Abstract
Background and aim
Bariatric surgery is the most effective treatment for obesity but is invasive and associated with serious complications. Endoscopic sleeve gastroplasty (ESG) is a less invasive weight loss procedure to reduce the stomach volume by full-thickness sutures. ESG has been adopted in many countries, but implementation at Scandinavian centres has not yet been documented. We performed a clinical pilot trial at a Norwegian centre with the primary objective to assess the feasibility of the ESG procedure.
Patients and methods
We included the first 10 patients treated with ESG at a Norwegian centre in a single-arm pilot study. The eligibility criteria were either a body mass index (BMI) of 40–49.9 kg/m2, BMI 35–39.9 kg/m2 and at least one obesity-related comorbidity, or BMI 30–34.9 kg/m2 and type 2 diabetes. Patient follow-up resembled the scheme used for bariatric surgery at the center, including dietary plans and outpatient visits.
Results
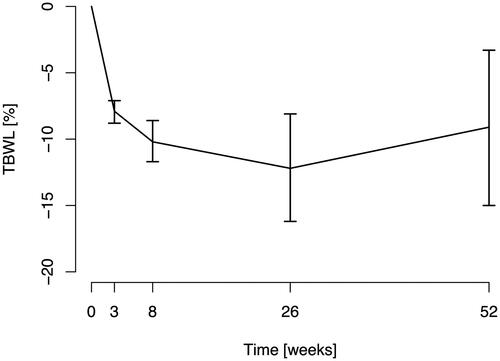
All procedures were technically successful except for one patient who had adhesions between the stomach and anterior abdominal wall, related to a prior hernia repair, resulting in less-than-intended stomach volume reduction. Mean total body weight loss (TBWL) after 26 and 52 weeks was 12.2% (95% CI 8.1–16.2) and 9.1% (95% CI 3.3 − 15.0). One patient experienced a minor suture-induced diaphragmatic injury, which was successfully managed conservatively.
Conclusions
This first Scandinavian clinical trial of ESG, documenting the implementation of the procedure at a Norwegian center, demonstrated acceptable feasibility and safety, with large variations in individual weight loss during the 52-week follow-up period.
Introduction
Bariatric surgery is the most effective and enduring treatment for obesity [Citation1–4]. With laparoscopic sleeve gastrectomy (LSG), the most used surgical weight-loss procedure today [Citation5], the stomach volume is reduced by vertical stapled resection. Patients typically lose approximately 25% of body weight, and obesity-related conditions are commonly improved or reversed [Citation2–4]. However, LSG may cause complications including gastric leakage and chronic gastroesophageal reflux disease [Citation6,Citation7].
Although 700,000 patients undergo weight-loss surgery yearly [Citation5,Citation8], there is considerable hesitancy for surgery as a treatment for weight loss. Less than 1% of patients eligible for, and with access to surgery, choose this option [Citation9,Citation10]. Less invasive and safer therapies are therefore desirable.
Endoscopic sleeve gastroplasty (ESG), first described in 2013 [Citation11], is a transoral procedure to reshape the stomach and reduce stomach volume with a suture device (OverStitch; Apollo Endosurgery, US) mounted on a gastroscope. Mechanisms for weight loss involve reduced stomach capacity, delayed gastric emptying and alterations to gut neuroendocrine and autonomic signalling [Citation12,Citation13]. Multiple clinical studies have shown total body weight loss (TBWL) at 52 weeks after the ESG procedure to range from 13 to 18% and an adverse event rate of 2–3% [Citation14–17]. The ESG procedure has been adopted in many countries as an endoscopic bariatric treatment modality, but implementation at Scandinavian centres has not yet been documented.
We performed a clinical pilot trial at a Norwegian obesity center to assess the feasibility and safety of the ESG procedure. The trial was intended to inform a planned randomized trial comparing ESG, sequential treatment with a novel intragastric balloon [Citation18], and LSG.
Patients and methods
Study design and aims
The study was a single-arm feasibility pilot study, intended to precede a randomized controlled trial. We planned to recruit ten patients from the Morbid Obesity Center at Vestfold Hospital Trust in Tønsberg, Norway. The primary study aim was to test the feasibility of the ESG procedure. The secondary aims were to evaluate study infrastructure requirements, which included registration of procedure safety and efficacy data, to prepare for a planned randomized trial. TBWL was assessed at 26 and 52 weeks after the procedure.
Patients
Patients eligible for the trial were men and women 18 years or older at the date of enrolment who were referred for treatment at the study centre and either had:
BMI 40–49.9 kg/m2;
BMI 35–39.9 kg/m2 and at least one obesity-related comorbidity; or
BMI 30–34.9 kg/m2 and type 2 diabetes with hemoglobin A1c (HbA1c) >53 mmol/mol (7%).
These inclusion criteria followed the eligibility criteria for bariatric surgery at the study center. Predefined exclusion criteria are shown in . Women of childbearing age were required to document a negative pregnancy test.
Table 1. Exclusion criteria.
Endoscopic sleeve gastroplasty procedure
Prior to the initiation of the ESG procedure, a diagnostic gastroscopy was performed. The ESG procedure was cancelled if there was evidence of significant upper GI disease: reflux esophagitis ≥ Los Angeles grade C; achalasia; hiatal hernia ≥5 cm; peptic ulcer disease; gastritis; duodenitis; gastroparesis or stenosis; or other conditions that, in the opinion of the investigator, could render the subject unsuited for the procedure. A 4-month course of pantoprazole (Somac 40 mg od) was started 3 days prior to the ESG procedure.
The ESG procedure was performed with a commercially available suture device for ESG (OverStitch, Apollo Endosurgery, US) as described previously [Citation16]. The suture device was mounted on a double channel gastroscope (GIF-2T160; Olympus Corp., Japan) and introduced orally to the stomach through an esophageal overtube (Apollo Endosurgery, US).
All ESG procedures for the trial were performed at the Intervention Centre of Oslo University Hospital, Oslo, Norway. Two senior endoscopists (CJT and LA) with a large experience in interventional endoscopy, but no prior experience with ESG, performed the procedures. Proper training was organized by a senior endoscopist from the United States who represented the device company and who also attended and supervised the local endoscopists during the first five procedures in the trial.
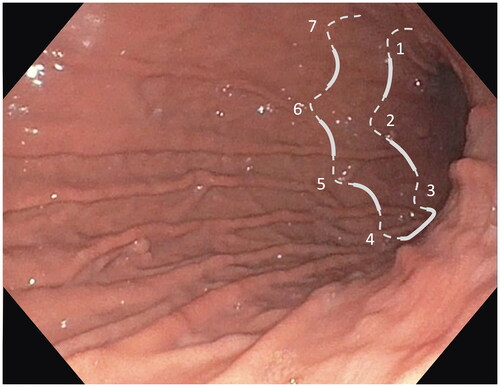
After identification of the anatomy and landmarks in the stomach markings with APC was made along the anterior and posterior walls of the stomach to guide the suture lines and ensure similar lumen reduction among patients. Starting at the level of the incisura and moving proximally, the greater curvature was pleated by sequential sutures forming a U-pattern, which was made by 6–8 full-thickness stitches with a non-absorbable material, running between the anterior and posterior stomach wall (). The stomach was thus reduced except for the fundus, which was left intact to provide a reservoir, as described previously [Citation16].
Figure 1. Endoscopic image of the stomach with an illustration of a U-pattern suture made by 6–8 full thickness stitches. The greater curve of the stomach was pleated by sequential U-pattern sutures starting at the level of the incisura and moving proximally.
The procedure was performed with general anaesthesia and endotracheal intubation. Compression stockings were used for the prevention of venous thromboembolism. Antibiotic prophylaxis was provided with one perioperative dose of cefalotin 2 g iv. All participants were scheduled for hospital admission for 1 day following the procedure. A 3-day course of aprepitant (Emend 125 mg day 1 and 80 mg day 2 and 3) and a 5-day course of ondansetron (Zofran 8 mg TID) was prescribed to reduce post-procedure nausea and vomiting. Participants were instructed to follow a liquid diet for 2 weeks after the procedure, before progressing to pureed food, and to gradually introduce solid food after 4 weeks [Citation19].
Lifestyle intervention and follow-up
All participants received a standardized 1200 kcal/day diet plan prepared by a nutritionist [Citation20]. Follow-up study visits were scheduled at 3, 8, 26 and 52 weeks after the ESG procedure. All visits included lifestyle guidance and clinical examination with anthropometric measures, blood pressure, laboratory analyses, medication registration, and assessment of adverse events.
Ascertainment of outcome measures
Feasibility and safety
Procedure feasibility was assessed by the investigators as the technical success of endoscopic suturing and imbrication of the stomach wall to achieve the intended gastroplasty configuration. Adverse event assessment was recorded during hospitalization and follow-up visits according to the Clavien-Dindo grading system. Serious adverse events were defined as Clavien-Dindo grade 3b or higher [Citation21,Citation22].
Anthropometric measures
Body weight was measured with patients wearing light clothing and no shoes. Height was measured using wall-mounted stadiometers. Height was measured to the nearest 0.5 cm and weight to the nearest 0.1 kg.
Ethics and approvals
The study was approved by the Regional Committees for Medical and Health Research Ethics of South-East Norway (2018/2566) and the institutional Data Protection Office. All patients provided written informed consent before enrolment. The OverStitch suture device is CE-approved.
Statistical analyses
We analyzed the predefined clinical endpoints for each patient. We used descriptive statistics and calculated means, standard deviations (SD) and 95% confidence intervals (CI) where applicable.
Results
Between June and September 2019, eleven participants were deemed eligible initially and provided informed consent to participate. One patient was diagnosed with Barrett’s esophagus at the gastroscopy performed prior to the ESG procedure and therefore excluded from the study. Thus, 10 patients underwent the procedure and are included in our analyses. Patient characteristics at enrolment are shown in .
Table 2. Patient characteristics at baseline.
Feasibility
All ten procedures were successful in performing suturing as intended, except for one patient (patient no. 9, ) who had adhesions between the stomach and anterior abdominal wall. The adhesions were probably caused by a prior laparoscopic umbilical hernia repair and resulted in a smaller-than-intended reduction of the gastric volume. The mean procedure time was 133 min (SD 30). No adverse events occurred during the procedures.
Table 3. TBWL is compared to weight at baseline.
Weight loss
The BMI and TBWL results for each patient are presented in . The mean total body weight loss (TBWL) 26 weeks after the ESG procedure was 12.2% (95% CI 8.1–16.2) and 15.5 kg (95% CI 9.4–21.6), respectively. 52 weeks after the procedure, the mean TBWL was 9.1% (95% CI 3.3–15.0) and 11.7 kg (95% CI 4.1–19.3), respectively (). There were large differences in weight loss between individual patients, with a TBWL ranging from 0.9% weight gained to 27.4% weight lost at week 52, .
Adverse events
There were no serious adverse events. Three patients were admitted for one additional day following the procedure. Two patients complained of nausea which was relieved with iv. antiemetics and fluids. The third patient (patient no. 8, ) complained of left shoulder pain which was aggravated by respiration. A CT scan demonstrated small air bubbles adjacent to the gastric fundus and in the supradiaphragmatic extrapleural space, consistent with a minor diaphragmatic injury. There were no signs of gastric leakage or pneumothorax. The patient was pain relieved with NSAIDs and discharged the next day with a course of peroral ciprofloxacin. The pain recurred after 4 weeks, and a new CT scan was performed which showed resorption of the air and was otherwise inconspicuous. The pain subsided a few days later, and the subsequent course was uneventful.
Discussion
This first Scandinavian clinical trial of the ESG procedure, documenting the initial experience at implementation at a Norwegian center, demonstrated acceptable feasibility and safety, but varying weight loss during the 52-week follow-up period.
The study includes the very first ESG procedures performed by two endoscopists which are reflected by relatively long procedure times. A learning curve effect on procedure time has been reported by others [Citation23], and considerably shorter procedure times are expected after a break-in period of approximately five procedures. The ESG procedure is complex and challenging. Prior experience with other interventional endoscopic procedures is an advantage and should be a prerequisite for implementing the ESG procedure, in our opinion.
We observed that the stomach was adherent to the anterior abdominal wall in one case (patient no. 9), leading to less gastric volume reduction, which was probably caused by adhesions from a prior laparoscopic umbilical hernia repair with mesh implantation. The patient did not experience significant weight loss, with a TBWL of 0.3% at 52 weeks, and was referred to laparoscopic Roux-en-Y gastric bypass surgery after the study was concluded. During surgery, adhesions between the stomach and anterior abdominal wall were dissected. The operation was otherwise uncomplicated, and the postoperative course was uneventful. It is important to recognize that umbilical hernia repairs, even when performed laparoscopically, can cause adhesions [Citation24,Citation25] and limit the effectiveness of ESG.
This study was not designed to evaluate procedure efficacy on weight loss. However, our data indicate a lower TBWL than expected. The only randomized controlled trial of ESG including 209 participants, showed a mean TBWL of 13.6% after 52 weeks compared to 0.8% in the control group who only received lifestyle intervention therapy. In our study, the mean TBWL was 9.1% after 52 weeks. Three patients (30%) had a TBWL of less than 5% at week 52, which is considered clinically insignificant [Citation26]. The mean TBWL in this study decreased by 25% from weeks 26 to 52. A large study demonstrated TBWL to increase by 9% during the same period [Citation15].
There are multiple possible reasons for the varying and tapering efficacy we observed. A study of 133 patients showed that less than 50% of patients had an intact gastroplasty on gastroscopy 6 months after ESG, and less than 25% of patients had an intact gastroplasty after 12 months [Citation27]. We did not prepare the mucosa with diathermy or APC to promote adaption of the stomach walls and thereby reduce dehiscence which has been proposed to improve weight loss [Citation28,Citation29]. Another reason could be the follow-up schedule frequency, which was designed to resemble a clinical real-world setting similar to the schedule used for LSG at the participating center. Consequently, the patients in this study received less frequent follow-up consultations compared to patients in other studies [Citation17], which have been demonstrated to induce less weight loss [Citation30].
In conclusion, this first Scandinavian clinical trial of the ESG procedure, including the first cases performed from implementation at our clinic, proved acceptable feasibility and safety, but with large variations in individual weight loss, and decreasing TBWL from week 26 to 52. Based on our experience and evidence indicating poor gastroplasty longevity [Citation27], we decided not to proceed from this pilot study to the intended randomized controlled trial comparing ESG to gastric balloon therapy and laparoscopic sleeve gastrectomy.
Disclosure statement
The suture devices utilized for this study were granted by Apollo Endosurgery (Austin, Texas, USA).
Additional information
Funding
References
- Flum DR, Khan TV, Dellinger EP. Toward the rational and equitable use of bariatric surgery. JAMA. 2007;298(12):1442–1444.
- Jakobsen G, Småstuen M, Sandbu R, et al. Association of bariatric surgery vs medical obesity treatment with long-term medical complications and obesity-related comorbidities. JAMA. 2018;319(3):291–301.
- Nguyen NT, Varela JE. Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol. 2017;14(3):160–169.
- Schauer PR, Bhatt DL, Kirwan JP, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-Year outcomes. N Engl J Med. 2017;376(7):641–651.
- Angrisani L, Santonicola A, Iovino P, et al. IFSO worldwide survey 2016: primary, endoluminal, and revisional procedures. Obes Surg. 2018;28(12):3783–3794.
- Thaher O, Croner RS, Driouch J, et al. Reflux disease following primary sleeve gastrectomy: risk factors and possible causes. Updates Surg. 2023. Online ahead of print. DOI:10.1007/s13304-023-01477-9
- Jung JJ, Jackson T, Gordon L, et al. Intraoperative leak test is associated with lower postoperative bleed rate in primary sleeve gastrectomy: a propensity matched analysis of primary and revision bariatric surgery using the MBSAQIP database. Surg Endosc. 2022;36(1):753–763.
- Angrisani L, Santonicola A, Iovino P, et al. Bariatric surgery survey 2018: similarities and disparities Among the 5 IFSO chapters. Obes Surg. 2021;31(5):1937–1948.
- Bhogal SK, Reddigan JI, Rotstein OD, et al. Inequity to the utilization of bariatric surgery: a systematic review and meta-analysis. Obes Surg. 2015;25(5):888–899.
- Welbourn R, Le Roux CW, Owen-Smith A, et al. Why the NHS should do more bariatric surgery; how much should we do? BMJ. 2016;353:i1472.
- Abu Dayyeh BK, Rajan E, Gostout CJ. Endoscopic sleeve gastroplasty: a potential endoscopic alternative to surgical sleeve gastrectomy for treatment of obesity. Gastrointest Endosc. 2013;78(3):530–535.
- Abu Dayyeh BK, Acosta A, Camilleri M, et al. Endoscopic sleeve gastroplasty alters gastric physiology and induces loss of body weight in obese individuals. Clin Gastroenterol Hepatol. 2017;15(1):37–43.e1.
- Vargas EJ, Rizk M, Gomez-Villa J, et al. Effect of endoscopic sleeve gastroplasty on gastric emptying, motility and hormones: a comparative prospective study. Gut. 2022. Online ahead of print. DOI:10.1136/gutjnl-2022-327816
- Lopez-Nava G, Galvao MP, Bautista-Castano I, et al. Endoscopic sleeve gastroplasty FOR obesity treatment: two years OF experience. Arq Bras Cir Dig. 2017;30(1):18–20.
- Alqahtani A, Al-Darwish A, Mahmoud AE, et al. Short-term outcomes of endoscopic sleeve gastroplasty in 1000 consecutive patients. Gastrointest Endosc. 2018;89(6):1132–1138. DOI:10.1016/j.gie.2018.12.012
- Sartoretto A, Sui Z, Hill C, et al. Endoscopic sleeve gastroplasty (ESG) Is a reproducible and effective endoscopic bariatric therapy suitable for widespread clinical adoption: a large, international multicenter study. Obes Surg. 2018;28(7):1812–1821.
- Abu Dayyeh BK, Bazerbachi F, Vargas EJ, et al. Endoscopic sleeve gastroplasty for treatment of class 1 and 2 obesity (MERIT): a prospective, multicentre, randomised trial. Lancet. 2022;400(10350):441–451.
- Tønnesen CJ, Hjelmesæth J, Hofsø D, et al. A novel intragastric balloon for treatment of obesity and type 2 diabetes. A two-center pilot trial. Scand J Gastroenterol. 2022;57(2):232–238.
- Lopez-Nava G, Sharaiha RZ, Vargas EJ, et al. Endoscopic sleeve gastroplasty for obesity: a multicenter study of 248 patients with 24 months follow-up. Obes Surg. 2017;27(10):2649–2655.
- Barstad LH, Johnson LK, Borgeraas H, et al. Changes in dietary intake, food tolerance, hedonic hunger, binge eating problems, and gastrointestinal symptoms after sleeve gastrectomy compared with after gastric bypass; 1-year results from the Oseberg study-a randomized controlled trial. Am J Clin Nutr. 2022;117(3):586–598. DOI:10.1016/j.ajcnut.2022.11.016
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213.
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196.
- Hill C, El Zein M, Agnihotri A, et al. Endoscopic sleeve gastroplasty: the learning curve. Endosc Int Open. 2017;5(9):E900–e4.
- Bieber EJ, Levrant S. The risk of anterior abdominal wall adhesions in patients with previous umbilical hernia repair. J Am Assoc Gynecol Laparosc. 1994;1(4, Part 2):S4.
- Ngo P, Cossa JP, Largenton C, et al. Ventral hernia repair by totally extraperitoneal approach (VTEP): technique description and feasibility study. Surg Endosc. 2021;35(3):1370–1377.
- Stevens J, Truesdale KP, McClain JE, et al. The definition of weight maintenance. Int J Obes (Lond). 2006;30(3):391–399.
- Pizzicannella M, Lapergola A, Fiorillo C, et al. Does endoscopic sleeve gastroplasty stand the test of time? Objective assessment of endoscopic ESG appearance and its relation to weight loss in a large group of consecutive patients. Surg Endosc. 2020;34(8):3696–3705.
- Razzak FA, Mahmoud T, Ghazi R, et al. Argon plasma coagulation prior to endoscopic sleeve gastroplasty for weight loss. VideoGIE. 2022;7(12):445–447.
- Itani MI, Farha J, Sartoretto A, et al. Endoscopic sleeve gastroplasty with argon plasma coagulation: a novel technique. J Dig Dis. 2020;21(11):664–667.
- Lopez-Nava G, Asokkumar R, Rull A, et al. Bariatric endoscopy procedure type or follow-up: what predicted success at 1 year in 962 obese patients? Endosc Int Open. 2019;7(12):E1691–e8.