Abstract
Background and Aims
Proton pump inhibitors (PPI) affect the gastrointestinal microbiota, which is thought to play a role in the pathogenesis of ulcerative colitis (UC). Previous studies suggest an association between PPI use and risk of incident UC as well as disease course. The aim of the study was to examine if PPI exposure is associated with disease course in UC patients.
Methods
A national cohort consisting of all newly diagnosed UC patients from 2010 to 2020 was defined combining data from Norwegian registries. PPI exposure was included as a time dependent variable with a 30 day time lag from starting the drug. Outcomes were starting advanced therapies including anti-TNF, systemic glucocorticoids, any additional systemic anti-inflammatory medication and undergoing colectomy during follow-up. Time-dependent Cox regressions included the variables PPI use, first systemic glucocorticoid prescription, first UC hospitalization, age-groups and sex.
Results
The study cohort consisted of 10,149 patients with median age 40 years (IQR 27–56) and 56% males. PPI use independently increased the risk of starting advanced therapies (HR 1.54, 95% CI 1.36–1.73, p < 0.005), starting systemic glucocorticoids (HR 1.20, 95% CI 1.07–1.34, p < 0.005), starting any additional anti-inflammatory treatment (HR 1.18, 95%CI 1.05–1.32, p < 0.01) and undergoing colectomy (HR 1.52, 95%CI 1.17–1.98, p < 0.005).
Conclusions
PPI use was associated with unfavorable outcomes including advanced therapy initiation, additional anti-inflammatory medications and undergoing colectomy. Although further studies are needed, the evidence suggests that PPIs could affect the course of UC and should be used cautiously in UC patients.
Introduction
Inflammatory bowel disease (IBD) consists of ulcerative colitis (UC) and Crohn’s disease (CD) and are believed to occur in genetically predisposed individuals with influence from environmental factors as well as the gastrointestinal (GI) microbiome. Numerous studies suggest that the microbiome is important in the pathogenesis of IBD [Citation1] and many of the genetic polymorphisms associated with IBD cause altered handling of microorganisms [Citation2]. Consequently, it is relevant to study how factors affecting the GI microbiome influence IBD. Antibiotics may cause long-lasting alterations in the fecal bacterial composition [Citation3] and their use has been identified as a risk factor for later development of IBD [Citation4,Citation5]. However, proton pump inhibitors (PPIs) alter the microbiome from the stomach [Citation6] to the feces [Citation7] and PPI use is the single factor that influences fecal microbial composition to the largest extent at population level [Citation8,Citation9]. It has thus been of interest to study how PPI use may affect IBD. Early life PPI exposure has also been found to increase the risk of later development of IBD [Citation10]. PPI use is associated with an increased risk of incident IBD [Citation11], at least the first two years after treatment start [Citation12]. Furthermore, PPI use is associated with altered clinical course of IBD, mainly in patients with CD, but PPI use was found to be associated with altered use of medication in UC patients as well [Citation13]. IBD patients using PPI when starting infliximab were less likely to achieve remission in multivariable and propensity score matched analyses of patients participating in randomized controlled studies [Citation14]. Whereas PPI use seemed to negatively affect patients with CD at both 30 and 54 weeks, the effect of PPI use was significant in patients with UC at 30 weeks only [Citation14]. Since PPIs may be prescribed to treat CD patients with involvement of the upper GI tract, reverse causation may be problematic in population-based studies of CD patients.
Considering a possible negative impact of PPI on UC it is of concern that the prescriptions of PPIs have nearly doubled over the past decade in Norway so that more than 10% of the Norwegian population received a prescription of a PPI in 2020 [Citation15]. It was therefore of interest to study if PPI use was associated with the course of UC in a national patient cohort.
Materials and methods
Data sources
All inpatient and outpatient hospital contacts in Norway are registered in the Norwegian Patient Registry (NPR) and it is mandatory to report diagnoses and clinical procedures. In addition, all prescription drugs sold in Norway are registered by their Anatomical Therapeutic Chemical (ATC) codes as well as preparation name in either NPR and/or for dispensed drugs the Norwegian Prescription Database (NorPD). Data from these two registries were combined. The NPR uses unique personal identification numbers from 2008 on, which makes it possible to follow individual patients over time. The patients were followed up until 31 December 2020.
Patients
The dataset included every inpatient and outpatient hospital event at public and private institutions for all patients who received their first UC diagnosis (ICD-10 code K51) between 1 January 2010 and 31 December 2020. For further analyses UC was defined as having received a UC diagnosis (K51) at least once and a prescription of oral 5-ASA (ATC-codes A07EC02 (mesalazine, oral preparations only), A07EC03 (olsalazin), A07EC04 (balsalazid)) or sulfasalazine (A07EC01) after the UC diagnosis. The dispensal of oral 5-ASA or sulfasalazine defined the date of UC diagnosis.
To ensure identification of incident UC and a correct time of diagnosis, patients were excluded if they received oral 5-ASA or oral budesonide more than 30 days before a K51 diagnosis, or if they had a CD diagnosis (ICD-10 code K50) in NPR during the entire study period.
Patients who had received azathioprine, methotrexate or advanced therapies as defined in before the first UC diagnosis were considered prevalent UC and therefore excluded. However, patients with use of rectal 5-ASA or rectal glucocorticoids before oral 5-ASA were perceived as ulcerative proctitis with subsequent progression to colitis and were not excluded.
Table 1. Anti-inflammatory medications and colectomy procedures defined as endpoints.
Exposure
The exposure was defined as PPI use (ATC-code A02B C) of at least 0.5 defined daily dose (DDD) for 28 days after the first prescription of oral 5-ASA. A lag time of 30 days was included to avoid reverse causation and as the effect on UC was not expected to be immediate. The exposure time was extended 60 days after the duration of the prescription calculated as 0.5 DDD/day. A 0.5 DDD equivalents to omeprazole 10 mg, pantoprazole 20 mg, lansoprazole 15 mg, esomeprazole 15 mg https://www.whocc.no/atc_ddd_index/?code=A02BC. The indication for PPI prescriptions during the study period was assessed by using the codes from the International classification of primary care (ICPC) and International classification of diseases (ICD).
Outcomes
Outcomes were defined as the use of advanced therapies (adalimumab, infliximab, golimumab, vedolizumab, ustekinumab and tofacitinib), glucocorticoids, any additional systemic anti-inflammatory medication or colectomy after the first prescription of oral 5-ASA (). All such events occurring from 30 days after start of PPI exposure were considered an outcome. The use of rectal glucocorticoids and rectal 5-ASA was not included in any analyses.
Statistical analyses
Time-dependent Cox regressions included the variables PPI use (time-dependent), first systemic glucocorticoid prescription (time-dependent), first UC hospitalization (time-dependent), age and sex. In the other time-dependent Cox regressions using systemic glucocorticoids and colectomy as outcomes, advanced therapies were also included as a variable. Patients were followed from their first 5-ASA prescription until the outcome of interest, death or December 2020, whichever came first.
In the main analysis PPI exposure was included as a time dependent variable with a 30 day time lag from starting the drug, however, a sensitivity analysis using outcomes from 0 days and 60 days were also performed with similar findings (Supplementary Table 1 and 2). In an additional analysis patients who dispensed a non-steroid anti-inflammatory drug (NSAID) (ATC-code M01A) up to 30 days before or during PPI exposure were excluded (Supplementary Table 3). In a further sensitivity analysis, all patients having dispensed PPI before the UC diagnosis were excluded (Supplementary Table 4).
Ethical considerations
The study was approved by the NPR, the Norwegian Data Protection Authority and the Regional Committees for Medical and Health Research Ethics of South-East Norway (2016/113). The dataset does not contain data that can identify individual patients and consent from the patient population was therefore not needed.
Results
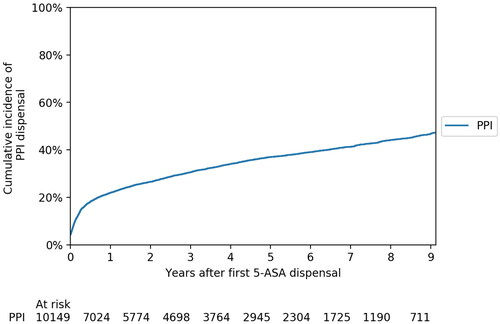
There were 10,149 patients with incident UC in the study cohort. The median age was 40 years (IQR 27–56) and 56% were males. The cumulative incidence of PPI use after UC diagnosis is illustrated in . Approximately 4% of the patients were exposed for PPI at the time of UC diagnosis. The predominant indications for PPI prescription were esophageal disease and gastroesophageal reflux disease, gastric ulcer and duodenal ulcer ().
Table 2. Indications for prescription of proton pump inhibitors in patients with ulcerative colitis given as number of prescriptions (n) and %.
In a Cox proportional hazard analyses PPI use independently increased the risk of starting glucocorticoids (HR 1.20, 95% CI 1.07–1.34, p < 0.005), starting advanced therapies (HR 1.54, 95% CI 1.36–1.73, p < 0.005), starting any additional anti-inflammatory treatment (HR 1.18, 95%CI 1.05–1.32, p < 0.01) and undergoing colon surgery (HR 1.52, 95%CI 1.17–1.98, p < 0.005) ().
Table 3. Multivariate analyses by Cox proportional hazards model with 30 days lag time from dispensal to outcome, identifying factors independently associated with the risk of starting systemic glucocorticoids, advanced therapies (biologics and JAK-inhibitors), starting any anti-inflammatory treatment and colectomy.
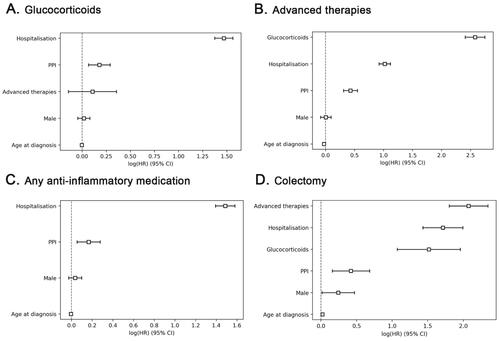
The strongest risk factor for starting glucocorticoids was hospitalization (HR 4.35 95%CI 3.96–4.77, p < 0.005), while young age also was a significant risk factor (). Use of glucocorticoids was the strongest risk factor for starting treatment advanced therapies (HR 13.19, 95%CI 11.12–15.63, p < 0.005) and younger age was also a significant risk factor in this analysis.
Figure 2. Factors independently associated with the risk of starting glucocorticoids (a), advanced therapies (B), any anti-inflammatory treatment (C) or colectomy (D) in patients with ulcerative colitis assessed by cox proportional hazard multivariate analyses.
In the sensitivity analysis using outcomes occurring without time lag and with 60 days lag after staring PPI, the findings were similar (Supplementary Tables 1 and 2). However, the HR of PPI use in the analysis with starting glucocorticoids as outcome was higher using 60 days time lag HR 1.37 (1.20–1.57) versus no time lag HR 1.24 (1.12–1.38) and similarly for starting any anti-inflammatory treatment with a 60 day lag HR 1.21 (1.07–1.36) versus no time lag HR 1.22 (1.10–1.36). In analyses where patients exposed to NSAID were excluded, PPI use remained a significant risk factor for starting advanced therapies HR 1.66 (1.47–1.88) and undergoing colectomy HR 1.64 (1.25–2.15), but PPI use was no longer a risk factor for starting glucocorticoids or starting any anti-inflammatory treatment (Supplementary Table 3). In analyses where patients with any dispensal of PPI before the UC diagnosis (n = 2822) were excluded, PPI use remained a significant risk factor for all defined outcomes (Supplementary Table 4).
Discussion
In this national cohort of newly diagnosed UC, PPI use as a time-dependent variable was associated with unfavorable outcomes defined as starting advanced therapies, systemic glucocorticoids and undergoing colectomy. Several other factors expected to be associated with disease exacerbation were included in the analyses and could thereby be adjusted for throughout the observation period, including hospitalization and the use of systemic glucocorticoids or advanced therapies which were all considered markers of more severe UC.
The indications for PPI prescription were dominated by esophageal diseases and gastroesophageal reflux disease (GERD) prescribed from primary and specialist health care, respectively. The current study was not designed to assess overuse of PPIs, but previous studies have identified overuse in both hospital [Citation16] and outpatient settings [Citation17]. PPI prescriptions in Norway nearly doubled during the study period [Citation15] and overuse in this UC population also seems probable.
The impact of PPI use on the course of UC has been evaluated in only few studies previously. PPI was found to be associated with IBD-related hospitalization and surgery in a large case control study [Citation18]. In a cohort study PPI use was associated with a change in medication against UC [Citation13]. In a recent meta-analysis of IBD patients treated with infliximab in five randomized controlled trials, PPI users were less likely to achieve week 30 remission in multivariate analyses as well as propensity score-matched analyses [Citation14]. The association was significant for UC patients after 30 weeks but not after 54 weeks. The current study therefore provides significant new data supporting that PPI use may alter the clinical course of UC.
The possible effects of PPI on UC risk and disease course could be mediated by alterations in the GI microbiome. Inactivation of bacteria in the stomach is primarily dependent on the intragastric pH [Citation19,Citation20] and PPI use is associated with an increased risk of infections with bacteria causing clinical gastroenteritis [Citation21]. Others have proposed that the effect of PPI on IBD could be mediated by a higher frequency of bacterial infections leading to symptomatic bacterial gastroenteritis [Citation14] that may cause or be perceived as IBD flares. PPI use also increases the risk of small-intestinal bacterial overgrowth [Citation22] as well as Clostridium difficile infection in patients randomized to receive PPI [Citation23]. The fecal microbiome of PPI users has a different composition [Citation24] and reduced diversity [Citation8, Citation25] compared to non-users. Similarities between the fecal microbiome of PPI users and UC patients include the reduced diversity [Citation26] and that both groups have been found to have decreased abundances of the genus Fecalibacterium [Citation27]. This genus is dominated by the butyrate producing F. prausnitzii and its depletion has been linked to IBD and inflammation in numerous studies [Citation28,Citation29]. The association between UC and an altered microbiome is indisputable. A causal relationship between GI microbiota and IBD is supported by the finding of genetic polymorphisms associated with IBD in genes coding for proteins involved in antimicrobial defense [Citation30,Citation31]. The effects of fecal transplantation do also support a role of the microbiota [Citation32–34], however, identifying characteristics of the microbiota administered to actual responders seems crucial.
In analysis where patients exposed to NSAIDs were excluded PPI use remained a significant risk factor for starting advanced therapy or undergoing colectomy but was no longer a risk factor for starting glucocorticoids or any anti-inflammatory treatment. There is evidence that NSAID use may cause intestinal ulcerations per se but the perceived risk of UC exacerbations has more recently been questioned [Citation35]. However, the combined use of PPI and NSAID exacerbates intestinal injury caused by NSAIDs alone [Citation36,Citation37] and our findings could be explained by PPIs being particularly harmful in UC patients with concomitant use of NSAIDs. In sensitivity analyses where patients exposed to PPI before the UC diagnosis PPI remained a risk factor with similar HRs for the various outcomes.
Strengths of the study include the large samples size consisting of a national cohort of newly diagnosed UC patients. The data sources are reliable and only prescriptions that were dispensed were used in the study. We also excluded patients with a CD diagnosis to minimize the possibility of reverse causation between IBD with worse prognosis and PPI use. The sensitivity analyses using 0 and 60 days lag time reduced the possibility of reverse causation and produced similar results to analyses with 30 days lag time. Furthermore, UC flares have clinically little in common with symptoms of diseases where PPIs are indicated, and we find it unlikely that the use of PPI as stress ulcer prophylaxis in hospitalized patients could have influenced the main findings. Limitations of the study include the inherent possibility of residual confounding as the patients were not randomized to receive PPI or not. We also underline that identifying risk factors using Cox proportional hazard analyses does not prove any causal relationship. Although randomized studies would provide evidence of causality it seems unlikely that such studies will be performed. Disease severity could not be assessed by clinical and endoscopic scoring at the time of starting PPI, however proxies of disease severity (first hospitalization and first glucocorticoid use) were included in the analyses for the entire observation period.
In conclusion, in this national cohort study we found that PPI use was associated with unfavorable outcomes such as starting additional anti-inflammatory medications as well as undergoing colectomy. Although further studies are needed, the evidence suggests that PPIs could affect the course of UC and should be used cautiously in UC patients [Citation38–42].
Author contributions
Conceptualization, RF; methodology, RF, SSL, MLH; formal analysis, SSL; data curation, SSL; writing—original draft preparation, RF; writing—review and editing, RF, SSL, MLH. All authors have read and agreed to the published version of the manuscript.
Institutional review board statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Norwegian Data Protection Authority and the Regional Committees for Medical and Health Research Ethics, NPR and NorPD.
Supplemental Material
Download PDF (62.4 KB)Acknowledgements
Authorship Statement: Reidar Fossmark is acting as the submission’s guarantor and takes responsibility for the integrity of the work as a whole. All authors approved the final version of the manuscript.
Disclosure statement
The author RF has received lecture fee from Takeda. SSL reports research funding from Takeda during the conduct of the study. MLH reports investigator research grants from Takeda, Pfizer, Tillotts and Ferring, and speaker fees from Tillotts, Ferring, Galapagos, BMS, MSD and AbbVie as well as Advisory board honoraria from Takeda and AbbVie.
Data availability statement
The data underlying this article cannot be shared publicly due to terms set by the Norwegian Data Protection Authority and the Regional Committees for Medical and Health Research Ethics of South-East Norway
Additional information
Funding
References
- Lloyd-Price J, Arze C, Ananthakrishnan AN, et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature. 2019;569(7758):655–662. doi: 10.1038/s41586-019-1237-9.
- Caruso R, Lo BC, Núñez G. Host-microbiota interactions in inflammatory bowel disease. Nat Rev Immunol. 2020;20(7):411–426. doi: 10.1038/s41577-019-0268-7.
- Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA. 2011;108 Suppl 1(Suppl 1):4554–4561. doi: 10.1073/pnas.1000087107.
- Nguyen LH, Örtqvist AK, Cao Y, et al. Antibiotic use and the development of inflammatory bowel disease: a national case-control study in Sweden. Lancet Gastroenterol Hepatol. 2020;5(11):986–995. doi: 10.1016/S2468-1253(20)30267-3.
- Örtqvist AK, Lundholm C, Halfvarson J, et al. Fetal and early life antibiotics exposure and very early onset inflammatory bowel disease: a population-based study. Gut. 2019;68(2):218–225. doi: 10.1136/gutjnl-2017-314352.
- Sharma BK, Santana IA, Wood EC, et al. Intragastric bacterial activity and nitrosation before, during, and after treatment with omeprazole. Br Med J (Clin Res Ed). 1984;289(6447):717–719. doi: 10.1136/bmj.289.6447.717.
- Clooney AG, Bernstein CN, Leslie WD, et al. A comparison of the gut microbiome between long-term users and non-users of proton pump inhibitors. Aliment Pharmacol Ther. 2016;43(9):974–984. doi: 10.1111/apt.13568.
- Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65(5):740–748. doi: 10.1136/gutjnl-2015-310376.
- Zhernakova A, Kurilshikov A, Bonder MJ, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352(6285):565–569. doi: 10.1126/science.aad3369.
- Schwartz NRM, Hutfless S, Herrinton LJ, et al. Proton pump inhibitors, H(2) blocker use, and risk of inflammatory bowel disease in children. J Pediatr Pharmacol Ther. 2019;24(6):489–496. doi: 10.5863/1551-6776-24.6.489.
- Xia B, Yang M, Nguyen LH, et al. Regular use of proton pump inhibitor and the risk of inflammatory bowel disease: pooled analysis of 3 prospective cohorts. Gastroenterology. 2021;161(6):1842–1852.e1810. doi: 10.1053/j.gastro.2021.08.005.
- Abrahami D, Pradhan R, Yin H, et al. Proton pump inhibitors and the risk of inflammatory bowel disease: population-based cohort study. Gut. 2023;72(7):1288–1295. doi: 10.1136/gutjnl-2022-328866.
- Juillerat P, Schneeweiss S, Cook EF, et al. Drugs that inhibit gastric acid secretion may alter the course of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36(3):239–247. doi: 10.1111/j.1365-2036.2012.05173.x.
- Lu TX, Dapas M, Lin E, et al. The influence of proton pump inhibitor therapy on the outcome of infliximab therapy in inflammatory bowel disease: a patient-level meta-analysis of randomised controlled studies. Gut. 2021;70(11):2076–2084. doi: 10.1136/gutjnl-2020-321609.
- (NorPD) NPD. http://www.reseptregisteret.no/Prevalens.aspx. Vol. 2022.
- Kelly OB, Dillane C, Patchett SE, et al. The inappropriate prescription of oral proton pump inhibitors in the hospital setting: a prospective Cross-Sectional study. Dig Dis Sci. 2015;60(8):2280–2286. doi: 10.1007/s10620-015-3642-8.
- Koggel LM, Lantinga MA, Büchner FL, et al. Predictors for inappropriate proton pump inhibitor use: observational study in primary care. Br J Gen Pract. 2022;72(725):e899–e906. doi: 10.3399/BJGP.2022.0178.
- Shah R, Richardson P, Yu H, et al. Gastric acid suppression is associated with an increased risk of adverse outcomes in inflammatory bowel disease. Digestion. 2017;95(3):188–193. doi: 10.1159/000455008.
- Giannella RA, Broitman SA, Zamcheck N. Gastric acid barrier to ingested microorganisms in man: studies in vivo and in vitro. Gut. 1972;13(4):251–256. doi: 10.1136/gut.13.4.251.
- Wilder-Smith CH, Spirig C, Krech T, et al. Bactericidal factors in gastric juice. Eur J Gastroenterol Hepatol. 1992;4:885–891.
- Hassing RJ, Verbon A, de Visser H, et al. Proton pump inhibitors and gastroenteritis. Eur J Epidemiol. 2016;31(10):1057–1063. doi: 10.1007/s10654-016-0136-8.
- Lo WK, Chan WW. Proton pump inhibitor use and the risk of small intestinal bacterial overgrowth: a meta-analysis. Clin Gastroenterol Hepatol. 2013;11(5):483–490. doi: 10.1016/j.cgh.2012.12.011.
- Moayyedi P, Eikelboom JW, Bosch J, et al. Safety of proton pump inhibitors based on a large, multi-year, randomized trial of patients receiving rivaroxaban or aspirin. Gastroenterology. 2019;157(3):682–691.e2. doi: 10.1053/j.gastro.2019.05.056.
- Shi YC, Cai ST, Tian YP, et al. Effects of proton pump inhibitors on the gastrointestinal microbiota in gastroesophageal reflux disease. Genomics Proteomics Bioinf. 2019;17(1):52–63. doi: 10.1016/j.gpb.2018.12.004.
- Gubatan J, Boye TL, Temby M, et al. Gut microbiome in inflammatory bowel disease: role in pathogenesis, dietary modulation, and Colitis-Associated Colon cancer. Microorganisms. 2022;10(7):1371. doi: 10.3390/microorganisms10071371.
- Morgan XC, Tickle TL, Sokol H, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13(9):R79. doi: 10.1186/gb-2012-13-9-r79.
- Takagi T, Naito Y, Inoue R, et al. The influence of long-term use of proton pump inhibitors on the gut microbiota: an age-sex-matched case-control study. J Clin Biochem Nutr. 2018;62(1):100–105. doi: 10.3164/jcbn.17-78.
- Quévrain E, Maubert MA, Michon C, et al. Identification of an anti-inflammatory protein from Faecalibacterium prausnitzii, a commensal bacterium deficient in crohn’s disease. Gut. 2016;65(3):415–425. doi: 10.1136/gutjnl-2014-307649.
- Zhao H, Xu H, Chen S, et al. Systematic review and meta-analysis of the role of Faecalibacterium prausnitzii alteration in inflammatory bowel disease. J Gastroenterol Hepatol. 2021;36(2):320–328. doi: 10.1111/jgh.15222.
- Cohen LJ, Cho JH, Gevers D, et al. Genetic factors and the intestinal microbiome guide development of microbe-based therapies for inflammatory bowel diseases. Gastroenterology. 2019;156(8):2174–2189. doi: 10.1053/j.gastro.2019.03.017.
- Khor B, Gardet A, Xavier RJ. Genetics and pathogenesis of inflammatory bowel disease. Nature. 2011;474(7351):307–317. doi: 10.1038/nature10209.
- Kump P, Wurm P, Grochenig HP, et al. The taxonomic composition of the donor intestinal microbiota is a major factor influencing the efficacy of faecal microbiota transplantation in therapy refractory ulcerative colitis. Aliment Pharmacol Ther. 2018;47(1):67–77. doi: 10.1111/apt.14387.
- Uygun A, Ozturk K, Demirci H, et al. Fecal microbiota transplantation is a rescue treatment modality for refractory ulcerative colitis. Medicine (Baltimore). 2017;96(16):e6479. doi: 10.1097/MD.0000000000006479.
- Paramsothy S, Kamm MA, Kaakoush NO, et al. Multidonor intensive faecal microbiota transplantation for active ulcerative colitis: a randomised placebo-controlled trial. Lancet. 2017;389(10075):1218–1228. doi: 10.1016/S0140-6736(17)30182-4.
- Cohen-Mekelburg S, Van T, Wallace B, et al. The association Between nonsteroidal anti-inflammatory drug use and inflammatory bowel disease exacerbations: a true association or residual bias? Am J Gastroenterol. 2022;117(11):1851–1857. doi: 10.14309/ajg.0000000000001932.
- Wallace JL, Syer S, Denou E, et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141(4):1314–1322.e5. doi: 10.1053/j.gastro.2011.06.075.
- Marlicz W, Loniewski I, Grimes DS, et al. Nonsteroidal anti-inflammatory drugs, proton pump inhibitors, and gastrointestinal injury: contrasting interactions in the stomach and small intestine. Mayo Clin Proc. 2014;89(12):1699–1709. doi: 10.1016/j.mayocp.2014.07.015.
- Freedberg DE, Toussaint NC, Chen SP, et al. Proton pump inhibitors alter specific taxa in the human gastrointestinal microbiome: a crossover trial. Gastroenterology. 2015;149(4):883–885.e889. doi: 10.1053/j.gastro.2015.06.043.
- Martinsen TC, Fossmark R, Waldum HL. The phylogeny and biological function of gastric juice-microbiological consequences of removing gastric acid. Int J Mol Sci. 2019;20(23):6031.
- Levine A, Wine E, Assa A, et al. Crohn’s disease exclusion diet Plus partial enteral nutrition induces sustained remission in a randomized controlled trial. Gastroenterology. 2019;157(2):440–450.e448. doi: 10.1053/j.gastro.2019.04.021.
- Olaisen M, Spigset O, Flatberg A, et al. Mucosal 5-aminosalicylic acid concentration, drug formulation and mucosal microbiome in patients with quiescent ulcerative colitis. Aliment Pharmacol Ther. 2019;49(10):1301–1313. doi: 10.1111/apt.15227.
- Biedermann L, Brulisauer K, Zeitz J, et al. Smoking cessation alters intestinal microbiota: insights from quantitative investigations on human fecal samples using FISH. Inflamm Bowel Dis. 2014;20(9):1496–1501. doi: 10.1097/MIB.0000000000000129.