Abstract
Objective
Patients with an ileostomy may experience postoperative electrolyte derangement and dehydration but are presumed to stabilise thereafter. We aimed to investigate the prevalence of sodium depletion in stable outpatients with an ileostomy and applied established methods to estimate their fluid status.
Methods
We invited 178 patients with an ileostomy through a region-wide Quality-of-Life-survey to undergo outpatient evaluation of their sodium and fluid status. The patients delivered urine and blood samples, had bioelectrical impedance analysis performed and answered a questionnaire regarding dietary habits.
Results
Out of 178 invitees, 49 patients with an ileostomy were included; 22 patients (45%, 95% CI, 31–59%) had unmeasurably low urinary sodium excretion (<20 mmol/L), indicative of chronic sodium depletion, and 26% (95% CI, 16–41%) had plasma aldosterone levels above the reference value. Patients with unmeasurably low urinary sodium excretion had low estimated glomerular filtration rates (median 76, IQR 63–89, mL/min/1.73m2) and low venous blood plasma CO2 (median 24, IQR 21–26, mmol/L), indicative of chronic renal impairment and metabolic acidosis. Bioelectrical impedance analysis, plasma osmolality, creatinine and sodium values were not informative in determining sodium status in this population.
Conclusion
A high proportion of patients with an ileostomy may be chronically sodium depleted, indicated by absent urinary sodium excretion, secondary hyperaldosteronism and chronic renal impairment, despite normal standard biochemical tests. Sodium depletion may adversely affect longstanding renal function. Future studies should investigate methods to estimate and monitor fluid status and aim to develop treatments to improve sodium depletion and dehydration in patients with an ileostomy.
Sodium depletion in otherwise healthy persons with an ileostomy was identified in a few publications from the 1980s. The magnitude of the problem has not been demonstrated before. The present study quantifies the degree of sodium depletion and secondary hyperaldosteronism in this group, and the results may help guide clinicians to optimise treatment. Sodium depletion is easily assessed with a urine sample, and sequelae may possibly be avoided if sodium depletion is detected early and treated. This could ultimately help increase the quality of life in patients with an ileostomy.
IMPACT AND PRACTICE RELEVANCE STATEMENT
Introduction
Patients with an ileostomy can experience sodium depletion and suffer from various degrees of dehydration long after surgery [Citation1,Citation2]. This has been known for at least six decades [Citation3]. The symptoms may be silent, and patients are frequently presumed to be healthy. Sodium depletion remains a neglected condition [Citation4] and, if left untreated, the condition may develop into severe secondary hyperaldosteronism [Citation5]. Measuring urine sodium excretion is a simple and inexpensive method to identify sodium depletion in patients with an ileostomy [Citation6].
No gold standard for measuring dehydration exists [Citation7]. Plasma (P-) osmolality has been proposed as a clinical surrogate marker [Citation8, Citation9], but it fails to identify isotonic dehydration [Citation10]. Blood urea nitrogen (BUN):creatinine ratio, blood (B-) haemoglobin and P-sodium are often used by clinicians, but none of the methods have been validated as markers of dehydration [Citation11]. Assessment of total body water (TBW) with bioelectrical impedance analysis (BIA) resistance is a potential new tool to help clinicians evaluate hydration status.
In the present study, we investigated the prevalence of sodium depletion in stable outpatients with an ileostomy and assessed established methods for estimating their fluid status.
Materials & methods
In this population-based, cross-sectional study, we invited 178 adult outpatients with an ileostomy to a clinical examination of their sodium and fluid status. All participants were recruited through a Quality of Life (QoL)-survey as previously described [Citation12]. Patients were eligible if they were at least 18 years old, had had an ileostomy for at least six weeks, lived in the Central Denmark Region and could read, speak and understand Danish. They were ineligible if they were pregnant, admitted to a hospital or received parenteral fluid or nutrition regularly.
Survey responders who accepted the invitation came to the Department of Hepatology and Gastroenterology at Aarhus University Hospital, Denmark, between September 2017 and March 2018. The participants delivered a urine sample and had a venous blood sample taken. Both were analysed at the Department of Clinical Biochemistry, Aarhus University Hospital. The participants had their body composition assessed with a stand-on, multi-frequency BIA (Seca mBCA 515, Seca GmbH, Hamburg, Germany), where the fat-free mass index (FFMI) and total body water (TBW) were assessed along with whole-body resistance measured by the device at a frequency of 50 kHz. Hand grip strength (HGS) was measured with a digital hand dynamometer (JAMAR Plus, Patterson Medical Supply, Cedarburg, WI, USA), with patients standing and their arms straight down along their bodies. The highest measurement of three with their dominant hand was registered. The participants answered a questionnaire about their habitual food and fluid intake during the past three months. This questionnaire was created locally by a registered dietitian. It was designed partly to assess dietary intake of sodium and partly to explore the patients’ intakes of items that are presumed to affect their sodium and fluid balance (e.g., oral rehydration solutions [ORS], hypo-osmolar fluids, sugary beverages, psyllium husks, fruit and vegetables). Information regarding reasons for ileostomy formation, estimates of remaining bowel length, comorbidities and regular medication use was obtained from the patients’ medical records. The patients’ remaining bowel length was estimated to be 350 cm if they had colectomy without ileal resection and the length had not been measured during surgery. We subtracted 40 cm from this length if the patients had previously had an ileo-anal reservoir (pouch) and no remaining length was noted in the record.
Outcomes
The primary outcome was urinary sodium excretion. Secondary outcomes were biochemical parameters (P-aldosterone, P-sodium, P-creatinine, P-carbamide, P-albumin, B-haemoglobin, estimated glomerulus filtration rate [eGFR] and venous blood P-total CO2), BIA-measures (height, weight, waist circumference, FFMI, TBW and resistance at 50 kHz), HGS and dietary intake (sodium intake, use of psyllium husks, intake of fruits and vegetables, amount and types of fluids).
Data handling and statistical analyses
All patients provided written informed consent. The study was performed in agreement with the Declaration of Helsinki. The protocol was approved by the Central Denmark Regional Committee on Health Research Ethics and the Danish Data Protection Agency (j.no. 1-10-72-290-16).
Research Electronic Data Capture (REDCap), an electronic data capture tool hosted at Aarhus University (www.redcap.au.dk) [Citation13,Citation14], was used to collect all data. STATA 17 software (StataCorp. College Station, TX, USA) was used for descriptive and comparative statistics. Figures were made in GraphPad Prism 9.4.1 (GraphPad Software, San Diego, CA, USA). Results are presented as mean (±SD) for parametric data and median and interquartile range (IQR) for nonparametric data. Percentages are presented with 95% confidence intervals (95% CI). Data distribution and variables determined the statistical tests. Chi2-tests or Fisher’s Exact tests were used for categorical variables, Spearman’s rank correlation coefficient or linear regression for continuous data, and Student’s t-tests or Wilcoxon signed-ranks tests were applied to analyse continuous data in groups. p Values below .05 were considered statistically significant.
Results
Patient characteristics
A total of 178 survey responders were invited to this study, and 49 patients with an ileostomy agreed to participate (Supplementary Figure S1). The participants had a median age of 60 years (IQR 51–67), and 61% (95% CI, 47–74) were women (). The participants had had their ileostomy for a median of five years (IQR 2–13), and the primary reason for ileostomy formation was ulcerative colitis (67%, 95% CI, 53–79).
Table 1. Participant characteristics.
Thirty-two participants (65%, 95% CI, 51–78) had an end-ileostomy and no ileal resection. Eight participants (16%, 95% CI, 8–30) had previously had a pouch, and 9 participants (18%, 95% CI, 10–32) had ileal resection without previously having a pouch.
Regular medications and comorbidities were extracted from the patients’ electronic medical records. Sixteen percent (95% CI, 8–30) of the participants used antimotility medication, and 20% (95% CI, 11–34) used antisecretory medication. There were no statistically significant differences in antimotility (p = .225) or antisecretory (p = .444) medications between patients with complete small bowel and those with a previous pouch or ileal resections. Four percent (95% CI, 1–15) used 5-aminosalicylic acid and 6% (95% CI, 2–18) used prednisolone. No participants used biological drugs or GLP-analogues. Twelve percent (95% CI, 6–25) had hypertension, and 12% (95% CI, 6–25) were diagnosed with osteopenia or osteoporosis.
Biochemistry
The participants had a median P-sodium of 140 mmol/L (IQR 139–142), and two patients (4%, 95% CI, 1–15) had hyponatraemia. Median P-aldosterone was 734 pmol/L (IQR 305–1862), and 26% (95% CI, 16–41) had levels above the reference value. The median venous total CO2 was 25 mmol/L (IQR 23–27), and 20% (95% CI, 11–34) had CO2 levels below the reference range. The participants had a median estimated glomerulus filtration rate (eGFR) of 81 ml/min/1.73m2 (IQR 68–90), a median P-creatinine of 78 µmol/L (IQR 67–88), and six participants (12%, 95% CI, 6–25) had levels above the creatinine reference range. The mean P-potassium was 3.96 mmol/L (±0.22). One patient’s result was below the reference value (2%, 95% CI, 0.3–14), and no patients had P-potassium levels above the reference range. The median P-albumin was 37 g/L (IQR 36–39), 16% (95% CI, 8–30) had albumin levels below reference values, and no patients had albumin levels above reference values. Mean B-haemoglobin was 8.4 mmol/L (± 0.7) for women and 9.1 mmol/L (± 0.7) for men. Three participants (6% (95% CI 2–18) had levels below reference values, and one patient (2% (95% CI, 0.3–14) had high levels of haemoglobin.
Urinary sodium
Twenty-two participants (45%, 95% CI, 31–59) were sodium-depleted, demonstrated by unmeasurably low (0–20 mmol/L) urinary sodium excretion ().
Figure 1. Urinary sodium excretion in 49 patients with an ileostomy, measured in a morning or midday spot urine sample. Circles indicate patients with end-ileostomy and complete small bowel, squares indicate patients with end-ileostomy after a previous pouch, and triangles indicate patients with end-ileostomy and ileal resections. Open marks indicate U-Na values ≤ 20 mmol/L, and closed marks indicate U-Na values >20 mmol/L. U-Na: urinary sodium.
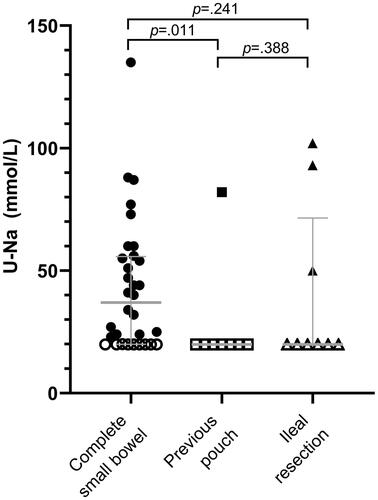
History of having a pouch or small bowel resections was statistically significantly associated with absent urinary sodium excretion (p = .016 and p = .002, respectively) (). Twenty-eight percent (95% CI, 15–47) of patients with an end-ileostomy and a complete small bowel remaining had unmeasurably low urinary sodium excretion, while the percentage for patients with ileal resections was 77% (95% CI, 49–92).
Table 2. Categorisation of patients with and without measurable natriuresis.
Patients with absent U-Na had 2.1 mmol/L lower P-sodium than patients with U-Na >20 mmol/L (p < .001), and patients with abnormal high P-aldosterone had 1.9 mmol/L lower P-sodium than patients with normal P-aldosterone (p = .006), although only one patient with unmeasurably low U-Na had abnormally low P-sodium. The positive predictive value for P-sodium detecting urinary sodium excretion <20 mmol/L was 50%, and the negative predictive value was 55%.
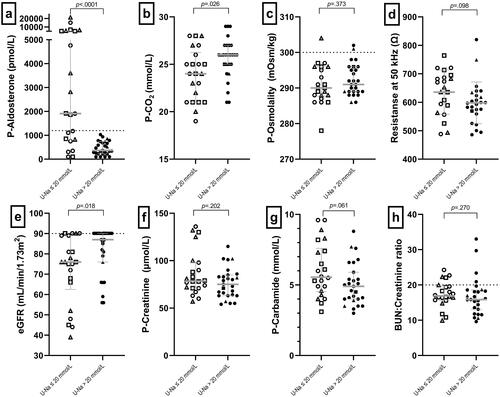
All participants with P-aldosterone above the reference range had U-Na <20 mmol/L. Patients with unmeasurably low urinary sodium excretion had a 1558 pmol/L higher median P-aldosterone than those with urinary sodium above 20 mmol/L (p < .001), indicative of secondary hyperaldosteronism (). The risk of having secondary hyperaldosteronism was statistically significantly higher for patients with ileal resection than for patients with a true end-ileostomy (p < .001). Patients with U-Na ≤20 mmol/L had median 2 mmol/L lower total CO2 (p = .026), indicating chronic metabolic acidosis (). Patients with low urinary sodium had a median eGFR of 76 mL/min/1.73m2 compared with a median of 86 mL/min/1.73m2 in those with U-Na >20 mmol/L (p = .018), indicating that low urinary sodium excretion may predispose to reduced kidney function (). We observed no statistically significant differences in P-creatinine levels between the two subgroups (median difference 3.5 µmol/L, p = .202) (), P-carbamide levels (median difference 0.65 mmol/L, p = .063) (), or BUN:creatinine ratio (median difference 1.8, p = .063) (). Likewise, there were no statistically significant differences in P-osmolality (1.3 mOsm/kg, 95% CI, −1.6–4.1, p = .373) (), or in resistance measured by BIA (38 Ω, 95% CI, −83–8, p = .098) (). There was no significant difference between FFMI in those with low U-Na and high U-Na (p = .315) or eGFR between high or low FFMI (p = .201) (Supplementary Figure S2).
Figure 2. Comparison of paraclinical variables in patients with and without measurable natriuresis. Dotted lines indicate reference values. Circles indicate patients with end-ileostomy and complete small bowel, squares indicate patients with end-ileostomy after a previous pouch, and triangles indicate patients with end-ileostomy and ileal resections. Open marks indicate U-Na values ≤20 mmol/L, and closed marks indicate U-Na values >20 mmol/L. CO2: carbon dioxide; eGFR: estimated glomerulus filtration rate; BUN: blood urea nitrogen; P: plasma; U-Na: urinary sodium.
Assessment of hydration status
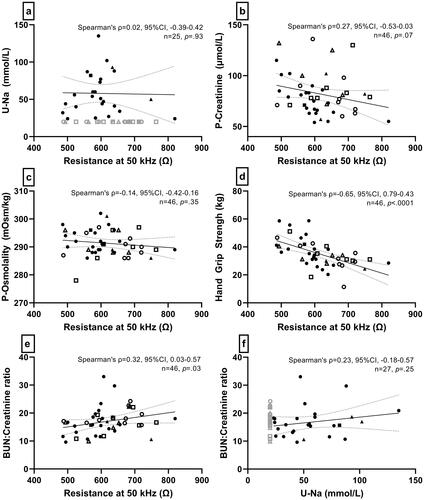
Whole-body resistance measured by BIA did not correlate with urinary sodium (Spearman’s Rho= −0.02, p = .923) () or with P-aldosterone (Spearman’s Rho= −0.03, p = .834). Likewise, it did not correlate with P-creatinine (Spearman’s Rho= −0.27, p = .070) () or P-osmolality (Spearman’s Rho= −0.14, p = .347) (). In contrast, we found a moderate, inverse correlation between resistance and HGS (Spearman’s Rho= −0.65, p < .001) (). After adjusting for potential confounders (sex, age and BMI), the model suggested that HGS decreases 4.5 kg for every 100 Ω (95% CI, −8.8– −0.3 kg, p = .038), indicating that decreasing muscle function is associated with decreasing fluid status assessed with BIA. There was a low, positive correlation between resistance and BUN:creatinine-ratio (Spearman’s Rho = 0.33, p = .027) (). There was no correlation between urine sodium and BUN:creatinine-ratio (Spearman’s Rho= −0.23, p = .251) (). All participants had Z-scores above sex-stratified reference means from the literature, and 34.8% were above 1.96 SD (Supplementary Figure S3), indicating that all patients with an ileostomy had lower fluid status than 50% of the reference population, and 34.8% had a lower fluid status than 97.5% of the reference population.
Figure 3. Correlations between different dehydration assessment methods. Resistance is an estimate of hydration (TBW) measured with bioelectrical impedance analysis. Grey marks are not included in the analysis. Circles indicate patients with end-ileostomy and complete small bowel, squares indicate patients with end-ileostomy after a previous pouch, and triangles indicate patients with end-ileostomy and ileal resections. Open marks indicate u-Na values ≤20 mmol/L, and closed marks indicate u-Na values >20 mmol/L. BUN: blood urea nitrogen; P: plasma; U-Na: urinary sodium; TBW: total body water.
Dietary intake
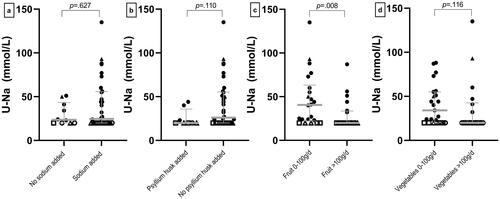
Thirty-two patients (76%, 95% CI, 60–87) reported that they added extra sodium to their diet, which was not associated with higher urinary sodium excretion (p = .622) (). Nineteen percent (95% CI, 10–34) added psyllium husks to their diet (), and 44% (95% CI, 29–60) ate more than 100 g vegetables every day () also without association to U-Na (p = .110 and p = .116, respectively). Forty-eight percent (95% CI, 33–63) ate more than 100 g of fruit every day, and this was statistically significantly associated with low U-Na (p = .008) ().
Figure 4. Dietary intake and association with urinary sodium. Comparison of patients with ileostomy who (a) added table salt to their diet or not, (b) ingested psyllium husks or not, (c) had an intake of fruit under or above 100 g/d and (d) had an intake of vegetables under or above 100 g/d. Circles indicate patients with end-ileostomy and complete small bowel, squares indicate patients with end-ileostomy after a previous pouch, and triangles indicate patients with end-ileostomy and ileal resections. Open marks indicate u-Na values ≤ 20 mmol/L, and closed marks indicate U-Na values >20 mmol/L. U-Na: urinary sodium excretion.
Nineteen percent (95% CI, 10–34) of the participants estimated that their daily fluid intake was 1–1.5 L/d, 33% (95% CI, 20–49) estimated that it was 1.5–2 L/d, and 21% (95% CI, 11–37) estimated that it was 2–2.5 L/d. The highest fluid intake was seen in patients with a previous pouch and ileal resection (p = .024). Fifty percent (95%CI, 11–89) of patients with a previous pouch had an intake above 2 L/d, and 88% (95%CI, 36–99) patients with ileal resection had an intake above 2 L/d. Most fluid intakes consisted of water, followed by coffee or tea, and non-diet soft drinks like juice and soda (Supplementary Figure S4). Eighty-three percent (95% CI, 68–92) never used oral rehydration solutions, and 17% (95% CI, 8–32) ingested 0–1.5 L/d. Too few data were available to assess the association between intake of ORS and U-Na.
Comparison with the survey cohort
The patients who agreed to participate in the present study were comparable with those in the QoL-survey cohort [Citation12] from which the study participants were recruited, with no statistically significant differences in sex (p = .427), age (p = .908), QoL (p = .093), or depressive symptoms (p = .504) between the two groups, indicating a representative sample on these parameters.
Discussion
In this cross-sectional study of stable outpatients with an ileostomy, we found that almost half of the participants had no measurable urine sodium excretion. A fourth had secondary hyperaldosteronism, indicative of severe chronic sodium depletion. Patients with unmeasurably low urinary sodium had lower eGFR and venous CO2, indicating reduced kidney function and metabolic acidosis. Other markers, such as P-sodium, creatinine, carbamide, albumin and haemoglobin did not help identify the sodium-depleted patients. Small bowel resection, including previously having had an ileo-anal pouch, strongly predicted low urinary sodium excretion and secondary hyperaldosteronism. Still, a fourth of patients with a true end-ileostomy without small bowel resection were also sodium-depleted.
Our findings align with previous studies. Low natriuresis [Citation1–3,Citation15] and high aldosterone levels in patients with an ileostomy have been frequently demonstrated [Citation5,Citation16–18], but no previous study has assessed the prevalences in a population-based group of patients with an ileostomy. Sodium absorption is highly correlated with remaining small bowel length [Citation19], and extensive ileal or jejunal resection increase the risk of chronic sodium depletion. Aldosterone is a steroid hormone acting as a mineralocorticoid to regulate renal sodium reabsorption [Citation20]. Mineralocorticoid adrenal activity can increase tenfold following the formation of an ileostomy, and high aldosterone levels indicate long-term sodium depletion [Citation21]. Patients with an ileostomy usually have normal P-sodium levels, possibly attributed to an adaption to their condition with intracellular sodium and potassium depletion [Citation22]. It may also result from haemoconcentration, secondary to a reduced plasma volume, which could explain high values of albumin and haemoglobin as well. It is therefore valuable for diagnosis and treatment monitoring to look at urinary sodium excretion in this patient group. The patients may experience fatigue, cramps, headache and dizziness along with abnormal urine or blood samples, such that using a combination of patient-reported outcomes and paraclinical variables is probably the best way to identify those who could benefit from treatment. Albeit rarely, patients may complain of fatigue and light-headedness and have hyperkalaemia, metabolic acidosis and high aldosterone levels, which may be due to pseudohypoaldosteronism type 3 caused by mineralocorticoid resistance [Citation23–25].
Calculations of eGFR solely based on P-creatinine, sex and age are inaccurate. Both dietary intake and muscle mass influence P-creatinine, and eGFR calculated with P-creatinine may be overestimated in patients with low muscle mass [Citation26]. Precision and accuracy can be improved by combining the regular (CDK-EPI) formula with measurements of cystatin-C [Citation27]. We found no statistically significant differences in eGFR due to muscle mass indicated by BIA-measured FFMI, but we cannot exclude the risk of biased eGFR. Clinical interpretations of eGFR based on P-creatinine should include an assessment of the patient’s nutrition status and body composition. Reduced urinary sodium secretion may be an early warning sign for reduced kidney function in patients with an ileostomy, and future studies should investigate the association between urinary sodium excretion and kidney function.
We found no collinearity between any of the dehydration assessment methods. If a patient seemed dehydrated using one method, they were not likely to score as dehydrated with another method. Using BIA, we focussed on whole-body resistance measured at 50 kHz as a surrogate measure of TBW to avoid prediction equations that are based on several assumptions, e.g., hydration status, which can be compromised during disease. All patients in this study had whole-body resistances below sex-stratified reference means prepared in a German population [Citation28]. This could either indicate that the patients generally had a low fluid status or that this German reference material was incomparable with our patient group. There was a low correlation between resistance measured with BIA and the BUN:creatinine-ratio and a moderate correlation between resistance and HGS. The latter indicates that dehydration reduces arm muscle function. Resistance did not correlate with urinary sodium secretion, which is in keeping with previous findings demonstrating no correlation between urinary sodium and total body water, even though total exchangeable sodium is highly correlated with body water/kg body weight [Citation2]. Low sodium status may change the resistivity of body fluids measured with BIA, which can affect the resistance values and complicate the interpretation of the results. Some BIA devices can estimate intracellular fluid, which may correlate better with sodium status if the patients are indeed intracellularly depleted. Also, segmental measurements may better monitor fluid status in these patients because the torso can skew the picture due to a higher proportion of fluid here than in the extremities. Further studies should determine if multi-frequency segmental BIA raw parameters are better at monitoring hydration status in patients with an ileostomy and assess which reference values are best suited to evaluate these patients.
Having an ileostomy independently predicts acute kidney injury and chronic kidney disease, and the decreased kidney function may not return to baseline following hospital discharge [Citation29]. This highlights the importance of identifying and treating the subgroup of patients with an ileostomy who develop sodium depletion. Some patients are told to add extra sodium to their diet in order to improve their sodium balance, but the current study, as well as a previous investigation [Citation30], indicate that this recommendation is insufficient to maintain sodium balance for some patients with an ileostomy. Rather, the sodium balance is affected by the types of oral fluids they ingest [Citation31–33]. Treatment with ORS may normalise both urinary sodium and P-aldosterone levels [Citation31,Citation34,Citation35], and postoperative treatment with ORS can decrease the risk of dehydration and sodium depletion [Citation36]. In the current study, less than 20% of the patients reported regular use of ORS. Patients should be informed about the possibility of drinking ORS following surgery, although the taste is not always tolerated, and there is a need for studies demonstrating long-term compliance and efficacy. Antimotility medication can decrease intestinal fluid and sodium losses and is recommended as part of the treatment of high stomal output [Citation37]. Surprisingly, only 16% of the patients in this present study are prescribed antimotility medication, which may indicate a general undertreatment of outpatients with an ileostomy.
Our study has limitations. We could not assess 24-h urine excretions. Pragmatically, we used a single urine sodium measurement to estimate natriuresis, and although this measure does not reflect sodium status in people without an ileostomy, it is a fair approximation in patients with an ileostomy [Citation30]. The device used for analyses of urinary sodium was not validated to measure values below 20 mmol/L, which makes the low values less informative. We did not measure the participants’ blood pressure or bone density, but we had access to their medical history and regular medications, thus allowing us to infer which patients had hypertension or osteopenia/-porosis. We cannot rule out selection bias which could arise if patients with high stomal output and repeated episodes of dehydration were more likely to participate. If this was the case, though, we would expect that the participants’ reported regular medication use would be higher. We therefore assume that the subgroup in this present study is representative of patients with an ileostomy in the Central Denmark Region.
In conclusion, nearly half of all patients with an ileostomy were sodium-depleted and a fourth had developed secondary hyperaldosteronism. The risk was highest in those with ileal resection, but patients with an end-ileostomy and complete small bowel could also be sodium-depleted. Plans for postoperative follow-up should be implemented for this patient group and include assessment of urinary sodium excretion, P-aldosterone, GFR and P-total CO2. It is challenging to assess fluid status clinically. Although urinary sodium excretion is a valuable indicator of chronic sodium depletion in patients with an ileostomy, it is probably not enough to identify dehydration. Future studies should identify valid hydration assessment methods and interventions to improve sodium absorption in patients with an ileostomy.
Author contributions
CLR, CLH and TLW designed the study. CLR acquired and analysed the data, and all authors contributed to the interpretation of the data. CLR wrote the first manuscript draft. All authors revised it critically for important intellectual content and contributed to manuscript revision. All authors approved the final version for submission.
| Abbreviations | ||
| BIA | = | bioelectrical impedance analysis |
| B | = | blood |
| BUN | = | blood urea nitrogen |
| BMI | = | body mass index |
| CI | = | confidence interval |
| CO2 | = | carbon dioxide |
| ECW | = | extracellular water |
| eGFR | = | estimated glomerulus filtration rate |
| FFMI | = | fat-free mass index |
| HGS | = | hand grip strength |
| IBD | = | inflammatory bowel disease |
| kHz | = | kilohertz |
| ORS | = | oral rehydration solution |
| P | = | plasma |
| QoL | = | quality of life |
| R | = | resistance |
| TBW | = | total body water |
| U-Na | = | urinary sodium |
Supplemental Material
Download PDF (339.8 KB)Acknowledgements
We thank Frederik Kraglund for statistical assistance and the Department of Clinical Biochemistry at Aarhus University Hospital for analyses of urine and blood samples. We want to express gratitude to the Stoma clinic at the Department of Surgery at Aarhus University Hospital. We are also grateful for the participants in our studies and hope that the results may help improve the treatment of patients with an ileostomy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Ng DHL, Pither CAR, Wootton SA, et al. The “not so short-bowel syndrome”: potential health problems in patients with an ileostomy. Color Dis. 2013;15:1154–1161.
- Clarke AM, Chirnside A, Hill GL, et al. Chronic dehydration and sodium depletion in patients with established ileostomies. Lancet. 1967;2(7519):740–743.
- Gallagher N, Harrison D, Skyring A. Fluid and electrolyte disturbances in patients with long-established ileostomies. Gut. 1962;3(3):219–223.
- Carmichael D, Few J, Peart S, et al. Sodium and water depletion in ileostomy patients. Lancet. 1986;2(8507):625–626.
- Ladefoged K, Olgaard K. Sodium homeostasis after small-bowel resection. Scand J Gastroenterol. 1985;20(3):361–369.
- Delin K, Aurell M. Sodium and water depletion in ileostomy patients. Lancet. 1986;328(8512):924.
- Cheuvront SN, Kenefick RW, Charkoudian N, et al. Physiologic basis for understanding quantitative dehydration assessment. Am J Clin Nutr. 2013;97(3):455–462.
- Lacey J, Corbett J, Forni L, et al. A multidisciplinary consensus on dehydration: definitions, diagnostic methods and clinical implications. Ann Med. 2019;51(3-4):232–251.
- Volkert D, Beck AM, Cederholm T, et al. ESPEN practical guideline: clinical nutrition and hydration in geriatrics. Clin Nutr. 2022;41(4):958–989.
- Armstrong LE. Assessing hydration status: the elusive gold standard. J Am Coll Nutr. 2007;26(5 Suppl):575S–584S.
- Armstrong LE, Kavouras SA, Walsh NP, et al. Diagnosing dehydration? Blend evidence with clinical observations. Curr Opin Clin Nutr Metab Care. 2016;19(6):434–438.
- Rud CL, Baunwall SMD, Bager P, et al. Patient-reported outcomes and health-related quality of life in people living with ileostomies: a population-based, cross-sectional study. Dis Colon Rectum. 2022;65(8):1042–1051.
- Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)-a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- Hill GL, Mair WSJ, Goligher JC. Cause and management of high volume output salt-depleting ileostomy. Br J Surg. 1975;62(9):720–726.
- Ladefoged K, Ølgaard K. Fluid and electrolyte absorption and renin-angiotensin-aldosterone axis in patients with severe short-bowel syndrome. Scand J Gastroenterol. 1979;14(6):729–735.
- Kennedy HJ, Al-Dujaili EA, Edwards CR, et al. Water and electrolyte balance in subjects with a permanent ileostomy. Gut. 1983;24(8):702–705.
- Delin K, Fasth S, Andersson H, et al. Factors regulating sodium balance in proctocolectomized patients with various ileal resections. Scand J Gastroenterol. 1984;19(2):145–149.
- Nightingale JMD, Lennard-Jones JE, Walker ER, et al. Jejunal efflux in short bowel syndrome. Lancet. 1990;336(8718):765–768.
- Booth RE, Johnson JP, Stockand JD. Aldosterone. Adv Physiol Educ. 2002;26(1-4):8–20.
- Huber FX, Stern J, Hinz U, et al. Effects of restorative proctocolectomy on renal and adrenal function. Dis Colon Rectum. 1999;42(10):1318–1324.
- Turnberg LA, Morris AI, Hawker PC, et al. Intracellular electrolyte depletion in patients with ileostomies. Gut. 1978;19(6):563–568.
- Riepe FG. Pseudohypoaldosteronism. In: Maghnie M, Loche S, Cappa M, editors. Hormone resistance and hypersensitivity: from genetics to clinical management. Endocr dev. Basel: Karger; 2013. p. 86–95.
- Vantyghem MC, Hober C, Evrard A, et al. Transient pseudo-hypoaldosteronism following resection of the ileum: normal level of lymphocytic aldosterone receptors outside the acute phase. J Endocrinol Invest. 1999;22(2):122–127.
- Niyazov D, Shawa H. A case of postileostomy hypovolemia presenting as pseudohypoaldosteronism with complete resolution after ostomy reversal. AACE Clin Case Reports. 2017;3(1):e5–e7.
- Nankivell BJ, Nankivell LFJ, Elder GJ, et al. How unmeasured muscle mass affects estimated GFR and diagnostic inaccuracy. EClinicalMedicine. 2020;29-30:100662.
- Trans JG, Krogstrup NV, Oltean M, et al. A comparison of four established GFR formulas to estimate measured GFR and changes in GFR in adult kidney transplant recipients. Scand J Clin Lab Invest. 2022;82(4):296–303.
- Bosy Westphal A, Danielzik S, Dörhöfer RP, et al. Phase angle from bioelectrical impedance analysis: population reference values by age, sex, and body mass index. JPEN J Parenter Enteral Nutr. 2006;30(4):309–316.
- Li L, Lau KS, Ramanathan V, et al. Ileostomy creation in colorectal cancer surgery: risk of acute kidney injury and chronic kidney disease. J Surg Res. 2017;210:204–212.
- Pedersen AKN, Rud C, Wilkens TL, et al. A single urine sodium measurement may validly estimate 24-hour urine sodium excretion in patients with an ileostomy. JPEN J Parenter Enteral Nutr. 2020;44(2):246–255.
- Newton CR, Gonvers JJ, McIntyre PB, et al. Effect of different drinks on fluid and electrolyte losses from a jejunostomy. J R Soc Med. 1985;78(1):27–34.
- Spiller RC, Jones BJM, Silk DBA. Jejunal water and electrolyte absorption from two proprietary enteral feeds in man: importance of sodium content. Gut. 1987;28(6):681–687.
- Rud C, Pedersen AKN, Wilkens TL, et al. An iso-osmolar oral supplement increases natriuresis and does not increase stomal output in patients with an ileostomy: a randomised, double-blinded, active comparator, crossover intervention study. Clin Nutr. 2019;38(5):2079–2086.
- Newton CR, Drury P, Gonvers JJ. Incidence and treatment of sodium depletion in ileostomists. Scand J Gastroenterol Suppl. 1982;17:159–160.
- Kudoh K, Shibata C, Funayama Y, et al. Oral rehydration solution normalizes plasma renin and aldosterone levels in patients with ulcerative colitis after proctocolectomy. J Anus Rectum Colon. 2017;1(3):78–83.
- Migdanis A, Koukoulis G, Mamaloudis I, et al. Administration of an oral hydration solution prevents electrolyte and fluid disturbances and reduces readmissions in patients with a diverting ileostomy after colorectal surgery. Dis Colon Rectum. 2018;61(7):840–846.
- Nightingale JMD. Review: how to manage a high-output stoma. Frontline Gastroenterol. 2022;13(2):140–151.
