Pancreatic cystic lesions have become well-known and very common findings to gastroenterologists, radiologists, pathologists and surgeons involved in hepatobiliary and pancreatic disorders. Indeed, an increasing number of new – often incidental – cysts are identified on a weekly basis, due to the increasing number of cross-sectional imaging used in the population. It is estimated that incidental pancreatic cysts are detected in around 2% of computed tomography (CT) scans and in more than 3% of all magnetic resonance imaging (MRI) scans [Citation1]. Indeed, about 50% of MRI scans may contain multiple, incidental and small cysts, for which two-thirds are ≤5 mm and about 90% may be ≤10 mm in size [Citation2]. For such small cysts, the cancer risk is almost negligible [Citation2]. Furthermore, many cysts may often only obtain the shape of a ‘cystic lesion’ and as such being indeterminate in their characteristics – referred to as the ‘shape of water’ [Citation3]. Hence, in order to better define management most cysts are now discussed in multidisciplinary ‘pancreatic cyst meetings’ in order to arrive at the best work-up or further surveillance. Importantly, some defined cysts, but not all, are declared as potential precursor lesions to pancreatic cancer [Citation4]. Despite the progress made over the past several decades, there is still considerable controversy around how to best manage cystic lesions of the pancreas, with discrepancies noted across the several existing (at least 5) updated and new society guidelines in use [Citation5].
Several of the pancreatic cyst sub-entities, such as the intraductal papillary mucinous neoplasia (IPMN), have now become household names for most specialists regularly dealing with this entity. This was not always the case, as at the end of the 1990s, several clinical series still used the blunt distinction between “cystadenoma” and “cystadenocarcinoma” of the pancreas [Citation6–8].
In Scandinavia, early surgical reports using the nomenclature term of IPMN seems to have emerged from Aarhus, Denmark reporting on 6 total pancreatectomies for IPMN in 2001 [Citation9]. A case report in 2008 from Oslo, Norway found an IPMN in a resected ectopic pancreatic tissue, likely the first report of this sort [Citation10]. A comprehensive review of the updated knowledge of IPMN was reported in 2010 [Citation11]. Since then, both monocentric series [Citation12] and nationwide registry studies [Citation13] have been reported from Scandinavia, highlighting several ongoing issues with this entity. Meanwhile, the number of new IPMNs, and hence the prevalence in the population, is increasing year on year.
Having obtained a deeper interest in the pancreas as a medical student as I was given the topic to present to the class (Propädeutisches Seminar der Anatomie; ‘Exokrines Pancreas’; University of Freiburg, 1997/98). Not only was I exposed to ‘the Whipple procedure’ and development of pancreatic cancer as a disease, but this testifies to how a topic in medical school can stick to a student. An introduction of pancreatic cysts further emerged from attending a conference lecture held by prof Mike Sarr from the Mayo Clinic (‘Pancreatic cysts’, Gastrointestinal Symposium, Stavanger, 1998) being a pioneer institution on the topic back in the days [Citation14–16]. (A further argument that we should invite and involve medical students to medical conferences). However, most cysts were still rather rare and of relatively large size at the time, but it was clear that the field was rapidly emerging to clinicians.
Only some years later my initial academic ‘contribution’ to the pancreas was initiated, as I presented a quiz case to the annual meeting of the Norwegian Society of Pathology ( and ). Being a novice yet budding PhD student at the time and working in the Department of Pathology, I submitted a case of a patient presenting with jaundice, found to have a dilated main pancreatic duct on MRI scan (), an atrophic pancreatic body and tail and, a 42 mm mass in the pancreatic head (). The patient had a pancreatoduodenectomy and an uneventful post-operative course.
Figure 1. Preoperative diagnosis of main duct IPMN. (A) MRI scan showing a universally dilated main pancreatic duct (arrows) in head, body and tail. (B) CT scan showing a 42 mm mass in the pancreatic head (arrow) and (C) an atrophic pancreatic body and tail with dilated duct (arrow).
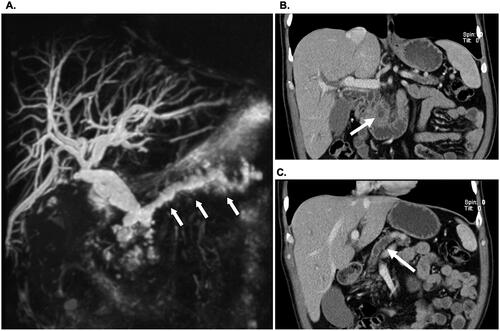
Figure 2. Histomorphological findings of an IPMN. (A) The IPMN is shown in the main pancreatic duct (arrow), with (B) positive staining for epithelial marker cytokeratin (CK)-19. Encircled area resembles the area for the immunohistochemistry markers to the right (middle column). (C) Part with invasive pancreatic adenocarcinoma and high-grade dysplasia in the pancreatic resection margin (inlets). (D) Areas of Pancreatic Intraepithelial Neoplasia (PanIN), with loss of cytokeratin-20, an epithelial marker. Presented to the annual meeting of the Norwegian Society of Pathology (Bergen, December 2004).
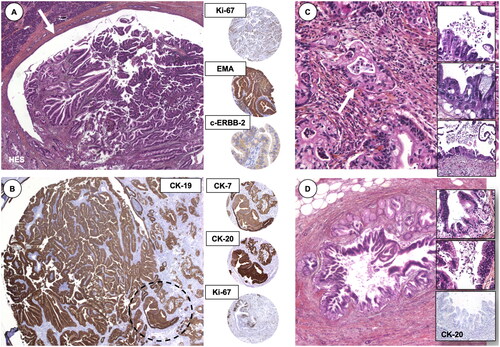
The submitted case was anonymously delivered for other pathologists to suggest the final diagnosis, with representative slides (the main panels in ) delivered for review (without immunohistochemistry). Of interest, some 35 pathologists provided their pre-conference suggested diagnosis, of which 18 (51%) had a suggestion that would resemble the final diagnosis; an IPMN with invasive adenocarcinoma. One has to keep in mind, the IPMN was very much still a ‘new kid on the block’ in both pathology and surgery back in the days [Citation17]. I suspect a similar case today would have achieved close to perfect match-rate among pathologists.
The pancreatoduodenectomy specimen showed an invasive adenocarcinoma from a main duct IPMN (pT2 N0 Mx). There was high-grade dysplasia (then named as ‘carcinoma in situ’) in the resection margin and additional findings of pancreatic intraepithelial neoplasia (PanIN; ) in the specimen. Recall, the ‘PanIN’-model suggested by the Johns Hopkins group [Citation18] had only been described a few years earlier, with the first set of consensus nomenclature suggested in 2000 [Citation19]. The use of immunohistochemistry markers may seem arbitrary today, such as staining for c-erbb-2 (today better known as HER-2 or, human epidermal growth factor receptor 2) [Citation20], but this marker was reported to play a role in the differential of these lesions to regular adenocarcinoma at the time, described early on as positive in two-thirds of IPMT [sic] [Citation21] and later to be related to invasive rather than the more benign type of IPMN [Citation22]. Proliferation marker Ki-67 was also reported to correlate with the invasive pattern of precursor lesions [Citation23]. A few years later, as a PhD student and keen to have learned of the progressive, precursor model to pancreatic cancer along the similar lines of the well-thought out adenoma-carcinoma sequence of colorectal cancer, I enthusiastically compiled a review of the PanIN-progression model in 2006 [Citation24].
Of note, in 2004, the first consensus guidelines for both pancreatic intraepithelial neoplasia (PanIN) and intraductal papillary mucinous neoplasia (IPMN) were reported [Citation25]. At the same time, the surgeons reported an exponential increase in the number of cases being resected, now reporting under the terminology of IPMN [Citation26], rather than ‘cystadenoma’ or ‘cystadenocarcinoma’. Since then, the literature has surged with new data and reports on IPMNs. There are almost 4000 hits in PubMed for IPMN, of which almost 2700 of these are produced after 2010. Furthermore, there are no lack of guidelines. Rather, a review and comparison of five of the most frequent in use suggest that there is inconsistency and overlap, redundancy and discrepancy in the terminology and proposed actions to take for patients with IPMN [Citation5]. Hence, the current landscape of decision-making is bound to be one of both over- and under-treatment. Truly, the rapid increase in incidental cysts is becoming a considerable disease burden and one that needs to be tackled by other means than the current frequent use of scans.
Among all IPMNs, the ones of the main duct and mixed types are thought to be at higher risk of malignant transformation and to have invasive cancer (but how risky are they?), while the side-branch IPMNs are thought to be more innocent (yet, not entirely, so how to pick the bad ones from the good ones?) [Citation27]. Data suggest that presence of one worrisome feature or high risk stigmata is associated with malignancy in 1 in every 5 IPMN [Citation28], with increased risk for every added accumulating risk feature. Furthermore, it is still common to see high numbers of serous cysts in resected pancreatic cyst series (up to one third in modern series), suggesting there is still room for improvement with the predictive accuracy for cysts designation and prediction. In a large series of almost 1300 cysts resected at Massachusetts General Hospital, 23 different cyst-entity diagnosis were entertained over 3 decades, but 80% were IPMNs, mucinous cystadenomas or serous cysts [Citation29]. While the diagnostic accuracy has improved with CT, MRI and MRCP, endoscopic ultrasound (EUS), and more lately with advances in biopsy and next-generation sequencing [Citation29], there is still risk concerning over- and under-treatment. Cyst-fluid protein biomarkers are emerging as predictive tools [Citation30], and multimodal test panels have shown ability to select better for resection or observation [Citation31], yet is lacking external validity. GNAS mutations have a higher prevalence than KRAS mutations in IPMNs [Citation32], and both mutations have a higher diagnostic accuracy than CEA for diagnosing mucinous cysts [Citation33]. Hence, a combination of genetic and molecular markers may be used for early detection or, for targeted prevention in future studies. As such, there may be a window of opportunity for earlier detection and timely intervention for patients at risk for pancreatic cancer [Citation34].
Going from a rare entity to a burgeoning rise in incidence, the workload of new and returning IPMNs seen in most clinics these days seems to reach almost unsustainable levels. We need stopping rules for surveillance and de-escalation of current care patterns. Most likely, well over 90% of these IPMNs that are being detected may never become clinically relevant. We may curse the entity as a plague and a torture. However, it may just as well be a blessing in disguise.
Pancreatic cancer is known for its late presentation, with few patients amenable to surgical care and, a notoriously difficult to treat cancer with systemic agents. As IPMNs are known precursor lesions to pancreatic cancer, we may take advantage of the early events of presentation, by focused research into its development, drivers of progression and, look for modes of prevention and better ways of prediction of progression [Citation34]. Indeed, the increase in prevalence through the prevalent use of imaging studies of IPMNs may be a blessing in disguise, and one that may able us to investigate the precursor steps and risks to pancreatic cancer. We should count our blessings, even if they are in disguise.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zhu S, Wang WT, Shang XS, et al. Difference analysis in prevalence of incidental pancreatic cystic lesions between computed tomography and magnetic resonance imaging. BMC Med Imaging. 2019;19(1):43.
- Kromrey ML, Bülow R, Hübner J, et al. Prospective study on the incidence, prevalence and 5-year pancreatic-related mortality of pancreatic cysts in a population-based study. Gut. 2018;67(1):138–145.
- Salvia R, Marchegiani G. Evolving management of pancreatic cystic neoplasms. Br J Surg. 2020;107(11):1393–1395.
- Søreide K, Marchegiani G. Clinical management of pancreatic premalignant lesions. Gastroenterology. 2022;162(2):379–384.
- Aziz H, Acher AW, Krishna SG, et al. Comparison of society guidelines for the management and surveillance of pancreatic cysts: a review. JAMA Surg. 2022;157(8):723–730.
- Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg. 1997;226(3):248–257. discussion 257–260.
- Borgne L, de Calan J, Partensky L. C. Cystadenomas and cystadenocarcinomas of the pancreas: a multiinstitutional retrospective study of 398 cases. French surgical association. Ann Surg. 1999;230(2):152–161.
- Meyer W, Köhler J, Gebhardt C. Cystic neoplasms of the pancreas–cystadenomas and cystadenocarcinomas. Langenbecks Arch Surg. 1999;384(1):44–49.
- Bendix Holme J, Jacobsen NO, Rokkjaer M, et al. Total pancreatectomy in six patients with intraductal papillary mucinous tumour of the pancreas: the treatment of choice. HPB (Oxford). 2001;3(4):257–262.
- Røsok BI, Rosseland AR, Grzyb K, et al. Laparoscopic resection of an intraductal papillary mucinous carcinoma in ectopic pancreatic tissue. J Laparoendosc Adv Surg Tech A. 2008;18(5):723–725.
- Dongbin L, Fei L, Werner Josefin B, et al. Intraductal papillary mucinous neoplasms of the pancreas: diagnosis and management. Eur J Gastroenterol Hepatol. 2010;22(9):1029–1038.
- Ånonsen K, Sahakyan MA, Kleive D, et al. Trends in management and outcome of cystic pancreatic lesions - analysis of 322 cases undergoing surgical resection. Scand J Gastroenterol. 2019;54(8):1051–1057.
- Aronsson L, Andersson B, Andersson R, et al. Intraductal papillary mucinous neoplasms of The pancreas: a nationwide Registry-Based study. Scand J Surg. 2018;107(4):302–307.
- Loftus EV, Jr., Olivares-Pakzad BA, Batts KP, et al. Intraductal papillary-mucinous tumors of the pancreas: clinicopathologic features, outcome, and nomenclature. Members of the pancreas clinic, and pancreatic surgeons of Mayo clinic. Gastroenterology. 1996;110(6):1909–1918.
- Sarr MG, Carpenter HA, Prabhakar LP, et al. Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg. 2000;231(2):205–212.
- Sarr MG, Kendrick ML, Nagorney DM, et al. Cystic neoplasms of the pancreas: benign to malignant epithelial neoplasms. Surg Clin North Am. 2001;81(3):497–509.
- Adsay NV. The "new kid on the block": intraductal papillary mucinous neoplasms of the pancreas: current concepts and controversies. Surgery. 2003;133(5):459–463.
- Wilentz RE, Iacobuzio-Donahue CA, Argani P, et al. Loss of expression of Dpc4 in pancreatic intraepithelial neoplasia: evidence that DPC4 inactivation occurs late in neoplastic progression. Cancer Res. 2000;60(7):2002–2006.
- Hruban RH, Adsay NV, Albores-Saavedra J, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25(5):579–586.
- Day JD, Digiuseppe JA, Yeo C, et al. Immunohistochemical evaluation of HER-2/neu expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasms. Hum Pathol. 1996;27(2):119–124.
- Sessa F, Solcia E, Capella C, et al. Intraductal papillary-mucinous tumours represent a distinct group of pancreatic neoplasms: an investigation of tumour cell differentiation and K-ras, p53 and c-erbB-2 abnormalities in 26 patients. Virchows Arch. 1994;425(4):357–367.
- Islam HK, Fujioka Y, Tomidokoro T, et al. Immunohistochemical study of genetic alterations in intraductal and invasive ductal tumors of the pancreas. Hepatogastroenterology. 2001;48(39):879–883.
- Klein WM, Hruban RH, Klein-Szanto AJ, et al. Direct correlation between proliferative activity and dysplasia in pancreatic intraepithelial neoplasia (PanIN): additional evidence for a recently proposed model of progression. Mod Pathol. 2002;15(4):441–447.
- Søreide K, Immervoll H, Molven A. [Precursors to pancreatic cancer. ]. Tidsskr Nor Laegeforen. 2006;126(7):905–908.
- Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28(8):977–987.
- Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal papillary mucinous neoplasms of the pancreas: an increasingly recognized clinicopathologic entity. Ann Surg. 2001;234(3):313–321. discussion 321–312.
- Aunan JR, Jamieson NB, Søreide K. Observation or resection of pancreatic intraductal papillary mucinous neoplasm: an ongoing tug of war. World J Gastrointest Oncol. 2019;11(12):1092–1100.
- Zelga P, Hernandez-Barco YG, Qadan M, et al. C. Number of worrisome features and risk of malignancy in intraductal papillary mucinous neoplasm. J Am Coll Surg. 2022;234(6):1021–1030.
- Roldán J, Harrison JM, Qadan M, et al. Evolving trends in pancreatic cystic tumors: a 3-Decade Single-Center experience With 1290 resections. Ann Surg. 2023;277(3):491–497.
- McIntyre CA, Rodrigues C, Santharaman AV, et al. Multiinstitutional validation study of cyst fluid protein biomarkers in patients With cystic lesions of the pancreas. Ann Surg. 2022;276(2):e129–e132.
- Springer S, Masica DL, Dal Molin M, et al. A multimodality test to guide the management of patients with a pancreatic cyst. Sci Transl Med. 2019;11(501):eaav4772.
- Wu J, Matthaei H, Maitra A, et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med. 2011;3(92):92ra66.
- McCarty TR, Paleti S, Rustagi T. Molecular analysis of EUS-acquired pancreatic cyst fluid for KRAS and GNAS mutations for diagnosis of intraductal papillary mucinous neoplasia and mucinous cystic lesions: a systematic review and meta-analysis. Gastrointest Endosc. 2021;93(5):1019–1033.e5. e1015.
- Søreide K, Ismail W, Roalsø M, et al. Early diagnosis of pancreatic cancer: clinical premonitions, timely precursor detection and increased Curative-Intent surgery. Cancer Control. 2023;30:107327482311547.
