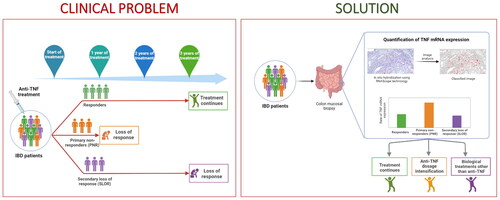
Abstract
Background and aims
Tumour necrosis factor-α (TNF) antagonists have improved the management of inflammatory bowel disease (IBD), however, their usage and administration persist to be suboptimal. Here, we examined the relationship between tissue-specific TNF mRNA expression in mucosal biopsies from IBD patients and anti-TNF treatment response.
Methods
Archived tissue samples from patients with luminal IBD that had all been or were in treatment with anti-TNF were included (18 adults and 24 paediatric patients). Patients were stratified into three groups according to anti-TNF response: responders, primary non-responders (PNR) and secondary loss of response (SLOR). TNF mRNA was detected using RNAscope in situ hybridisation (ISH) and the expression was quantified using image analysis.
Results
The ISH analysis showed varying occurrence of TNF mRNA positive cells located in lamina propria and often with increased density in lymphoid follicles (LF). Consequently, expression estimates were obtained in whole tissue areas with and without LF. Significantly higher TNF mRNA expression levels were measured in adults compared to paediatric patients in both the analyses with and without LF (p = .015 and p = .016, respectively). Considering the relation to response, the adult and paediatric patients were evaluated separately. In adults, the TNF expression estimates were higher in PNRs compared to responders with and without LF (p = .017 and p = .024, respectively).
Conclusion
Our data indicate that adult PNR have significantly higher TNF mRNA levels than responders. This suggests that higher anti-TNF dose may be considered for IBD patients with high TNF mRNA expression estimates from the start of treatment.
Graphical Abstract
Introduction
Inflammatory bowel diseases (IBD) such as Crohn’s disease (CD) and ulcerative colitis (UC) are characterised by intestinal inflammation. Even though the precise pathogenesis of IBD is uncertain, studies show that a blend of genetics, environmental factors and microbiome plays an important role in the onset of IBD [Citation1]. The interaction between intestinal wall and luminal antigens activates an abnormal acute inflammatory response driven by the innate immune system. This leads to the secretion of proinflammatory cytokines such as Tumour necrosis factor-α (TNF) and thereby results in tissue damage and activation of the adaptive immune system [Citation2]. Anti-TNF therapy has been shown to reduce symptoms, heal mucosal ulcers, decrease hospitalisations and surgeries and reduce the use of corticosteroids [Citation3]. However, 30–40% of patients do not respond to anti-TNF agents and 30–50% of initial responders lose response over time [Citation4,Citation5]. For selecting candidates for switching from ongoing conventional treatment to anti-TNF therapy, it is demanding that anti-TNF therapeutic response, drug withdrawal and relapse after anti-TNF termination is considered in the IBD patients [Citation5–9].
Predicting TNF response is challenging, mainly due to the multiple factors that can lead to primary or secondary treatment failure. TNF protein can be measured in blood and tissue, but some studies have measured and emphasised the potential of using the mucosal TNF transcript as a biomarker of disease activity [Citation6,Citation10]. Martınez-Borra et al. measured TNF in serum and found increased levels in a subset of IBD patients not responding to anti-TNF therapy, but also suggested that better understanding of the presence of TNF-secreting lamina propria cells may better identify the subgroup of patients who would benefit from anti–TNF-treatment [Citation11]. TNF mRNA is reported to be expressed at a low level in many cell types in the intestinal mucosa, such as immune cells and epithelial cells [Citation6,Citation12,Citation13]. TNF is expressed in intestinal lymphoid tissues, where it is considered to have a regulatory effect on the conservation of microarchitecture, local immune cell function and differentiation in the colorectal mucosa [Citation14]. This diversity of TNF-expressing cells in the mucosal biopsies is likely to lead to variation in the levels of TNF expression. For instance, accidental sampling of biopsies with lymphoid follicles (LF) or Peyer’s Patches with a high number of macrophages or biopsies with an increased presence of eosinophils and Paneth cells, is likely to result in relatively higher TNF or TNF mRNA levels.
Using in situ hybridisation (ISH) and image analysis, the main objective of our study was to determine if TNF mRNA levels in intestinal biopsies of paediatric and adult IBD patients could be a biomarker in predicting the clinical response to anti-TNF therapy.
Materials and methods
Patient samples
Archived formalin-fixed paraffin-embedded (FFPE) biopsies of 42 IBD patients in ongoing or previous anti-TNF treatment were retrospectively included based on response type and grouped according to sex, age and IBD subtype. All the patients included in this study received TNF treatment based on a synthesis of clinical activity, endoscopic and/or radiological evaluation. Most patients had steroid failure or steroid dependency prior to biological treatment. Samples were not obtained as part of a planned study but were all a part of routine diagnostic from the affected areas of the colon or rectum. One biopsy per patient was chosen based on the availability and as standardised as possible regarding the location of the affected tissue. As the efficiency of anti-TNF therapy depends on factors like age, sex, disease type, disease severity, dosage etc.[Citation9], our selected patient cohort included both adults and paediatric patients, CD and UC, and males and females. Patients with fistulising or penetrating disease were excluded. Biopsies were collected prior to initiation of biological treatment and all patients were naïve to biological treatment when biopsies were collected. Patients were classified into three groups: responders, primary non-responders (PNR) and secondary loss of response (SLOR). Responders received anti-TNF treatment for a minimum of three years and continued with the treatment with no signs of loss of response. PNRs had received induction treatment at zero, two and six weeks. After the induction treatment if there was no sufficient response, regulation of anti-TNF dosage or another TNF agent (adalimumab or golimumab) were prescribed. The patients in the PNR group didn’t show any or sufficient sign of clinical response in combination with blood test and foecal marker analyses. The median time of PNRs on TNF treatment were around six months. None of the PNR’s received TNF for more than one year. After treatment failure with TNF agents, PNR patients were put on other biologics such as Vedolizumab, Ustekinumab etc. SLOR group of patients received a minimum of three years of anti-TNF treatment and lost response afterward. Drug levels were estimated every six months, and if there was any sign of non-response or if the trough levels were below 1 mg/ml, supplementary tests for antidrug antibodies were performed. Loss of response was not due to antibody formation or side effects. All patients included in this study were on TNF treatment and were all followed closely in the outpatient’s clinic according to the standard procedures. All patients were followed with blood tests, calprotectin and clinical tests such as Simple clinical colitis activity index (SCCAI) (for UC and IBDU patients) and Harvey Bradshaw Index (for CD patients) for adults and abbreviated Paediatric Crohn’s Disease Activity Index (abbrPCDAI) (for CD patients) and Paediatric Ulcerative Colitis Activity Index (PUCAI) (for UC and IBDU patients) for the paediatric patients according to timelines systematically. Patients were followed at Department of Gastroenterology, Copenhagen University Hospital - Herlev and Gentofte, Denmark or the Department of Paediatric and Adolescence Medicine, Copenhagen University Hospital - Hvidovre, Hvidovre, Denmark.
Using haematoxylin and eosin-stained slides histological disease activity was assessed as per the validated Nancy index [Citation15] for UC patients. In CD patients histological disease activity was assessed using a modified version of the Global Histological Activity Scores (GHAS) [Citation16]. All CD patients had active disease defined as neutrophils in lamina propria, neutrophils in epithelium or ulcerations.
In situ hybridisation (ISH)
TNF mRNA ISH was performed using RNAscope® 2.5 HD technology (Advanced Cell Diagnostics, CA) [Citation17] in a Ventana Discovery Ultra instrument using Fast Red chromogenic substrate [Citation18,Citation19]. In brief, FFPE sections were cut at 5 µm and air dried overnight. The TNF probe (RNAscope® Probe- Hs-TNF-a, Target region: 70–1456, 20 zz pairs) was incubated on sections as per the manufacturer’s directions, using Amp-2 and Amp-3 steps both at 16 min and Amp-5 for 8 min. For a subset of cases, RNAscope probes for the housekeeping gene PPIB, and the bacterial dapB were used as positive and negative controls, respectively. Two runs with adults and paediatrics separately were conducted to enable direct comparison of expression estimates between response groups in both adults and paediatrics. A subset of samples was used in both runs to enable comparisons between adults and paediatrics. Digital whole slides were obtained using a 20X objective in a bright-field slide scanner (Axio Scan.Z1; Zeiss).
Quantitative image analysis
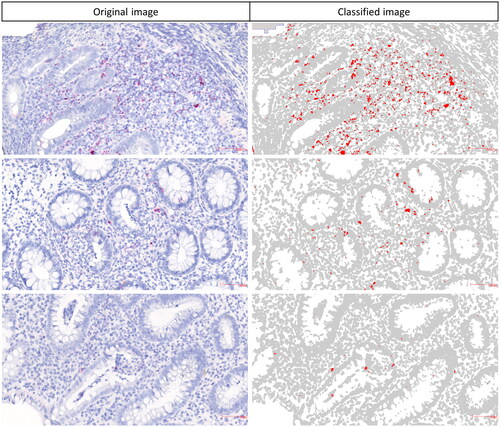
To obtain relative TNF mRNA expression estimates from the ISH analyses, we submitted digital slides for image analysis (Visiopharm, Denmark) as also described previously [Citation20,Citation21]. To reduce the risk of sampling bias, we analysed the levels of TNF mRNA expression in the colorectal tissue biopsies with and without LF. We identified all LFs on the tissue slides and outlined them as region of interest (ROI)-2, and the rest of the tissue area without LFs was defined as ROI-1. Not all samples contained LF. A colour segmentation profile was designed to discriminate the red ISH signal from the blue nuclei, connective tissue and background stain (see also ). The colour segmentation profile was applied to all ROI-1 and ROI-2. The median area of ROI-1 and ROI-2 in individual samples among all tissue slides was 7.7 mm2 (range: 0.9 to 227 mm2). Unspecific precipitations were cleared from the ROIs by manual exclusion. For each slide, we obtained area fractions (these are considered expression estimates that reflect expression levels) of the red ISH signal relative to the area of the corresponding ROI. The outline of ROIs was performed blinded by two authors (LBR and JPJ). An outline of the image analysis procedure with the colour segmentation is presented in Supplementary Figure 1. Average biological variance of TNF expression estimates among all tissue samples were found to be 81% (range 61% −102%), whereas the technical variance with the repeated set of 6 samples, using serial sections were only about 16% (range 5% − 37%).
Figure 1. Quantification of TNF mRNA levels in IBD tissue. Digital slides of the TNF mRNA ISH stained sections were submitted to colour segmentation-based image analysis. Original images with the TNF mRNA ISH signal (left) and corresponding colour classified images (right). Relative expression estimates were obtained as area fractions (red area/total area of ROI). Precision of the colour segmentation system at different signal intensities are shown here.
Statistics
Descriptive statistics were used to determine the baseline characteristics of the sample cohort. Mann-Whitney U-test or Kruskal-Wallis Rank Sum test were used to compare the TNF expression values between two or three different groups. Graphics with statistical details were generated using the R packages ggstatsplot [Citation22] and ggplot2. All statistical analyses were performed using R (version 4.1.2). A two-tailed p value < .05 was considered statistically significant.
Results
In this study we performed in situ expression analyses of TNF mRNA on FFPE sections from tissue samples of 42 IBD patients. The demographic data and baseline clinical characteristics of the patients are summarised in .
Table 1. Baseline characteristics of the IBD patient population.
TNF mRNA localisation
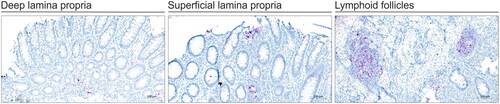
TNF mRNA transcripts were visible by RNAscope ISH as red stain primarily observed in cells located within the lamina propria (LP) and in LF (). The ISH signal appeared as red signal dots or as a dense chromogenic precipitate in cells with increased expression. TNF mRNA expression in superficial and deep lamina propria was common in adults and paediatric patients in all response groups. Most of the adult PNRs had TNF expression primarily in the deep lamina propria and LFs.
Figure 2. TNF mRNA ISH in IBD colonic tissue. All tissue biopsies were collected before initiation of anti-TNF therapy. ISH for TNF mRNA was done using RNAscope technology and performed on tissue from the three response groups: responders, primary non-responders (PNR) and secondary non-responders (SLOR). The TNF mRNA signal (red stain) is seen in superficial lamina propria (SLP), deep lamina propria (DLP) and lymphoid follicles (LF). Tissue sections were counterstained with haematoxylin. The ISH images are representative for different response groups among adult IBD patient samples.
TNF mRNA expression among adults and paediatric patients
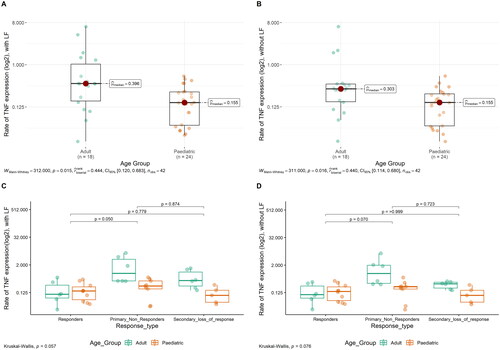
Examples of TNF mRNA ISH in the different response types of adult and paediatric IBD patients are shown in . To determine the quantitative TNF mRNA expression levels in the patient cohort, we obtained relative expression estimates using image analysis both including and excluding the LF. The median TNF mRNA expression estimates in adults were 0.396 when including LF and 0.303 when excluding LF from the calculation. In paediatric IBD, the median values for TNF mRNA expression, including and excluding the LF, were both 0.155 (See also )). IBD tissue from paediatric patients had significantly lower TNF mRNA expression compared to that from adults (p = .015, including LF, , p = .016, excluding the LF, ). Two cases among paediatric PNR had quite low TNF values compared to the other 7 PNR patients. Parameters such as age, sex, disease duration and inflammation scoring didn’t show any correlation to the low TNF values in the PNR paediatric group. Also, there was no apparent technical issues with the two samples that could indicate poor quality. The PPIB mRNA signal indicated positive reaction in all samples.
Figure 3. TNF mRNA ISH in adults and paediatric IBD colonic tissue. Examples of TNF mRNA ISH in the different response groups of adult and paediatric IBD patients. TNF mRNA expression (red stain) is shown in the lamina propria area. The examples suggest that, in adults, TNF expression is high in PNRs compared to responders and SLORs, moreover, in paediatric patients, a similar trend with high TNF expression in PNRs compared to responders and SLORs is noted. The examples also suggest that adults have higher TNF expression than paediatric patients. The TNF mRNA ISH signal was quantified using image analysis (see ).
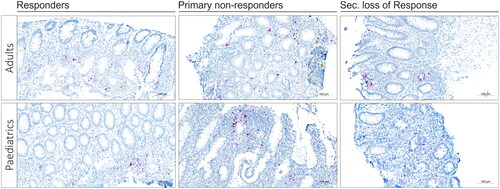
Figure 4. Relative TNF mRNA levels in IBD tissues with and without LF. TNF mRNA expression estimates from the ISH-stained slides were obtained in tissue areas with and without LF. All IBD patients included are divided into age groups (A and B) and response groups (C and D). Plots were made using ggstatsplot package from R. Each dot represents one patient.
TNF mRNA expression among different response types
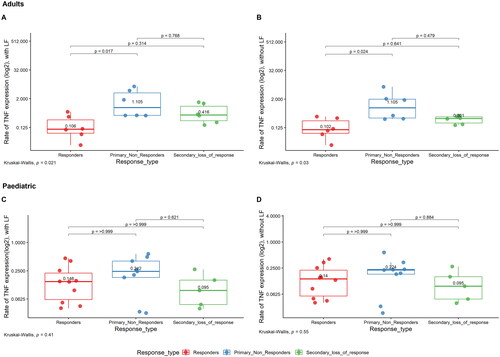
The median TNF mRNA expression estimates in adults were 0.106, 1.105 and 0.416 for responders, PNR and SLOR, respectively, when including LF and 0.102, 1.105 and 0.301 for responders, PNR and SLOR, respectively, when the LF was excluded from the calculation. Among adults, PNR had significantly higher TNF mRNA expression levels compared to responders with and without considering area of LF (p = .017 and p = .024, respectively) (). Among paediatric patients, when TNF mRNA expression levels were compared between responders, PNR and SLOR, there were no significant differences between the three groups with and without LF (), although a similar trend as that of adults with higher TNF expression in PNR was seen. Median values with LF for Responders, PNR and SLOR in paediatric patients were 0.146, 0.242 and 0.095, respectively. Median values without LF for responders, PNR and SLOR in paediatric patients were 0.14, 0.224 and 0.095, respectively. Considering the total patient cohort with harmonised results from two separate analytical runs, no significant difference in the TNF mRNA expression between responders, PNR and SLOR was found ().
Figure 5. Relative TNF mRNA levels in adult and paediatric IBD tissue. TNF mRNA expression estimates from the ISH-stained slides were obtained in the whole tissue area. A + B presents. TNF expression for adults in the different response groups, with and without LF, respectively. C + D presents TNF expression in paediatric patients in different response groups, with and without LF, respectively. Each dot represents one patient.
Discussion
In this study of tissue samples from biological treatment naïve IBD patients, we found that adults with no or minor response (PNR) to anti-TNF treatment surprisingly had higher TNF mRNA levels compared to responders and SLOR. A similar trend was observed in the paediatric group, however not significant. Anti-TNF treatment is accepted as a standard therapeutic option in IBD patients who are resistant to steroids or immunomodulators. Nevertheless, the primary and secondary non-response rates to anti-TNF antagonists in patients with IBD are high. The lack of effectiveness of anti-TNF treatment in about one-third of patients support the need for biomarkers to identify patient subgroups who will benefit from the drug. Our findings suggest that identifying PNRs among adult IBD patients, by measuring tissue specific TNF mRNA levels prior to initiating anti-TNF treatment, confers the possibility of adjusting the dosage of the anti-TNF therapy.
We hypothesise that among the three groups of anti-TNF non-responders, PNR and SLOR might be linked to alternative cytokine pathways in the development of IBD other than the inflammation driven by TNF. Lower levels of TNF in SLOR suggest that the disease might have entered a new TNF independent phase, hence biological treatments other than anti-TNF could be considered. Previous studies have shown that IL-23 blockade is effective in CD patients who do not respond to anti-TNF therapy [Citation23,Citation24]. On the other hand, responders and PNR have a TNF driven disease profile which could be treated with anti-TNF antagonists based on the levels of TNF present in the sample. Responders have the optimal amount of TNF for the existing dosage of anti-TNF, whereas in PNRs the amount of anti-TNF dosage could be intensified to meet the requirement.
The initial key finding of our study suggested that IBD may represent various diseases depending on age at diagnosis since adults exhibited higher TNF expression levels than the paediatric group. Age-related changes of expression are common, and in particular, genes that play roles in inflammation are overexpressed with age [Citation25]. In a previous study, we found that miR-21 was also significantly higher in adult than in paediatric UC patients [Citation20]. Interestingly, it has been reported that TNF may contribute to a positive regulation of miR-21 [Citation19,Citation26]. Taken together, TNF mRNA expression levels were significantly different in paediatric and adult IBD patients and were therefore considered separately in our study.
We found that adult PNRs on average had increased TNF levels compared to responders, and we propose that the therapeutic ant-TNF dosage may have been insufficient. In agreement with this finding, it has been reported that TNF concentrations in serum before treatment with infliximab were lower in responders compared to non-responders in fistulising CD patients aged 15–58 years [Citation11]. It remains to be shown if there is correlation between TNF mRNA levels and serum TNF in the same patient. This may however not be a simple correlation since the TNF measured in serum is likely a soluble form (sTNF, see below). Alternatively, both the tissue mRNA estimate, and the TNF serum parameter may be complementary measures to identify responders and non-responders. Indeed, it has been argued that the TNF concentration in serum is a possible overspill from that produced locally in the colon and may be representative of local productions [Citation27]. Yarur et al. [Citation28] also showed that there was a higher rate of mismatch with the serum to tissue anti-TNF levels in active IBD cases. Though these results are not implicating correlated results, local and systemic measurements of TNF levels are different.
The TNF mRNA encodes a transmembrane protein that, as a homo-trimer, forms TNF. The anti-TNF drug, infliximab, recognises the extracellular domain of TNF [Citation29]. The extracellular domain can be processed by TNF-alpha converting enzyme (TACE) [Citation30] to release a soluble form of TNF (sTNF). The expression of TNF protein in the colonic tissues is significantly higher in inflamed tissue compared to the matched uninflamed tissue [Citation31]. For efficient anti-TNF dosage, the ratio with TNF itself should be in favour of the drug. In inflamed tissue, the ratio of colonic tissue concentration of TNF to anti-TNF is high compared to uninflamed tissue [Citation31]. Yarur et al. [Citation28] found that the anti-TNF to TNF ratio in highly inflamed tissue of adults was lower as compared to uninflamed tissue, suggesting that there was insufficient anti-TNF to neutralise the TNF and that the local inflammation with high levels of TNF could serve as a reservoir for anti-TNF. These observations support our proposal that IBD patients with elevated TNF mRNA expression would benefit from intensified dosage.
Thus, our ISH-based determination of TNF mRNA levels in IBD samples suggested that it may help to identify some of the adult PNRs. To reduce variation in staining intensities, we employed automated chromogenic RNAscope and applied the same custom-developed image analysis application to all stained slides. It cannot be excluded, however, that tissue processing and storage may have individually affected the TNF mRNA content before being used for the ISH analysis. We noted a significant difference in size and numbers of LF in the colonic biopsies, which could lead to a possible sampling bias. Studies have shown a higher number of LF and Peyer’s patches in younger patients compared with older [Citation32,Citation33]. Hence, it is vital to consider the presence or absence of LFs when comparing the expression levels of molecular markers such as TNF, which can be highly expressed in LF particularly in adult IBD tissue. Our image analysis application required manual identification and separation of the LF regions from the mucosa (the ROIs). Automated identification and selection of the LF ROIs, e.g., by the increased cell density, may ease the image analysis procedure and would be beneficial in clinical routine settings where there is lack of IBD specialised pathologists.
It is also noted that since we had only one biopsy per patient, the intra individual variation cannot be assessed from our sample cohort. The unique sample cohort we used in this study had several inclusion criteria which limits the number of samples that were available to investigate. In real scenario of IBD cases, it is quite common to have FFPE biopsies collected and stored in the hospitals worldwide, our study has proven that it is possible to measure the TNF mRNA levels in archived FFPE biopsies using our technique. Hence, applying this technique on biopsies prior to initiation of anti-TNF treatment and there by regulating the dosage in an individualised manner could help in reducing the treatment burden for the patients and cutting down the cost in the health care systems.
Our study included a unique selection of 18 adult and 24 paediatric patients with close follow-up of the response to anti-TNF therapy over up to five years. Importantly, we demonstrated that it is possible to detect tissue specific TNF mRNA in 20-year-old archived biopsies, which provides the possibility to expand retrospective studies to include samples after anti-TNF treatment at different time points and to study the mechanism of various TNF antagonist therapies.
The data presented in this study suggest that knowing the tissue specific TNF mRNA expression levels prior to initiating anti-TNF treatment can help to optimise personalised dosage regimes for IBD patients. In addition, our data indicate that tissue specific TNF mRNA expression levels is higher in adult IBD patients compared to a paediatric IBD patients. Thus, our study supports the assumption that the molecular mechanisms of inflammation in IBD is different in adults and paediatric patients [Citation34]. We suggest that measuring TNF mRNA expression before initiating anti-TNF treatment in IBD patients, could be considered as a biomarker to support personalised medicine in the form of individualised dose-regulated treatment. Further investigation leading to insights on pathways and possible different mechanisms are warranted. Age, gender, disease subtype, disease activity etc. should be thoroughly investigated in a larger cohort of IBD patient samples to get more individualised dose regulated regimes.
Ethics statement
The study was conducted in accordance with the ethical standards, according to the Declaration of Helsinki and according to national and international guidelines. The study was approved by the Danish National Committee on Health Research Ethics (H-20032221) and the Danish Data Protection Agency (P-2020-737).
Authors contributions
No additional writing assistance was used for this manuscript. L.B.R., B.S.N., J.P.J., E.L., M.M. and E.H. all contributed to the conceptualisation and the design of study. L.B.R., E.L., M.M. and J.P.J collected samples, B.S.N., L.B.R. and J.P.J. designed experiments and analysed the data. J.P.J., L.B.R. and B.S.N. drafted the manuscript. E.L., M.M. and E.H. critically revised the manuscript for important intellectual content. All authors have approved the final version of the manuscript.
| Abbreviations | ||
| PCDAI | = | Paediatric Crohn’s Disease Activity Index |
| CD | = | Crohn’s Disease |
| FFPE | = | Formalin-fixed paraffin-embedded |
| GHAS | = | Global Histological Activity Scores |
| IBD | = | Inflammatory bowel disease |
| IBDU | = | Inflammatory bowel disease unclassified |
| IQR | = | Inter quartile range |
| ISH | = | In situ hybridisation |
| LF | = | Lymphoid follicles |
| LP | = | lamina propria |
| mRNA | = | Messenger RNA |
| PNR | = | Primary non-responders |
| PUCAI | = | Paediatric Ulcerative Colitis Activity Index |
| ROI | = | Region of interest |
| SCCAI | = | Simple clinical colitis activity index |
| SLOR | = | Secondary loss of response |
| sTNF | = | Soluble form of TNF |
| TACE | = | TNF-alpha converting enzyme |
| TNF | = | Tumor necrosis factor – α |
| UC | = | Ulcerative colitis |
Supplemental Material
Download PDF (195.1 KB)Acknowledgements
We would like to thank laboratory technicians Trine Møller, Bioneer A/S for her valuable technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data underlying this article are available in the article and in its online supplementary material.
Additional information
Funding
References
- De Souza HSP, Fiocchi C. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol. 2016;13(1):13–27.
- Gareb B, Otten AT, Frijlink HW, et al. Review: local tumor necrosis factor-α inhibition in inflammatory bowel disease. Pharmaceutics. 2020;12(6):539.
- Billmeier U, Dieterich W, Neurath MF, et al. Molecular mechanism of action of anti-tumor necrosis factor antibodies in inflammatory bowel diseases. World J Gastroenterol. 2016;22(42):9300–9313.
- Papamichael K, Gils A, Rutgeerts P, et al. Role for therapeutic drug monitoring during induction therapy with TNF antagonists in IBD. Inflamm Bowel Dis. 2015;21(1):182–197.
- Gisbert JP, Marín AC, McNicholl AG, et al. Systematic review with meta-analysis: the efficacy of a second anti-TNF in patients with inflammatory bowel disease whose previous anti-TNF treatment has failed. Aliment Pharmacol Ther. 2015;41(7):613–623.
- Olsen T, Goll R, Cui G, et al. Tissue levels of tumor necrosis factor-alpha correlates with grade of inflammation in untreated ulcerative colitis. Scand J Gastroenterol. 2007;42(11):1312–1320.
- Olsen T, Goll R, Cui G, et al. TNF-alpha gene expression in colorectal mucosa as a predictor of remission after induction therapy with infliximab in ulcerative colitis. Cytokine. 2009;46(2):222–227.
- Kennedy NA, Heap GA, Green HD, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal Crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4(5):341–353.
- Cui G, Florholmen J, Goll R. Could mucosal TNF transcript as a biomarker candidate help optimize anti-TNF biological therapy in patients with ulcerative colitis? Front. Immunol. 2022;13:2364.
- Olsen J, Gerds TA, Seidelin JB, et al. Diagnosis of ulcerative colitis before onset of inflammation by multivariate modeling of genome-wide gene expression data. Inflamm Bowel Dis. 2009;15(7):1032–1038.
- Martínez-Borra J, López-Larrea C, González S, et al. High serum tumor necrosis factor-α levels are associated with lack of response to infliximab in fistulizing Crohn’s disease. Am J Gastroenterol. 2002;97(9):2350–2356.
- Breese EJ, Michie CA, Nicholls SW, et al. Tumor necrosis factor α-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994;106(6):1455–1466.
- Murch SH, Braegger CP, Walker-Smith JA, et al. Location of tumour necrosis factor a by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993;34(12):1705–1709.
- Tumanov AV, Grivennikov SI, Kruglov AA, et al. Cellular source and molecular form of TNF specify its distinct functions in organization of secondary lymphoid organs. Blood. 2010;116(18):3456–3464.
- Jauregui-Amezaga A, Geerits A, Das Y, et al. A simplified Geboes score for ulcerative colitis. J Crohns Colitis. 2017;11:305–313.
- D’Haens GR, Geboes K, Peeters M, et al. Early lesions of recurrent Crohn’s disease caused by infusion of intestinal contents in excluded ileum. Gastroenterology. 1998;114(2):262–267.
- Wang F, Flanagan J, Su N, et al. RNAscope. J Mol Diagn. 2012;14(1):22–29.
- Anderson CM, Zhang B, Miller M, et al. Fully automated RNAscope in situ hybridization assays for formalin-fixed paraffin-embedded cells and tissues. J Cell Biochem. 2016;117(10):2201–2208.
- Møller T, James JP, Holmstrøm K, et al. Co-detection of miR-21 and TNF-α mRNA in budding cancer cells in colorectal cancer. Int J Mol Sci. 2019;20(8):1907.
- Malham M, James JP, Jakobsen C, et al. Mucosal microRNAs relate to age and severity of disease in ulcerative colitis. Aging. 2021;13(5):6359–6374.
- Thorlacius-Ussing G, Schnack Nielsen B, Andersen V, et al. Expression and localization of miR-21 and miR-126 in mucosal tissue from patients with inflammatory bowel disease. Inflamm Bowel Dis. 2017;23(5):739–752.
- Patil I. Visualizations with statistical details: the “ggstatsplot” approach. J Open Sour Softw. 2021;6(61):3167.
- Sands BE, Chen J, Feagan BG, et al. Efficacy and safety of MEDI2070, an antibody against interleukin 23, in patients with moderate to severe Crohn’s disease: a phase 2a study. Gastroenterology. 2017;153(1):77.e6–86.e6.
- Feagan BG, Sandborn WJ, D’Haens G, et al. Induction therapy with the selective interleukin-23 inhibitor risankizumab in patients with moderate-to-severe Crohn’s disease: a randomised, double-blind, placebo-controlled phase 2 study. Lancet. 2017;389(10080):1699–1709.
- de Magalhães JP, Curado J, Church GM. Meta-analysis of age-related gene expression profiles identifies common signatures of aging. Bioinformatics. 2009;25(7):875–881.
- Cottonham CL, Kaneko S, Xu L. miR-21 and miR-31 converge on TIAM1 to regulate migration and invasion of colon carcinoma cells. J Biol Chem. 2010;285(46):35293–35302.
- Murch SH, Lamkin VA, Savage MO, et al. Serum concentrations of tumour necrosis factor in childhood chronic inflammatory bowel disease. Gut. 1991;32(8):913–917.
- Yarur AJ, Jain A, Sussman DA, et al. The association of tissue anti-TNF drug levels with serological and endoscopic disease activity in inflammatory bowel disease: the ATLAS study. Gut. 2016;65(2):249–255.
- Scallon B, Cai A, Solowski N, et al. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J Pharmacol Exp Ther. 2002;301(2):418–426.
- Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385(6618):729–733.
- Berends SE, van Steeg TJ, Ahsman MJ, et al. Tumor necrosis factor-mediated disposition of infliximab in ulcerative colitis patients. J Pharmacokinet Pharmacodyn. 2019;46(6):543–551.
- Van Kruiningen HJ, Ganley LM, Freda BJ. The role of Peyer’s patches in the age-related incidence of Crohn’s disease. J Clin Gastroenterol. 1997;25(2):470–475.
- Van Kruiningen HJ, Brain West A, Freda BJ, et al. Distribution of Peyer’s patches in the distal ileum. Inflamm Bowel Dis. 2002;8:180–185.
- Ruel J, Ruane D, Mehandru S, et al. IBD across the age spectrum—is it the same disease? Nat Rev Gastroenterol Hepatol. 2014;11(2):88–98.
