, stimulants, and disease distribution and behavior
Serologic response was shown in 98.5% of patients on ustekinumab, 98.1% on vedolizumab, 95.2% on TNFi monotherapy, 87.1% on TNFi combined with methotrexate and 83.3% on TNFi combined with thiopurines (). Due to an insignificant number of patients treated with corticosteroids (<10), this compound was not included in the analyses. The response rate was significantly lower in CD compared to UC using TNFi monotherapy (93.6% vs.97.8%, p = 0.034) or TNFi in combination with thiopurines (76.7% vs. 92.9%, p = 0.031) (). In patients receiving two doses of mRNA-1273 or BNT162b2 vaccine, the response rate was 98.9% and 92.1% (p = 0.001), respectively (). Among current snuffers (19%), the overall response rate was 89.1%, and CD patients demonstrated a lower response rate compared to UC patients within this group (83.9% vs. 97.2% p = 0.046). The overall serologic response among current smokers was 93%, with no difference between CD and UC (93.3% vs. 90.9%, p = 0.773). No impact of disease distribution and behavior on response rate or serology level was shown (Supplementary Table 1).
Table 2. Serologic response and anti-Spike antibody levels after two-dose SARS-CoV-2 vaccination in all patients, and across diseases.
Impact of serum drug levels
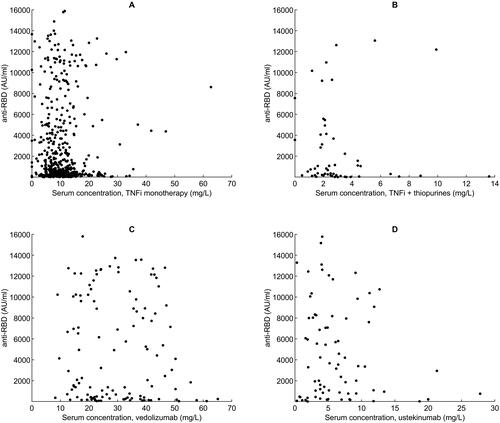
No associations between anti-Spike antibody levels and serum concentrations of TNFi in mono- or combination therapy with thiopurines, vedolizumab, and ustekinumab were demonstrated (). Patients treated with TNFi in combination with thiopurines (102/479 (21%)) with low 6-thioguanine nucleotide (6-TGN) levels (<3.5 pmol/8 × 108 red blood cells (RBC)) demonstrated a higher response rate (92.9%) than patients who had 6-TGN levels within the therapeutic range (≥3.5 pmol/8 × 108 RBC) (53.3%) (p = 0.003) ().
Figure 2. Anti-Spike antibody levels following two-dose SARS-CoV-2 vaccination related to serum concentrations of various biological therapies. Scatter plots demonstrating anti-Spike antibody levels (AU/ml) related to serum concentrations (mg/L) of A. TNFi monotherapy, B. TNFi + thiopurines, C. vedolizumab, D. ustekinumab. (TNFi, tumour necrosis factor inhibitor).
Predictors of response following two-dose SARS-CoV-2 vaccination
The distribution of covariates in terms of serologic response is shown in Supplementary Table 2. In the multiple regression model, UC as compared to CD (odds ratio (OR) 2.30, 95% confidence interval (CI), 1.05; 5.06)), BMI (OR 1.08 (95% CI, 1.01; 1.17), and mRNA-1273 vaccine as compared to BNT162b2 (OR 3.37, 95% CI, 1.15; 9.86) were associated with higher odds for humoral response following two-dose vaccination (). Older age (OR 0.94, 95% CI 0.92; 0.97) and patients on treatment with TNFi in combination with thiopurines (OR 0.18, 95% CI 0.07; 0.43) had lower odds to have humoral response (). TNFi monotherapy, disease activity (CRP, faecal calprotectin, disease indices) gender and smoking were not associated with humoral response.
Table 3. Predictors of serologic response following two-dose SARS-CoV-2 vaccination.
Discussion
This large, prospective observational study of IBD patients on biological therapy, addressed the influence of diagnosis and immunosuppressive medication on serologic response to two-dose SARS-CoV-2 vaccination. We demonstrated an overall high serologic response rate in the patients, though weaker than that of controls. The anti-Spike antibody levels were significantly lower in CD compared to UC patients. No association between serum concentrations of the biological drugs and the level of anti-SARS-CoV-2 antibody formation was found. Combination therapy with TNFi and thiopurines was associated with an impaired serological response, especially in patients with 6-TGN levels within the therapeutic range.
A lack of association between serum drug levels of any of the biologic drugs and the vaccine response in our study is in accordance with two smaller studies encompassing IBD patients, where neither timing of TNFi administration nor drug levels were associated with humoral vaccine response [Citation12,Citation18]. Hence, there seems to be no dose-dependent inhibition of the vaccine response related to serum levels of biologic medication. This important finding has implications for clinical practice, indicating that SARS-CoV-2 vaccine can be given regardless of proximity to drug administration.
The finding that CD patients had an attenuated serologic response to SARS-CoV-2 vaccine compared to UC patients have previously been shown in the Italian ESCAPE-IBD study including more than 1000 IBD patients, where the CD diagnosis was found to be an independent predictor of reduced seropositivity rates after two-dose SARS-CoV-2 vaccination [Citation8]. The same result was also demonstrated in a study by Kennedy et al. who examined the response to a single dose of a SARS-CoV-2 vaccine in 293 IBD patients treated with biologics [Citation2]. In our study, the use of immunosuppressive medication were well balanced between the UC and CD patients, so were age, time between vaccination and serum sampling, disease activity, smoking and type of vaccine received. According to the tender system for biologics in Norway, both CD and UC patients must fail TNFi treatment before prescription of other biologics [Citation19,Citation20], which contrasts many countries where UC patients often initiate their biologic treatment with vedolizumab. The mechanisms involved in impaired response to vaccines in IBD patients remain unclear but might be related to immunological alterations generated by the disease or the medical treatment. Although there are many similarities between UC and CD, there are also important pathophysiological differences [Citation21]. Moreover, CD is a more severe systemic disease, whereas UC is usually limited to colonic mucosal inflammation alone.
In accordance with previous studies, we found differences among the immunosuppressive drugs regarding SARS-CoV-2 vaccine response, with the lowest proportion of responders observed for treatment with TNFi in combination with thiopurines [Citation3,Citation5,Citation22]. These results are in line with the findings of the serologic response following other relevant vaccines in this patient group [Citation23]. The novel finding of an association between a high 6-TGN level and reduced SARS-CoV-2 vaccine response should be taken into consideration before SARS-CoV-2 vaccination and may indicate dose reduction/pausing of thiopurines before SARS-CoV-2 vaccination in vulnerable patients with stable disease.
In contrast to TNFi, the use of vedolizumab and ustekinumab were not associated with reduced serologic vaccine response in our study. This is in agreement with previous studies including a report by Alexander et al. who evaluated immunogenicity after two doses of SARS-CoV-2 vaccine in a large cohort of IBD patients receiving treatment with different immunosuppressive medications [Citation2,Citation6,Citation7,Citation12,Citation24]. Moreover, we also demonstrate a strong serologic response regardless of the route of administration of vedolizumab and the serum concentration of the drug, which has not been reported previously. An explanation for the high response rates in patients on vedolizumab may be due to its gut selectivity with subsequent limited systemic effects and no need for co-medication [Citation25]. This fact is reflected in general advice regarding vaccination in IBD, where recommendations differ for users of vedolizumab compared with other biologic drugs [Citation26].
BMI was shown to be an independent factor of importance to vaccine responsiveness among the IBD patients included in the study, as higher BMI was associated with a better vaccine response. We have no plausible explanation for this observation, and it is worth noting that an inverse association between BMI and serological response to influenza vaccine has been demonstrated in other populations [Citation27,Citation28]. In a recent study, central obesity defined according to waist circumference, was associated with lower titers in response to SARS-CoV-2 vaccine and not BMI [Citation29]. It might be speculated whether a higher BMI in IBD to some extent reflects a lower disease activity and hence a better health status, which again could influence responsiveness to the vaccine.
We could not confirm smoking as a predictor for low serologic vaccine response as demonstrated in a previous study [Citation2]. However, current snuffers demonstrated an overall low serologic response, with a lower response in CD than UC, which is a novel finding. We were, however, not able to assess snuffing in the regression model since it was recorded at one centre only.
Vaccination with mRNA-1273 as compared to BNT162b2 was found to induce higher anti-Spike antibody levels. Prior studies have suggested that mRNA-1273 may be more immunogenic than BNT162b2 in healthy subjects, which might provide an explanation for the present finding [Citation6,Citation30,Citation31].
In several recent studies, the association between older age and reduced response to vaccines has been demonstrated [Citation2,Citation10,Citation18]. This relationship was confirmed in our study. The significant contribution of gender to modulating vaccine induced immunity is well recognized [Citation32]. In general, females compared to males develop higher magnitude immune responses, with respect to antibody levels after anti-viral vaccinations [Citation33]. In a study including 248 Italian health care workers, it was demonstrated that gender was significantly associated with a difference in antibody response to SARS-CoV-2 BNT162b2 vaccine [Citation33,Citation34]. No difference was found between genders in our study, however, which is in accordance with other studies evaluating response to SARS-CoV-2 vaccines in IBD patients [Citation2,Citation10,Citation12].
It has been hypothesised that both long standing and active immune mediated inflammation may reduce seroconversion by influencing vaccine immunogenicity, as demonstrated in rheumatic diseases with other non-live vaccines such as influenza vaccine [Citation35,Citation36]. Our study observed no such effect.
Strengths of this study include the prospective study design, a broad inclusion, well characterised patients and a large sample size regarding patients and controls.
The main limitation of this study is the lack of data regarding cellular immune responses, which would have allowed elucidation of T cell mediated immune responses to SARS-CoV-2 vaccination. More follow-up data assessing both humoral and cellular responses and clinical outcomes over time in IBD patients are warranted [Citation37]. Moreover, some medications, such as the use of corticosteroids, were only used by a low number of patients. The IBD patients were slightly younger and had a lower proportion of female gender compared with the control group, raising the possibility of biased results. However, we have adjusted for age and gender in the multiple regression model. Due to the one-center recording of serum levels of thiopurines and snuffing, we were not able to test these variables in the multiple regression model.
In summary, we found that drug exposure assessed by serum drug concentrations did not impact the humoral immune response to SARS-CoV-2 vaccines in IBD patients. TNFi, especially in combination with thiopurines, were associated with an attenuated serological response, and serological response was significantly reduced in CD compared to UC patients. Our results indicate that SARS-CoV-2 vaccines can be provided without considering the timing of administration of biologic in IBD patients and will therefore aid decision-making regarding re-vaccinations and tailoring of medication in order to keep vulnerable IBD patients protected against serious SARS-CoV-2 infection.
Ethical approval
This study does not involve any commercial entity. It has been conducted in accordance with ethical principles that have their origin in the ‘Declaration of Helsinki’ and is consistent with applicable laws and regulations. The study is approved by an independent ethics committee (Regional Committees for Medical and Health Research Ethics South-East, reference numbers 235424, 135924, 204104, 233704) and by appropriate institutional review boards. All patients and controls provided written informed consent. All authors approved the final submitted version and take responsibility for the completeness and accuracy of the data and analyses. KKJ and JSB had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author contributions
KKJ, MLH, AWM and JJ were involved in conception and design of the study. KKJ, MLH, AWM, JJ, AC, PR, NB, DJW and KA were involved in the acquisition of the data. KKJ, MLH, AC, JŠB, PR, BM, IJ, NB, DJW, JTV, LAM, KEAL, KA, SWS, GLG, FLJ, AWM and JJ analysed and interpreted the data, drafted the article, critically revised for intellectual content, and approved the final version for submission. FLJ and NB developed the assay used for serological assessment and performed the laboratory procedures. JŠB was study statistician and performed the statistical analyses. KKJ and JŠB had full access to all the data in the study and took final responsibility for the decision to submit for publication.
| Abbreviations | ||
| AHUS | = | Akershus University Hospital |
| BMI | = | body mass index |
| CD | = | Crohn’s disease |
| CEPI | = | Coalition for Epidemic Preparedness Innovations |
| CRP | = | C-reactive protein |
| HBI | = | Harvey-Bradshaw Index |
| IBD | = | Inflammatory bowel disease |
| IMIDs | = | immune-mediated inflammatory diseases |
| NIPH | = | Norwegian Institute of Public Health |
| OUH | = | Oslo University Hospital |
| pMS | = | partial Mayo score |
| RBD | = | receptor-binding domain |
| SYSVAK | = | Norwegian Immunisation Registry |
| TNFi | = | tumor necrosis factor inhibitors |
| UC | = | ulcerative colitis |
Supplemental Material
Download PDF (104.4 KB)Acknowledgements
The authors thank the patients who have participated in this study, and all healthy controls who have donated serum samples. The authors are grateful for the time and effort they have invested in the project. The authors thank the patient representative in the study group: Roger Thoresen. Many people have contributed to the study design and implementation of the study at AHUS and OUH. The authors thank all study personnel, laboratory personnel and other staff involved at the clinical departments involved, particularly Synnøve Aure, Liv S. Sjue and Maren B. Sjåmo. The authors thank the staff at the Department of Immunology at OUH.
Disclosure statement
KKJ reports speaker bureaus from Roche, BMS and Janssen. MLH reports research grants from Tillotts, Ferring and Takeda; advisory board for Takeda; and speaker fees from AbbVie, Meda and Takeda. BM serves in the advisory board for AbbVie, Jansen, Hospira, Orion Pharma, Pfizer, Sandoz, Vifor Pharma and reports speaker fees from Takeda, Pfizer, Orion Pharma, Hospira, Ferring, and Cosmo Pharma. NB reported receipt of personal fees from Roche, Janssen, and Novartis. LAM reports funding from KG Jebsen foundation, support for infrastructure and biobanking from the university of Oslo and Oslo University Hospital and speaker bureaus from Novartis and Cellgene. KEAL reports personal fees from Amyra BMBH, Bioniz Pharmaceuticals Inc, Chugai Pharmaceutical Co., Ltd, Dr. Falk Pharma GMBH, Intrexon Actobios and Takeda California, Inc. KA reports personal fees from Takeda. GLG reports funding from The Karin Fossum foundation, Diakonhjemmet Hospital, Oslo University Hospital, Akershus University Hospital, Trygve Gydtfeldt og frues Foundation, South-East region Health authority, consulting fees from AbbVie and Pfizer, speaker fees from AbbVie, Pfizer, Sandoz, Orion Pharma, Novartis and UCB, and has served in advisory boards for Pfizer and AbbVie. JJ has served as a speaker, consultant, or advisory board member for Abbie, Astro Pharma, Boehringer Ingelheim, BMS, Ferring, Celltrion, Hikma, Janssen, Meda, MSD, Napp Pharma, Norgine, Novartis, Orion Pharma, Pfzer, Pharmacosmos, Roche, Takeda, Tillotts, and Sandoz. AC, JŠB, PR, IJ, DJW, JTV, SWS, FLJ and AWM report nothing to disclose.
Data availability statement
A de-identified patient data set can be made available to researchers upon reasonable request. The data will only be made available after submission of a project plan outlining the reason for the request. Project proposals can be submitted to the corresponding author. Data sharing will have to follow appropriate regulations.
Additional information
Funding
References
- Lamb CA, Kennedy NA, Raine T, IBD guidelines eDelphi consensus group, et al. British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106.
- Kennedy NA, Lin S, Goodhand JR, Contributors to the CLARITY IBD study, et al. Infliximab is associated with attenuated immunogenicity to BNT162b2 and ChAdOx1 nCoV-19 SARS-CoV-2 vaccines in patients with IBD. Gut. 2021;70(10):1884–1893.
- Jena A, Mishra S, Deepak P, et al. Response to SARS-CoV-2 vaccination in immune mediated inflammatory diseases: systematic review and meta-analysis. Autoimmun Rev. 2022;21(1):102927.
- Kappelman MD, Weaver KN, Boccieri M, PREVENT-COVID Study Group, et al. Humoral immune response to messenger RNA COVID-19 vaccines Among patients With inflammatory bowel disease. Gastroenterology. 2021;161(4):1340–1343.e2.
- Sakuraba A, Luna A, Micic D. Serologic response to coronavirus disease 2019 (COVID-19) vaccination in patients With Immune-Mediated inflammatory diseases: a systematic review and meta-analysis. Gastroenterology. 2022;162(1):88–108. e9.
- Alexander JL, Kennedy NA, Ibraheim H, et al. COVID-19 vaccine-induced antibody responses in immunosuppressed patients with inflammatory bowel disease (VIP): a multicentre, prospective, case-control study. Lancet Gastroenterol Hepat. 2022;7(4):342–352.
- Syversen SW, Jyssum I, Tveter AT, et al. Immunogenicity and safety of standard and Third-Dose SARS-CoV-2 vaccination in patients receiving immunosuppressive therapy. Arthritis Rheumatol. 2022;74(8):1321–1332.
- Macaluso FS, Principi M, Facciotti F, et al. Reduced humoral response to two doses of COVID-19 vaccine in patients with inflammatory bowel disease: data from ESCAPE-IBD, an IG-IBD study. Dig Liver Dis. 2022;55(2):154–159.
- Shiga H, Kakuta Y, Abe AK, et al. Response to COVID-19 vaccine is reduced in patients with inflammatory bowel disease, but improved with additional dose. J Gastroenterol Hepatol. 2022;38(1):44–51.
- Alexander JL, Moran GW, Gaya DR, Inflammatory Bowel Disease section of the British Society of Gastroenterology and the the Inflammatory Bowel Disease Clinical Research Group, et al. SARS-CoV-2 vaccination for patients with inflammatory bowel disease: a british society of gastroenterology inflammatory bowel disease section and IBD clinical research group position statement. Lancet Gastroenterol Hepatol. 2021;6(3):218–224.
- Lin S, Lau LH, Chanchlani N, et al. Recent advances in clinical practice: management of inflammatory bowel disease during the COVID-19 pandemic. Gut. 2022;71(7):1426–1439.
- Cerna K, Duricova D, Lukas M, et al. Anti-SARS-CoV-2 vaccination and antibody response in patients With inflammatory bowel disease on immune-modifying therapy: prospective Single-Tertiary study. Inflamm Bowel Dis. 2021;28(10):1506–1512.
- Chanchlani N, Lin S, Chee D, et al. Adalimumab and infliximab impair SARS-CoV-2 antibody responses: results from a therapeutic drug monitoring study in 11422 biologic-treated patients. J Crohns Colitis. 2021;16(3):389–397.
- Christensen IE, Jyssum I, Tveter AT, et al. The persistence of anti-Spike antibodies following two SARS-CoV-2 vaccine doses in patients on immunosuppressive therapy compared to healthy controls-a prospective cohort study. BMC Med. 2022;20(1):378.
- Norwegian Institute of Public Health. Norwegian Immunisation Registry (SYSVAK). https://www.fhi.no/en/hn/health-registries/norwegian-immunisation-registry-sysvak/
- Tran TT, Vaage EB, Mehta A, et al. Multiplexed measurement of binding- and neutralizing antibodies to SARS-CoV-2 variants in 12.000 post-vaccine sera. bioRxiv. 2022.
- Klaasen RA, Egeland EJ, Chan J, et al. A fully automated method for the determination of serum belatacept and its application in a pharmacokinetic investigation in renal transplant recipients. Ther Drug Monit. 2019;41(1):11–18.
- Edelman-Klapper H, Zittan E, Bar GIL, REsponses to COVid-19 vaccinE IsRaeli IBD group (RECOVER), et al. Lower serologic response to COVID-19 mRNA vaccine in patients With inflammatory bowel diseases treated With Anti-TNFalpha. Gastroenterology. 2022;162(2):454–467.
- Brkic A, Diamantopoulos AP, Haavardsholm EA, et al. Exploring drug cost and disease outcome in rheumatoid arthritis patients treated with biologic and targeted synthetic DMARDs in Norway in 2010-2019 - a country with a national tender system for prescription of costly drugs. BMC Health Serv Res. 2022;22(1):48.
- Anisdahl K, Svatun Lirhus S, Medhus AW, et al. First-line biologic treatment of inflammatory bowel disease during the first 12 months after diagnosis from 2010 to 2016: a norwegian nationwide registry study. Scand J Gastroenterol. 2021;56(10):1163–1168.
- Chang JT. Pathophysiology of inflammatory bowel diseases. N Engl J Med. 2020;383(27):2652–2664.
- Wellens J, Edmans M, Obolski U, et al. Combination therapy of infliximab and thiopurines, but not monotherapy with infliximab or vedolizumab, is associated with attenuated IgA and neutralisation responses to SARS-CoV-2 in inflammatory bowel disease. Gut. 2021;71(9):1919–1922.
- Muller KE, Dohos D, Sipos Z, et al. Immune response to influenza and pneumococcal vaccines in adults with inflammatory bowel disease: a systematic review and meta-analysis of 1429 patients. Vaccine. 2022;40(13):2076–2086.
- Melmed GY, Botwin Gj Sobhani K, Li D, et al. Antibody responses After SARS-CoV-2 mRNA vaccination in adults With inflammatory bowel disease. Ann Intern Med. 2021;174(12):1768–1770.
- Feagan BG, Rutgeerts P, Sands BE, GEMINI 1 Study Group, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699–710.
- Manser CN, Maillard MH, Rogler G, et al. Vaccination in patients with inflammatory bowel diseases. Digestion. 2020;101(Suppl 1):58–68.
- Sheridan PA, Paich HA, Handy J, et al. Obesity is associated with impaired immune response to influenza vaccination in humans. Int J Obes. 2012;36(8):1072–1077.
- Neidich SD, Green WD, Rebeles J, et al. Increased risk of influenza among vaccinated adults who are obese. Int J Obes. 2017;41(9):1324–1330.
- Watanabe M, Balena A, Tuccinardi D, et al. Central obesity, smoking habit, and hypertension are associated with lower antibody titres in response to COVID-19 mRNA vaccine. Diabetes Metab Res Rev. 2022;38(1):e3465.
- Steensels D, Pierlet N, Penders J, et al. Comparison of SARS-CoV-2 antibody response Following vaccination With BNT162b2 and mRNA-1273. JAMA. 2021;326(15):1533–1535.
- Caldera F, Knutson KL, Saha S, et al. Humoral immunogenicity of mRNA COVID-19 vaccines Among patients With inflammatory bowel disease and healthy controls. Am J Gastroenterol. 2022;117(1):176–179.
- Flanagan KL, Fink AL, Plebanski M, et al. Sex and gender differences in the outcomes of vaccination over the life course. Annu Rev Cell Dev Biol. 2017;33:577–599.
- Klein SL, Marriott I, Fish EN. Sex-based differences in immune function and responses to vaccination. Trans R Soc Trop Med Hyg. 2015;109(1):9–15.
- Pellini R, Venuti A, Pimpinelli F, et al. Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine. 2021;36:100928.
- Campos LM, Silva CA, Aikawa NE, et al. High disease activity: an independent factor for reduced immunogenicity of the pandemic influenza a vaccine in patients with juvenile systemic lupus erythematosus. Arthritis Care Res. 2013;65(7):1121–1127.
- Velikova T, Georgiev T. SARS-CoV-2 vaccines and autoimmune diseases amidst the COVID-19 crisis. Rheumatol Int. 2021;41(3):509–518.
- Ni L, Ye F, Cheng ML, et al. Detection of SARS-CoV-2-Specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52(6):971–977.e3.