Abstract
Objectives
Alcohol-related liver disease (ALD) is among the most common indications for liver transplantation (LTX) in Europe and North America, with good five-year survival rates post-LTX. Here we evaluated survival up to and beyond 20 years after LTX for patients with ALD compared to a comparison group.
Methods
Patients with ALD and a comparison group transplanted in the Nordic countries between 1982 and 2020 were included. Data were analyzed using descriptive statistics, Kaplan–Meier curves and predictors of survival were assessed with Cox-regressions.
Results
831 patients with ALD and 2979 patients in the comparison group were included in the study. Patients with ALD were older at the time of LTX (p < .001) and more likely to be male (p < .001). The estimated median follow-up time was 9.1 years for the ALD-group and 11.1 years for the comparison group. 333 (40.1%) patients with ALD and 1010 (33.9%) patients in the comparison group died during follow-up. The overall survival was impaired for patients with ALD compared to the comparison group (p < .001) and was evident for male and female patients, patients transplanted before and after 2005, and observed in all age-groups except patients over 60 years. Age at transplant, waiting time, year of LTX and country of LTX were associated with decreased survival after LTX for patients with ALD.
Conclusions
Patients with ALD have a decreased long-term survival following LTX. This difference was evident in most sub-groups of patients and warrants close follow-up of liver transplanted patients with ALD with focus on risk reduction.
Introduction
Alcohol-related liver disease (ALD) is one of the most common indications for liver transplantation (LTX) in Europe and North America with increasing numbers in the recent years [Citation1–3]. Listing criteria for LTX and access to organs differs between countries, but most countries and LTX programs require a period of abstinence, usually six months, before patients are listed for LTX [Citation4,Citation5]. This period of abstinence might in some cases improve the liver function of the patient and hence in some cases remove the need for LTX [Citation6,Citation7]. Pre-transplant abstinence is seen as a predictor of continuous abstinence after the transplantation [Citation8–10] along with other factors, such as psychiatric comorbidities and smoking [Citation8,Citation11].
It is difficult to both predict and measure the degree of alcohol consumption post-LTX and estimates for the percentage of patients that start drinking again range between 20 and 50% [Citation12]. It has been suggested that relapse to moderate alcohol consumption after LTX is not associated with significant graft damage or loss nor reduced survival [Citation13] while excessive alcohol consumption after LTX is known to reduce the long-term survival [Citation14]. Most studies regard all consumption of alcohol post-LTX as relapse, which is stricter than the definition of relapse normally used in the field of addiction medicine [Citation4,Citation15,Citation16] and highlights the importance of distinguishing between minor setbacks and relapse to heavy drinking [Citation12]. Some studies have shown no difference in relapse between ALD-patients and other patients post-LTX [Citation17,Citation18] while a study from Faure et al. showed that patients with a pre-transplant diagnosis of ALD had higher post-transplant consumption of alcohol compared to other patients [Citation19].
No significant differences in one-, three- and five-year survival after LTX have been reported for patients with ALD compared to other cirrhotic patients [Citation13,Citation18,Citation20–22]. Studies evaluating survival beyond the five-year period have inherent difficulties due to patients being lost to follow-up and other potential biases. A small study from Poland reported that LTX recipients with ALD had a negative outcome beyond the fifth-year post-transplant and this was not attributable to recidivism of alcohol abuse [Citation21]. Multiple studies have shown an increased frequency of cardiovascular diseases and de novo malignancies post-transplant among patients with ALD compared to other non-viral and non-malignant etiologies [Citation4,Citation6], which could have a clear negative impact on the survival for patients with ALD.
In this study we aimed to evaluate the long-term survival beyond the fifth-year post-transplant for patients with ALD compared to other cirrhotic patients in a large transplant registry with close to complete follow-up data facilitated by the single-payer Nordic health care systems [Citation23]. This gives us the unique opportunity to evaluate long-term survival also up to and beyond 20 years after transplantation. The practices for organ allocation and temporal changes in the transplant activity in the Nordic countries have previously been reported [Citation23]. Other aims of this study were to analyze differences in retransplantation rate and cause of death between patients with ALD and a comparison group and evaluate differences between the Nordic countries.
Materials and methods
Patients and diagnoses
Data from The Nordic Liver Transplant Registry (NLTR) was used as the basis for this study. NLTR includes all patients undergoing LTX in Denmark, Estonia, Finland, Iceland, Norway and Sweden from the first transplant in 1982. LTX are performed in all these countries except for Icelandic patients that have been transplanted in Denmark or Sweden. The registry includes prospective data on all listed patients from 1990 for patients transplanted in Denmark, Finland, Norway and Sweden and from 2016 for patients from Estonia. Prior to these timepoints all transplanted patients were registered retrospectively.
All adult patients (above 18 years of age) listed with alcoholic cirrhosis as primary diagnosis between 1982 and 2020 in the Nordic countries were included. Adult patients listed with a non-malignant, non-viral and non-acute liver diseases (primary sclerosing cholangitis (PSC), primary biliary cholangitis (PBC), autoimmune hepatitis (AIH), metabolic disease, cryptogenic cirrhosis, secondary biliary cirrhosis and cirrhosis of unknown cause) were included as a comparison group. To exclude patients with a potential viral etiology that were listed with another primary diagnosis, patients with positive serology for Hepatitis C virus (HCV) antibody, Hepatitis B virus surface (HBs) antigen or Hepatitis B virus core (HBc) antibodies were excluded. Patients listed for highly urgent LTX were excluded in both the ALD and comparison group. Patients who had developed hepatocellular carcinoma (HCC) on the basis of their ALD cirrhosis or the other cirrhotic indications were listed with HCC as the primary diagnosis and thus not included in this study. Complete follow-up data were available until 31 December 2020 or until death for all patients. Information about the causes of death is registered in each country based on clinical information from the local hospital, general practitioners and death certificates and added to NLTR when a patient dies. In this study the information listed in NLTR was used.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, N.Y., USA). Clinical and biochemical variables were compared between groups using the student T-test for continuous variables and the chi-square test for categorical variables. One-way ANOVA and post hoc tests with Bonferroni correction were used for testing differences in means between three or more groups. Survival analyses were performed with Kaplan–Meier plots and the Log-rank test was used to compare survival distributions. Median follow-up time was estimated using the reverse Kaplan–Meier approach. Actuarial survival was estimated using life tables. Variables predicting patient survival were analyzed using univariate and multivariate Cox-regressions. p Values <.05 were considered significant. In the analysis of causes of death, we only evaluated patients that died during the follow-up period and the cause of death was treated as a categorical variable without taking into account the time from LTX to time of death.
Results
Patients and diagnosis
In total 1028 patients were listed for LTX with ALD as the primary diagnosis. Sixty-five of these patients were excluded due to positive serology for Hepatitis B virus (HBV) or HCV, three patients due to listing for an urgent LTX and 129 patients because they did not end up undergoing transplant. Eight-hundred and thirty-one patients with ALD were thus included in the final study group (). The number of patients listed with ALD as their primary diagnosis was steadily increasing through the study period (Supplementary Figure 1A). The highest number of transplanted patients were transplanted in Sweden (n = 298) while the fewest were transplanted in Estonia (n = 13) (). When evaluated in relation to the population size, Estonia clearly had the lowest number of transplants due to ALD per million population while for the remaining countries it was on the same level with the highest number in Finland (). The mean age of the patients with ALD at the time of the first LTX was 55.7 years and the median age was 56.2 years (). The mean age at LTX increased from 49.0 years in the period from 1982 to 1990 (n = 5) to 57.7 years during the last five-year period from 2016 to 2020 (n = 238) (p = .017). After exclusion of the small number of Estonian patients (n = 13) the difference in mean age at LTX between the four other countries was statistically significant (p < .001). Norway had the highest mean age with 57.7 years (95% CI 56.6–58.9 years) and Denmark the lowest with 53.6 years (95% CI 52.6–54.6 years). The difference in mean age was statistically significant between Denmark and Sweden (p < .001), Denmark and Norway (p < .001) and Finland and Norway (p = .037).
Figure 1. Selection of patients with alcohol-related liver disease (ALD) in the study group and patients with another non-viral, non-malignant and non-acute liver disease in the comparison group from the Nordic Liver Transplant Registry (NLTR). Patients under 18 years of age, patients with other primary diagnoses, positive serology for Hepatitis B virus (HBV) or Hepatitis C virus (HCV), patients with urgent liver transplantation (LTX) and patients who did not receive a LTX were excluded from the primary analysis. Patients not receiving a LTX were included in the intention-to-treat analysis.
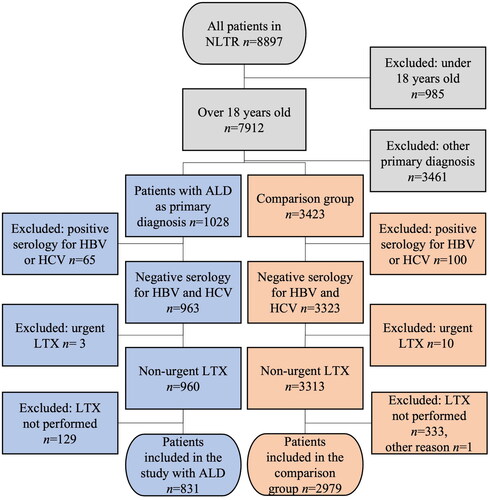
Table 1. Absolute and relative numbers of transplanted patients with alcohol-related liver disease (ALD).
Table 2. Clinical characteristics of patients with alcohol-related liver disease (ALD) and patients in the comparison group.
Among all patients who received a LTX in the Nordic countries during the study period ALD was the third most common indication (11.5%). PSC (16.1%) and HCC (11.7%) were the two most frequent diagnoses. The proportion of patients transplanted with ALD increased from 1.8% in the period from 1982 to 1990 (n = 5) to 13.9% in the period from 2016 to 2020 (n = 255) (p < .001).
Two thousand nine hundred and seventy-nine patients were included in the comparison group (). The most common diagnoses in this group were PSC (n = 1208), PBC (n = 590) and metabolic disease (n = 432). The mean age of the patients in the comparison group was 49.1 years and the median age was 50.4 years at the time of transplant (). The mean age at LTX increased from 46.2 years in the period from 1982 to 1990 (n = 124) to 50.1 years in the period 2016–2020 (n = 679) within the comparison group (p = .002).
Differences in clinical baseline variables in the ALD-group compared to the comparison group at the time of listing are shown in . Notably, the group transplanted for ALD were older at the time of first LTX (55.7 vs. 49.1, p < .001), more likely to be male (76.4% vs. 52.6%, p < .001) and had a higher body mass index (BMI) (26.2 vs. 24.7, p < .001). A higher percentage of the transplants in the ALD-group occurred in 2005 or later compared to the comparison group (71.2 vs. 63.8, p < .001) (Supplementary Figure 1B).
Donor characteristics and transplant
The mean donor age for the patients with ALD was 53.0 years compared to 47.8 years for the comparison group (p < .001). The donors were more frequently male (60.0% vs. 51.7%, p < .001) and had a higher mean BMI (25.3 vs. 24.6, p = .002) in the ALD-group compared to the comparison group.
Survival
The overall survival was impaired for patients with ALD compared to the comparison group (p < .001) (). The patient survival rates at 1, 5, 10, 15 and 20 years post liver transplantation in the ALD-group were 90%, 81%, 63%, 41% and 27%, respectively. In the comparison group the survival rates at 1, 5, 10, 15 and 20 years after LTX were 90%, 83%, 73%, 62% and 49%, and it was evident that the survival for ALD was similar to the comparison group in the first five years post transplantation (p = .18). The inferior survival in patients with ALD compared to the comparison group was still evident if PSC and PBC patients were removed from the comparison group (p < .001).
Figure 2. Patient survival in years after liver transplantation (LTX) for patients with alcohol-related liver disease (ALD) as the primary diagnosis compared to survival for patients with another non-viral, non-malignant and non-acute liver disease. KM-plot showing the overall survival in years after LTX. Actuarial survival is given for patients with ALD and the comparison group 1, 5, 10, 15, 20 and 25 years after LTX. The numbers shown below the survival graphs indicate the number of patients contributing to the analyses at that specific time point.
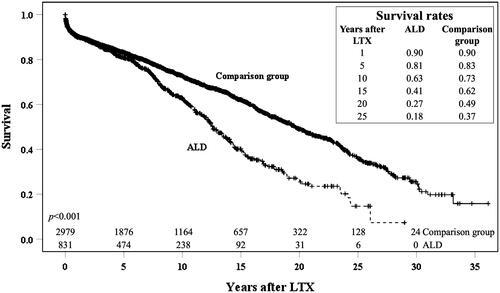
To rule out that this effect on survival was due differences in baseline characteristics in the two groups stratified analyses were performed. The observed difference in the survival between ALD-patients and the comparison group was present both for patients transplanted before 2005 and after 2005 (Supplementary Figure 3). Interestingly the survival differences were evident in even the youngest age group with patients under 40 years (nALD = 22 ncomp = 752, p < .001) as well as in patients between 40 and 50 years (nALD = 158 ncomp = 705, p = .004) and patients between 50 and 60 years (nALD = 397 ncomp = 834, p = .002) (Supplementary Figure 4). There was no difference in survival (nALD = 254 ncomp = 688, p = .78) for the ALD-patients over 60 years compared to the comparison group (Supplementary Figure 4). There were statistically significant differences in survival between ALD-patients and the comparison group in Sweden (nALD = 298 ncomp = 1363, p < .001), Denmark (nALD = 188 ncomp = 461, p < .001) and Norway (nALD = 146 ncomp = 614, p = .003), while there was lacking clear evidence for a difference in survival between the two groups in Finland (nALD = 186 ncomp = 518, p = .189) (Supplementary figure 5). No difference in survival was detected for Estonian patients (nALD = 13 ncomp = 23, p = .67) which is most likely due to the low number of patients in this sub-cohort (Supplementary figure 5). There was a significant difference in survival between the patients transplanted for ALD and the comparison group both for women (nALD = 196 ncomp = 1413, p = .008) and men (nALD = 635 ncomp = 1566, p < .001) (Supplementary Figure 6). The difference in long-term survival was still evident after adjusting for other variables in a multivariate Cox-regression analysis (p = .001) (). Comparison of graft survival between the two groups displayed a similar pattern as the patient survival for the respective groups (p < .001) (Supplementary Figure 2).
Table 3. Multivariate Cox-regression analyzing patient survival after liver transplantation (LTX) for patients with alcohol-related liver disease (ALD) and the comparison group.
Retransplantation
Among the patients with ALD 5.3% (n = 44) were retransplanted during the study period compared to 10.4% (n = 310) in the comparison group (p < .001). This difference could not explain the impaired patient survival between patients with ALD and the comparison group as the impaired survival between the two groups was evident for the graft survival as well (Supplementary Figure 2). The retransplantation rate within the first three months after LTX were similar within the two groups at respectively 2.3% (n = 19) for patients with ALD and 3.0% (n = 88) for the comparison group (p = .30). There was no difference in three-month patient survival after first LTX between the two groups (p = .69).
Causes of death
By the end of the study period 333 patients with ALD who had received a LTX had died. Cardiovascular or cerebrovascular conditions accounted for 14.7% (n = 49), liver complications for 13.5% (n = 45), de novo or recurrence of tumor for 19.2% (n = 64) and infections for 12.3% (n = 41) of the deaths while 14.1% had an unknown cause of death (n = 47) and 26.1% had other causes of death (n = 87). Causes of death for the patients with ALD did not differ significantly from the comparison group (). There were no differences in causes of deaths during the first five years following transplant when the two groups were compared (data not shown).
Table 4. Cause of death for patients with alcohol-related liver disease (ALD) and patients in the comparison group.
Predictors of patient survival
A univariate Cox-regression analyzing potential predictors for survival after LTX for patients with ALD can be found in . In this analysis waiting time, LTX-period and country were significantly associated with survival. The same variables, except Model for End-stage Liver Disease (MELD) score due to a high number of missing values, were included in a multivariate Cox-regression (). In this multivariate analysis recipient age, waiting time, LTX-period and country were significant predictors of survival.
Table 5. Univariate Cox-regression analyzing patient survival among patients with alcohol-related liver disease (ALD) after liver transplantation (LTX).
Table 6. Multivariate Cox-regression analyzing patient survival among patients with alcohol-related liver disease (ALD) after liver transplantation (LTX).
Intention-to-treat analysis
In an intention-to-treat analysis survival from entry on waiting list for patients listed with ALD (n = 960) was compared to survival for patients listed to the comparison group (n = 3313) after excluding patients under 18 years of age, patients with positive serology for HBV or HCV and patients with urgent LTX (). The difference between long term survival between the two groups was also evident in the intention-to-treat analysis. The patient survival 1, 5, 10 and 15 years after listing for the patients with ALD was 82%, 72%, 56% and 37% compared to 87%, 79%, 68% and 58% for the comparison group (p < .001).
Discussion
The main finding of this study is that patients with ALD have a decreased long-term survival compared to patients with other non-malignant, non-viral and non-acute end-stage liver diseases. This difference was present after adjusting for other covariates such as gender, year of transplantation and recipient age. There was no difference in the rates of acute death or retransplantation during the first three months post-transplant. Importantly, the two groups had similar survival rates during the first five years after LTX. Beyond this point the ALD-group shows a steeper decline in survival. The difference in long-term survival was not due to the lower retransplantation rate in the ALD-patients as the graft survival was similarly impaired.
Among the patients with ALD we observed that the age of the recipient, waiting time from acceptance to the waiting list to transplant, year of LTX and country of LTX were factors associated with a decreased survival time after LTX. Constant improvements in surgical techniques and immunosuppressive treatment post-LTX have been attributed to patients transplanted in recent years having a survival benefit following LTX [Citation24,Citation25] and this effect is also evident for patients with ALD [Citation13]. Since shorter waiting time is a significant contributor to survival indicates that all LTX programs should strive to keep the waiting time as low as possible.
In the four countries with a high number of transplantations (i.e., excluding Estonia) performed, Finland stands out as the only country where we did not observe a difference in the long-term survival between the ALD-patients and the comparison group. Finland also had higher median survival time after LTX compared to Sweden, Denmark and Norway for both the ALD-patients and the comparison group. These differences were also present after adjusting for other variables using multivariate Cox-regression. Given the data presently available in NLTR we were not able to evaluate potential explanatory factors regarding details on clinical status of the patients, surgical methods and differences in the medical follow up programs between the countries. The differences between the countries underline the importance of exchanging knowledge between the different transplant centers and exploring such differences in an effort to improve clinical practice.
ALD accounts for the majority of liver deaths in Europe [Citation26,Citation27] and for patients with end-stage ALD there are no medical treatment alternatives rendering liver transplantation the only curative treatment option available [Citation5]. Within the Scandiatransplant-area the access to liver-grafts has been adequate, thus there is less need to prioritize between different patient-groups. This situation might change in the future with a larger group of patients with end-stage liver disease being eligible for transplantation. Most transplant programs, including the programs in the Nordic countries, lack a systematic follow up and approach to relapse of alcohol consumption [Citation5,Citation28]. Potential differences in alcohol consumption-patterns between the patients with ALD and the comparison group in our study might to some extent explain the impaired survival we have shown. This warrants the importance of optimal selection of graft recipients and proper follow-up care after transplantation.
This study has some limitations regarding selection bias in the comparison group and confounders. The registration of clinical characteristics in NLTR such as pre-transplant comorbidities and smoking are limited. Previous studies have shown that a significantly higher proportion of patients with ALD are smoking both before and after LTX compared to patients transplanted for other liver diseases [Citation29,Citation30]. Continued smoking after LTX is associated with increased risk of death due to cardiovascular diseases and smoking-related malignancies and the incidence of cardiovascular events and de novo tumors are higher among patients with ALD than patients with other liver diseases after LTX [Citation13,Citation29,Citation31–33]. We did not find differences in cause of death in our study, but still believe that closer follow up of risk behavior and comorbidities among patients with ALD could reduce the gap in long-term survival. Standardization of reporting of clinical features pre- and post-transplant in recent years will allow more thoroughly investigation of the impact these factors have on the long-term survival post liver transplantation.
In conclusion, we have consistently demonstrated that the survival following LTX for ALD is just as good as for other cirrhotic indications in the first five years following transplant and is then followed by a steep decline which was present across different age-groups, gender and transplant centers. This finding strongly encourages close long-term medical follow-up of patients with ALD undergoing LTX with a focus on risk reduction.
| Abbreviations | ||
| AIH | = | autoimmune hepatitis |
| ALD | = | alcohol-related liver disease |
| BMI | = | body mass index |
| HBc | = | hepatitis B virus core |
| HBs | = | hepatitis B virus surface |
| HBV | = | hepatitis B virus |
| HCV | = | hepatitis C virus |
| LTX | = | liver transplantation |
| MELD | = | Model for End-stage Liver Disease |
| NLTR | = | The Nordic Liver Transplant Registry |
| PBC | = | primary biliary cholangitis |
| PSC | = | primary sclerosing cholangitis |
Supplemental Material
Download PDF (1.2 MB)Acknowledgements
The maintenance of the database system supporting NLTR has been performed by the Scandiatransplant team in Aarhus and we are especially grateful for the help and support provided by Ilse Duus Weinreich. Physicians, nurses and transplant coordinator at all the involved transplant center have provided invaluable support in keeping the registry updated.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Adam R, Karam V, Delvart V, et al. Evolution of indications and results of liver transplantation in Europe. A report from the european liver transplant registry (ELTR). J Hepatol. 2012;57(3):675–688.
- Kim WR, Lake JR, Smith JM, et al. OPTN/SRTR 2017 annual data report: liver. Am J Transplant. 2019;19 Suppl 2:184–283.
- Melum E. The nordic liver transplant registry (NLTR) annual report 2020 [internet]. Oslo (NO): Scandiatransplant; 2021 [cited 2022 Jan 14]. Available from: http://www.scandiatransplant.org/members/nltr/TheNordicLiverTransplantRegistryANNUALREPORT2020.pdf
- Mathurin P, Lucey MR. Liver transplantation in patients with alcohol-related liver disease: current status and future directions. Lancet Gastroenterol Hepatol. 2020;5(5):507–514.
- European Association for the Study of the Liver. EASL clinical practice guidelines: management of alcohol-related liver disease. J Hepatol. 2018;69(1):154–181.
- Stickel F, Datz C, Hampe J, et al. Pathophysiology and management of alcoholic liver disease: update 2016. Gut Liver. 2017;11(2):173–188.
- European Association for the Study of the Liver. EASL clinical practice guidelines: liver transplantation. J Hepatol. 2016;64(2):433–485.
- Chuncharunee L, Yamashiki N, Thakkinstian A, et al. Alcohol relapse and its predictors after liver transplantation for alcoholic liver disease: a systematic review and meta-analysis. BMC Gastroenterol. 2019;19(1):150.
- Tandon P, Goodman KJ, Ma MM, et al. A shorter duration of pre-transplant abstinence predicts problem drinking after liver transplantation. Am J Gastroenterol. 2009;104(7):1700–1706.
- Lindenger C, Castedal M, Schult A, et al. Long-term survival and predictors of relapse and survival after liver transplantation for alcoholic liver disease. Scand J Gastroenterol. 2018;53(12):1553–1561.
- Yates WR, Martin M, LaBrecque D, et al. A model to examine the validity of the 6-month abstinence criterion for liver transplantation. Alcoholism Clin Exp Res. 1998;22(2):513–517.
- Tome S, Lucey MR. Timing of liver transplantation in alcoholic cirrhosis. J Hepatol. 2003;39(3):302–307.
- Burra P, Senzolo M, Adam R, et al. Liver transplantation for alcoholic liver disease in Europe: a study from the ELTR (European Liver Transplant Registry). Am J Transplant. 2010;10(1):138–148.
- Cuadrado A, Fabrega E, Casafont F, et al. Alcohol recidivism impairs long-term patient survival after orthotopic liver transplantation for alcoholic liver disease. Liver Transpl. 2005;11(4):420–426.
- Neuberger J, Schulz KH, Day C, et al. Transplantation for alcoholic liver disease. J Hepatol. 2002;36(1):130–137.
- Maisto SA, Roos CR, Hallgren KA, et al. Do alcohol relapse episodes During treatment predict long-term outcomes? Investigating the validity of existing definitions of alcohol use disorder relapse. Alcohol Clin Exp Res. 2016;40(10):2180–2189.
- Bravata DM, Olkin I, Barnato AE, et al. Employment and alcohol use after liver transplantation for alcoholic and nonalcoholic liver disease: a systematic review. Liver Transpl. 2001;7(3):191–203.
- Mackie J, Groves K, Hoyle A, et al. Orthotopic liver transplantation for alcoholic liver disease: a retrospective analysis of survival, recidivism, and risk factors predisposing to recidivism. Liver Transpl. 2001;7(5):418–427.
- Faure S, Herrero A, Jung B, et al. Excessive alcohol consumption after liver transplantation impacts on long-term survival, whatever the primary indication. J Hepatol. 2012;57(2):306–312.
- Lucey MR, Schaubel DE, Guidinger MK, et al. Effect of alcoholic liver disease and hepatitis C infection on waiting list and posttransplant mortality and transplant survival benefit. Hepatology. 2009;50(2):400–406.
- Grat M, Lewandowski Z, Grat K, et al. Negative outcomes after liver transplantation in patients with alcoholic liver disease beyond the fifth post-transplant year. Clin Transplant. 2014;28(10):1112–1120.
- Gedaly R, McHugh PP, Johnston TD, et al. Predictors of relapse to alcohol and illicit drugs after liver transplantation for alcoholic liver disease. Transplantation. 2008;86(8):1090–1095.
- Fosby B, Melum E, Bjoro K, et al. Liver transplantation in the nordic countries - an intention to treat and post-transplant analysis from The nordic liver transplant registry 1982-2013. Scand J Gastroenterol. 2015;50(6):797–808.
- Dutkowski P, De Rougemont O, Mullhaupt B, et al. Current and future trends in liver transplantation in Europe. Gastroenterology. 2010;138(3):802- 9.e1-4.
- Adam R, Hoti E. Liver transplantation: the current situation. Semin Liver Dis. 2009;29(1):3–18.
- Sheron N. Alcohol and liver disease in Europe–simple measures have the potential to prevent tens of thousands of premature deaths. J Hepatol. 2016;64(4):957–967.
- Karlsen TH, Sheron N, Zelber-Sagi S, et al. The EASL-Lancet liver commission: protecting the next generation of europeans against liver disease complications and premature mortality. Lancet. 2022;399(10319):61–116.
- Addolorato G, Mirijello A, Leggio L, et al. Liver transplantation in alcoholic patients: impact of an alcohol addiction unit within a liver transplant center. Alcohol Clin Exp Res. 2013;37(9):1601–1608.
- Leithead JA, Ferguson JW, Hayes PC. Smoking-related morbidity and mortality following liver transplantation. Liver Transpl. 2008;14(8):1159–1164.
- van der Heide F, Dijkstra G, Porte RJ, et al. Smoking behavior in liver transplant recipients. Liver Transpl. 2009;15(6):648–655.
- Herrero JI, Pardo F, D'Avola D, et al. Risk factors of lung, head and neck, esophageal, and kidney and urinary tract carcinomas after liver transplantation: the effect of smoking withdrawal. Liver Transpl. 2011;17(4):402–408.
- Mangus RS, Fridell JA, Kubal CA, et al. Worse long-term patient survival and higher cancer rates in liver transplant recipients with a history of smoking. Transplantation. 2015;99(9):1862–1868.
- Addolorato G, Bataller R, Burra P, et al. Liver transplantation for alcoholic liver disease. Transplantation. 2016;100(5):981–987.
