Abstract
Background
Zenker’s diverticulum is a false diverticulum arising in the oesophago-pharyngeal junction. It may cause symptoms like dysphagia and regurgitation. In Central Norway, treatment is centralized to St. Olavs hospital, either as an endoscopic stapled oesophago-diverticulostomy procedure at the Department of Gastrointestinal Surgery or as laser diverticulostomy at the Department of Ear, Nose and Throat Surgery, depending on diverticulum size.
Methods
Retrospective, population-based, study from 2001–2020 on patients treated for Zenker’s diverticulum, at the time with a rigid endoscopic approach. Patients were identified through the in-hospital register for operations. The two treatment groups were compared on relevant pre-, intra-, and postoperative variables by review of the individual patient records.
Results
78 consecutive patients, 36 at Dept. of Ear, Nose and Throat Surgery and 42 at Dept. of Gastrointestinal Surgery, were treated with a total of 104 interventions. Crude incidence for a surgery-demanding Zenker’s diverticulum was 0.57 per 100 000 per year. The Dept. of Ear, Nose and Throat Surgery administered significantly less often prophylactic antibiotics than the Dept. of Gastrointestinal Surgery (p < 0.001), administered more frequently intraoperative dexamethasone (p < 0.001), and had significantly more postoperative infections (19.6% vs 3.4%, p = 0.01). No procedure-related mortality was registered. Although no standardized follow-up took place, at a median of 119 months elapsed, observed clinical recurrence was 35% for the endostapler treatment and 51% for the laser treatment procedure.
Conclusion
Both rigid endoscopic stapled oesophago-diverticulostomy and laser diverticulostomy are safe treatments for Zenker’s diverticulum, however with a substantial risk of recurrence.
Background
Zenker’s diverticulum (ZD), named after Friedrich Albert von Zenker [Citation1], is a false diverticulum arising from the posterior oesophagus in a triangle called the Killian’s dehiscence, formed by the cricopharyngeal muscle inferiorly and the inferior pharyngeal constrictor muscle laterally [Citation2]. An increased intraluminal pressure is considered important in the pathophysiology of ZD, however the cause of this is poorly understood [Citation3]. Motor abnormalities in the upper oesophageal sphincter has been suggested and is to date the most widely accepted hypothesis [Citation4–7]. ZD is estimated to have an annual incidence of approximately 2 per 100 000 and a prevalence between 0.01% and 0.11% [Citation8–10]. The real incidence is probably higher due to undiagnosed, clinically silent diverticula [Citation11]. The ZD occurs mainly among the elderly, 70–80 years old, and is uncommon before the age of 40 [Citation10–12].
Patients suffering from ZD can display a broad spectrum of symptoms. Dysphagia, reported in 80−90% of the patients [Citation8,Citation13], is often progressive as the diverticulum enlarges [Citation9], and may cause weight loss and malnutrition. Other common symptoms include hoarseness, chronic cough, halitosis, regurgitation, dysphonia, and aspiration pneumonia [Citation14]. Cervical borborygmus, often in combination with a palpable lump, is by many considered pathognomonic [Citation8,Citation9,Citation13]. The diagnosis is confirmed by radiological examination (barium swallow) and endoscopy. The operation criteria for ZD are in general dysphagia, regurgitation, and aspiration.
Open surgery with cricopharyngeal myotomy was for long the preferred treatment, but also associated with frequent complications [Citation15]. It has by far been replaced by rigid or flexible endoscopic procedures [Citation16–23], or recently, a submucosal tunnelling approach [Citation24–26]. The treatment of choice depends on multiple factors, in particular the depth of the diverticulum [Citation6,Citation9], but no randomized controlled trials and few long-term studies comparing different treatment options are found. To the best of our knowledge, the only population based study from Scandinavia on ZD, was a registry study from Finland, with no detailed information on the individual entries [Citation10].
Since the late nineties, treatment of ZD in Central Norway has been centralized to St. Olavs hospital, the university hospital of the region. The aims of the present study were to identify the surgical load for ZD at St. Olavs hospital during the last 20 years, and to compare the cohort treated at the Department of Ear, Nose and Throat Surgery (further referred to as ENT) to that at the Department of Gastrointestinal Surgery (further referred to as GIS) in terms of demographic variables, postoperative morbidity, and long-term patency rates.
Material and methods
Population-based, retrospective study comprising all patients treated for ZD in Central Norway from 1. Jan 2001 through 31. Dec 2020, at the Department of ENT and at the Department of GIS, St. Olavs hospital, the university hospital of the region. Central Norway on average comprised a population of around 690.000 inhabitants during the study period, some 13% of the Norwegian population. Based on the length from the septum apex to the bottom of the diverticulum as estimated at the preoperative examination, patients were selected for treatment by laser diverticulostomy at the ENT (<2 cm) or endoscopic stapling at the GIS (>2 cm), with the septum between the diverticulum and true oesophageal lumen divided using either a CO2 laser, used at 2-4 W on continuous mode, or an endo GIA™ device. Both procedures were performed endoscopically through a rigid diverticuloscope. One patient in the GIS group received planned open surgery due to a short and stiffened neck, impeding neck hyperextension required by the endostapling procedure. The study patients were identified by an institutional search on the relevant NOMESCO classification of surgical procedures. The search initially returned a total of 105 patients. Duplicates (n = 20) and misclassifications with oesophageal diverticula other than ZD (n = 7) were excluded, adding up to a study population of 78 unique patients. Primary interventions were JCA12 (Endoscopic stapled diverticulo-oesophagostomy, n = 36), aborted JCA12 (n = 5), ENC70 (Endoscopic laser diverticulo-hypopharyngostomy, n = 36), and JCA60 (Transcervical excision of oesophageal diverticulum, n = 1). An operation was considered “technically successful” if the procedure was not aborted. “Clinical success” was defined as symptom relief after a technically successful operation. Following either an unsuccessful primary operation or later recurrence of symptoms, 26 reoperations were performed, adding up to 104 procedures to evaluate.
No standardized follow-up took place, but by review of the individual patient records, data on patient demographics, symptoms, preoperative workup, intraoperative expenditures, and postoperative complications could be registered. Further, data on 90-day mortality, and, when available, time to recurrence of symptoms, were collected. Follow-up time was counted from the day of surgery until the day of censoring, 31.07.2022. Due to a study time span of two decades and mainly old patients at risk, no attempt to approach patients or relatives postoperatively was made.
Statistics
Continuous variables are summarised by median (range) and compared using the Mann-Whitney-U-test for independent samples. Categorical data were cross-tabulated and compared using the χ2 test or Fisher’s Exact test, as appropriate. Cumulative incidence of symptom recurrence was estimated by the Kaplan-Meier method, treating death as a competing event, i.e., retaining the patients in the risk set uncensored [Citation27]. Sex distribution was analysed by a one-sample binominal test, comparing to a hypothesised equal sex distribution. Missing data were handled by pairwise deletion for variables assumed to be missing at random (e.g., due to missing anaesthesia records). For variables with data assumed not to be missing at random (e.g., data on preoperative symptoms), no comparison between the departments were made due to risk of bias [Citation28]. The significance level was set to 0.05. Data were analysed using the Statistical Package for Social Sciences (SPSS, Version 27).
Ethics
The study was approved by the institutional board, and the manuscript prepared in accordance with the STROBE guidelines [Citation29].
Results
Demographics
The crude incidence for a surgery-demanding Zenker’s diverticulum in Central Norway was 0.57 per 100 000 per year, with subsets ranging from 0.00 (e.g. women aged <40) to 4.37 (men aged 80–89). Data on patient demographics and comorbidity for the n = 78 patients in the study cohort are displayed in , divided into the groups ENT (n = 36) and GIS (n = 42). The cohort consisted of 28 women (36%) and 50 men (64%), p = 0.02. Women were significantly older at operation compared to men, median 76 years (54 − 91) vs. 68 years (41 − 91), p < 0.001. No significant difference was found between the departments regarding median age (p = 0.733), sex distribution (p = 0.163), or ASA score (p = 0.365). Comorbidity was equally distributed among the departments, . The median post-procedural time elapsed was 119 months (19–251), 110 months (19–251) for the GIS patients and 126 (33–251) months for the ENT patients, p = 0.364.
Table 1. Demographics at index operation for patients operated for ZD at St. Olavs hospital during 2001–2020, by department, n = 78.
Preoperative examination
The length of the diverticulum was measured by barium swallow and intraoperatively using the endoscope for 42/42 of the GIS patients and 27/36 for the ENT patients. Median length from the septum apex to the bottom of the diverticulum was 35 mm (20–80) for the GIS patients, compared to 20 mm (5–50) for the ENT patients, p < 0.001, indicating that some of the ENT patients had their diverticulum underestimated at the preoperative examination, but still had the laser procedure performed beyond agreed criteria for diverticulum length. The most common position of the diverticulum was midline posterior (n = 39), followed by diverticula leaning to the left (n = 12), with no difference between the departments, . The main symptom was dysphagia, reported by all 78 patients offered surgery, followed by regurgitation, also reported by a large majority of the patients, .
Table 2. Diverticula characteristics and symptoms prior to index operation for patients operated for ZD at St. Olavs hospital during 2001–2020, by department, n = 78*.
Intraoperative expenditures
All the n = 104 procedures were performed under general anaesthesia. Data on prophylactic antibiotics and intraoperative use of dexamethasone are displayed in . Overall, 8/104 procedures were aborted: 7 at the Department of GIS and 1 at the Department of ENT, n = 2 due to endostapler dysfunction, n = 2 due to inadequate neck extension, n = 4 due to non-visualised diverticulum inlet. At the GIS, median operation time was 50 min (19–195) compared to 50 min (20–195) at the ENT, p = 0.848.
Table 3. Prophylactic antibiotics and intraoperative dexamethasone during the ZD procedures at St. Olavs hospital during 2001–2020, by department, n = 104.
Postoperative in-hospital morbidity and mortality
An average of 25% of the study patients experienced some postoperative morbidity, with no difference across the ENT/GIS cohorts, . Comparing the patients in the lower ASA-groups (ASA1 + 2) to the patients in the higher ASA group (ASA 3 + 4), the latter had a significantly higher postoperative morbidity rate, 16% vs 58% respectively, p = 0.01. A total of 8/104 procedures (8%) suffered from perforation, defined either by free air outside the oesophagus at CT or by contrast leak at barium swallow, with no differences across the departments, p = 0.134, . These patients were treated with nil per os for some days, and compared to others, had a significantly longer time to oral intake (9 days vs 1 day, p < 0.001) and hospital discharge (14 days vs 3 days, p < 0.001).
Table 4. Post-operative in-hospital morbidity for ZD procedures at St. Olavs hospital during 2001–2020, by department, n = 104.
A total of 11/104 procedures (11%) was counted to have postoperative infections, defined as either proven infection or suspected infection leading to treatment with antibiotics, and by default included all perforations. Of the 11 postoperative infections, eight were perforation-associated (GIS n = 2, ENT n = 6), two were cases of pneumonia, and one infection of unknown focus. Three of the patients received both antibiotics and steroids, three only antibiotics, four only steroids, and one neither. This pattern of distribution was not significantly different from those who did not experience a postoperative infection, p = 0.402. None of the perforation-associated infections developed into frank mediastinitis and could all be treated by medication only. Significantly fewer infections were registered at GIS (3%) compared to ENT (20%), p = 0.010, . No in-hospital or procedure-related death was recorded. One patient died of unrelated trauma within 90 days postoperatively.
Short and long-term outcomes
Analyses regarding symptom improvement and recurrence are restricted to the index procedures, . The GIS and the ENT had a technical success rate of 37/42 (88%) and 36/36 (100%), respectively, . Following a technically successful operation, the clinical success rate was 37/37 (100%) and 35/36 (97%), respectively, adding up to 72 patients with a clinically successful index operation. Of these, 31 (43%) had a clinical recurrence confirmed by manual review of the individual patient records, 13/37 (35%) at the GIS and 18/35 (51%) at the ENT, p = 0,23. The estimated cumulative rates of acknowledged recurrence are depicted in , stratified by operating department, log-rank p = 0.154. Most symptom recurrences occurred within five years (87%), with only four symptom recurrences acknowledged beyond five years (the latest after 11 years).
Figure 1. Estimated cumulative symptom recurrence for the clinically successful index procedures for ZD at St. Olavs hospital during 2001–2020, stratified by operating department, log rank = 0.154. Cumulative symptom recurrence was estimated treating death as a competing event, i.e., retaining the patients in the risk set uncensored. GIS: Department of Gastrointestinal Surgery; ENT: Department of Ear, Nose and Throat Surgery.
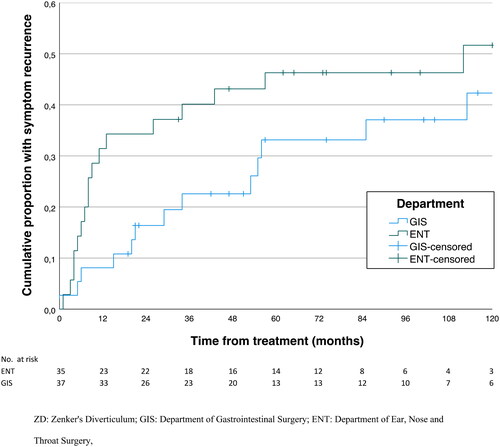
Table 5. Patient-reported symptom improvement at a median follow-up of 119 months, following a technical successful index procedure for ZD at St. Olavs hospital during 2001–2020, by department, n = 73.
Discussion
To the best of our knowledge, this is the first population-based study in Scandinavia to analyse the surgical load for ZD, comparing two standardized approaches for treatment over a time span of 20 years. A recent national audit from Finland was registry based, with limited information on individual entries [Citation10]. The crude incidence for a surgery demanding ZD in the present study was 0.57 per 100,000/year. Considering an annual incidence of approximately 2 per 100,000 [Citation8,Citation9], it follows that about one in four patients had surgical treatment of their ZD. Numbers from the Finnish study suggested that some 38% of the diagnosed diverticula received surgical treatment [Citation10].
A main finding of the present study was that the Department of ENT had significantly more postoperative infections compared to the Department of GIS (p = 0.01). The ENT administered less frequently prophylactic antibiotics (p < 0.001), preferentially clindamycin, which may be ineffective. GIS administered iv doxycycline + metronidazole, which is considered more appropriate considering the oral cavity mixed flora, in particular the gram-negative components [Citation30]. However, any oesophageal perforation was by default also counted as an infection. Although numbers are small, number of perforations at the Dept. of ENT was higher than at the Dept. of GIS (6 vs. 2), and was likely to drive the observed difference in infection rates. As this is a strict mechanical complication the administration of prophylactic antibiotics cannot explain the interdepartmental difference in infections.
The patients operated at the ENT more frequently received dexamethasone intraoperatively compared to patients operated at the GIS (p < 0.001). Dosages given during ZD-surgery (4−16 mg dexamethasone) are highly potent. However, Corcoran et al. demonstrated in a randomized multicentre trial with more than 8000 patients, non-inferiority for dexamethasone 8 mg vs no steroids intraoperatively, regarding surgical-site infection [Citation31]. Thus, the clinical significance of the interdepartmental difference in dexamethasone use is uncertain.
The overall number of complications did not differ between the departments, 22% at the GIS vs 28% at the ENT (p = 0.504). Rates referred in the literature differ significantly among studies, ranging from 4 − 31% for the endostapler treatment [Citation17,Citation32,Citation33] and 8 − 9% for the laser treatment [Citation33,Citation34]. Endostapler treatment at St. Olavs hospital falls within this range (22%), whereas the laser treatment seems to yield some more complications (28%) compared to that reported in the relevant literature, although numbers are small and confidence intervals correspondingly wide. Comorbidity indexes, such as the Charlson Comorbidity Index, include some variables inevitably incompletely recorded in a retrospective study spanning 20 years. ASA-group on the other hand, was routinely recorded by the anaesthesiologists, and has been shown to be a reliable predictor of complications and postoperative mortality for other surgical procedures [Citation35–37]. In the present study, patients in the ASA-low-group (ASA 1 and ASA 2) had overall fewer complications compared to the ASA-high-group (ASA 3 and ASA 4) when assessed for the study cohort as a whole (p = 0.01). No complications demanding any surgical interventions occurred.
Time to oral intake and hospital stay was significantly longer at the ENT compared to the GIS (p < 0.001). Although the number of perforations demanding nil per os for some days was higher for ENT patients, we believe the inequality mainly to rely on different in-house policies, based on the notion of a safer sealing of the mucosal cutting at the GIS. Leong et al. reported a 66% and 87% hospital discharge by the first and second postoperative day after uncomplicated endostapling, respectively [Citation38]. As emphasised by Crawley et al. the time to both oral intake and hospital discharge vary significantly between studies [Citation39], a testimony to a lack of much wanted standardisation.
As expected, patients operated at the Department of GIS had significantly longer diverticula than patients operated at the Department of ENT (median 35 mm vs. 20 mm), p < 0.001, with diverticulum length on par with that reported from similar studies [Citation16–18,Citation24]. No difference between the departments was found regarding symptom burden, with dysphagia and regurgitation as predominant. Technical success was lower for the endostapling technique than the laser technique, 88% vs. 100%, mainly due to non-visualized diverticulum inlet and inadequate neck extension, both shortcomings with the use of a rigid diverticuloscope used together with a straight 12 mm endostapler device.
When comparing recurrence rates, both criteria for recurrence as well as follow-up time must be considered, as witnessed by . The large majority of symptom recurrences occurred within five years postoperatively, but the latest 11 years after surgery. Rates of symptom recurrence reported differ significantly across studies. In a review from 2018, Ishaq et al. reported a recurrence rate of 12.9% for rigid endoscopy (both laser and endostapling) and 20% for flexible endoscopy [Citation6]. Recurrence rates for rigid endostapling reported in different studies are 12.8% (follow-up range 1-79 months) [Citation38], 24% (median follow up 63 months) [Citation17], 9.2% (27 months follow-up) [Citation34], and 10.0% (32 months follow-up) [Citation34]. Corresponding rates for the laser treatment are scarcely reported, but two recent meta-analyses comparing endostapling to laser treatments reported a 5% symptom recurrence rate [Citation40,Citation41]. In the present study, observed symptom recurrence rates both seem to be substantially higher, at GIS 35% and at ENT 51%. This might be contingent on a longer follow-up time and liberal criteria in defining symptom recurrence. The estimated cumulative proportion of patients with symptom recurrence, treating death as a competing event, demonstrated no difference between the departments, log-rank p = 0.154.
Treatment for ZD has changed over the years, and different flexible endoscopic techniques are now by many considered the standard of care [Citation6,Citation16,Citation42]. Although no prospective comparative studies between open surgery, rigid endoscopy and flexible endoscopy exists to show any superiority in terms of clinical outcome for one technique over the other, the European Society of Gastrointestinal Endoscopy (ESGE) Guidelines recommend flexible endoscopic techniques as the treatment for ZD [Citation43]. In accordance with this, we recently changed our policy, now using a flexible endoscopic stapling technique as described by Wilmsen et al. [Citation44].
Strengths of the present study include its population-based design, comprising all patients in Central Norway operated for ZD over a 20-year period. Median follow-up time was correspondingly long. Data on each patient was obtained individually from the electronic patient journals, and not limited to registry entries. Obvious limitations include its retrospective nature, and a lack of completeness of registration for some variables. Variables such as medication, comorbidity, demographics, and intraoperative expenditures were recorded accurately in the patient record, with a correspondingly low risk of information bias. Data on symptom improvement and symptom recurrence were often incompletely recorded, rendering the question of information bias relevant. Standardized follow-up with questioners and scoring tools would have improved the data quality of these variables, but by virtue, was not an option in the present study.
Conclusion
Crude incidence for a surgery-demanding Zenker’s diverticulum was 0.57 per 100 000 per year. Both rigid endoscopic stapled oesophago-diverticulostomy and laser diverticulostomy are safe treatments for ZD, however, sometimes with technical challenges, and with a substantial risk of recurrence. Modern guidelines advocate a flexible endoscopic approach.
Ethical approval and informed consent
The study was approved by the Data Protection Official/Officer and the institutional board at St. Olavs Hospital, who waved the requirement for individual consent, based on Norwegian legislation that consider non-interventional retrospective studies as quality assurance studies, and hence not embraced by the need for approval from the Regional Ethics committee.
| Abbreviations | ||
| ASA | = | American Society of Anesthesiologists |
| CT | = | Computerized tomography |
| ENT | = | Department of Ear, Nose and Throat Surgery |
| GIS | = | Department of Gastrointestinal Surgery |
| ZD | = | Zenker’s diverticulum |
Acknowledgements
The authors thank Sondre Erik Wennberg for English language editing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data are not publicly available due to data storage regulations. The authors will answer any question regarding the data on request.
Additional information
Funding
References
- Zenker FA. Handbuch der speciellen pathologie und therapie: handbuch der krankheiten des chylopoëtischen apparates. 1. Anhang. Krankheiten des oesophagus. Leipzig: Verlag von F. C. W. Vogel 1877.
- Ferreira L, Simmons DT, Baron TH. Zenker’s diverticula: pathophysiology, clinical presentation, and flexible endoscopic management. Dis Esophagus. 2008;21(1):1–8. doi: 10.1111/j.1442-2050.2007.00795.x.
- Scher R, Myssiorek D. Management of zenker and hypopharyngeal diverticula. Cham, Switzerland: Springer International Publishing AG; 2018.
- Ishaq S, Hassan C, Antonello A, et al. Flexible endoscopic treatment for Zenker’s diverticulum: a systematic review and meta-analysis. Gastrointest Endosc. 2016;83(6):1076–1089.e5. doi: 10.1016/j.gie.2016.01.039.
- Cook IJ, Gabb M, Panagopoulos V, et al. Pharyngeal (Zenker’s) diverticulum is a disorder of upper esophageal sphincter opening. Gastroenterology. 1992;103(4):1229–1235. doi: 10.1016/0016-5085(92)91508-2.
- Ishaq S, Sultan H, Siau K, et al. New and emerging techniques for endoscopic treatment of Zenker’s diverticulum: state-of-the-art review. Dig Endosc. 2018;30(4):449–460. doi: 10.1111/den.13035.
- Zhang LY, Wu PI-C, Szczesniak M, et al. Clinical utility of cricopharyngeal distensibility measurements during endoscopic myotomy for Zenker’s diverticulum. Gastrointest Endosc. 2021;93(2):390–397. doi: 10.1016/j.gie.2020.05.064.
- Hussain T, Maurer JT, Lang S, et al. Pathophysiologie, diagnose und therapie des Zenker-Divertikels. HNO. 2017;65(2):167–176. doi: 10.1007/s00106-016-0302-z.
- Bizzotto A, Iacopini F, Landi R, et al. Zenker’s diverticulum: exploring treatment options. Acta Otorhinolaryngologica Italica. 2013;33:219–229.
- Uoti S, Andersson SE-M, Robinson E, et al. Epidemiology and management of Zenker diverticulum in a low-threshold single-payer health care system. JAMA Otolaryngol Head Neck Surg. 2022;148(3):235–242. doi: 10.1001/jamaoto.2021.3671.
- Siddiq MA, Sood S, Strachan D. Pharyngeal pouch (Zenker’s diverticulum). Postgrad Med J. 2001;77(910):506–511. doi: 10.1136/pmj.77.910.506.
- Skrobić OM, Simić AP, Radovanović NS, et al. Current concepts in the anatomy and origin of pharyngeal diverticula. Acta Chir Iugosl. 2009;56(1):17–24. doi: 10.2298/aci0901017s.
- Law R, Katzka DA, Baron TH. Zenker’s diverticulum. Clin Gastroenterol Hepatol. 2014;12(11):1773–1782. doi: 10.1016/j.cgh.2013.09.016.
- Vogelsang A, Schumacher B, Neuhaus H. Therapy of Zenker’s diverticulum. Dtsch Arztebl Int. 2008;105(7):120–126.
- Chang CY, Payyapilli RJ, Scher RL. Endoscopic staple diverticulostomy for Zenker’s diverticulum: review of literature and experience in 159 consecutive cases. Laryngoscope. 2003;113(6):957–965. doi: 10.1097/00005537-200306000-00009.
- Al Ghamdi SS, Farha J, Moran RA, et al. Zenker’s peroral endoscopic myotomy, or flexible or rigid septotomy for Zenker’s diverticulum: a multicenter retrospective comparison. Endoscopy. 2022;54(4):345–351. doi: 10.1055/a-1518-7223.
- Bonavina L, Aiolfi A, Scolari F, et al. Long-term outcome and quality of life after transoral stapling for Zenker diverticulum. World J Gastroenterol. 2015;21(4):1167–1172. doi: 10.3748/wjg.v21.i4.1167.
- Costamagna G, Iacopini F, Bizzotto A, et al. Prognostic variables for the clinical success of flexible endoscopic septotomy of Zenker’s diverticulum. Gastrointest Endosc. 2016;83(4):765–773. doi: 10.1016/j.gie.2015.08.044.
- Hoffmann M, Fazel A, Mews K-G, et al. 32 Years of experience with CO2-LASER-assisted treatment for Zenker’s diverticulum – an update of 227 patients treated in Kiel. Clin Otolaryngol. 2017;42(3):592–596. doi: 10.1111/coa.12777.
- Li LY, Yang YT, Qu CM, et al. Endoscopic needle-knife treatment for symptomatic esophageal zenker’s diverticulum: a meta-analysis and systematic review. J Dig Dis. 2018;19(4):204–214. doi: 10.1111/1751-2980.12588.
- Parker NP, Misono S. Carbon dioxide laser versus stapler-assisted endoscopic Zenker’s diverticulotomy: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2014;150(5):750–753. doi: 10.1177/0194599814521554.
- Skaug HP, Geirdal AØ, Brøndbo K. Laser diverticulotomy for Zenker’s diverticulum—does it improve quality of life? Eur Arch Otorhinolaryngol. 2013;270(9):2485–2490. doi: 10.1007/s00405-013-2470-8.
- Zanghì S, Siboni S, Asti E, et al. Endoscopic stapling versus laser for Zenker diverticulum: a retrospective cohort study. Eur Arch Otorhinolaryngol. 2021;278(7):2625–2630. doi: 10.1007/s00405-020-06346-4.
- Budnicka A, Januszewicz W, Białek AB, et al. Peroral endoscopic myotomy in the management of zenker’s diverticulum: a retrospective multicenter study. J Clin Med. 2021;10(2):187. doi: 10.3390/jcm10020187.
- Repici A, Spadaccini M, Belletrutti PJ, et al. Peroral endoscopic septotomy for short-septum zenker’s diverticulum. Endoscopy. 2020;52(7):563–568. doi: 10.1055/a-1127-3304.
- Wagh MS, Draganov PV. How to approach a patient with a Zenker’s diverticulum. Gastroenterology. 2021;160(1):10–14. doi: 10.1053/j.gastro.2020.11.018.
- Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation. 2016;133(6):601–609. doi: 10.1161/CIRCULATIONAHA.115.017719.
- Kang H. The prevention and handling of the missing data. Korean J Anesthesiol. 2013;64(5):402–406. doi: 10.4097/kjae.2013.64.5.402.
- von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X.
- NorwegianDirectorateofHealth: Antibiotics in hospital. https://www.helsedirektoratet.no/retningslinjer/antibiotika-i-sykehus. 2013). Accessed 11. October 2021.
- Corcoran TB, Myles PS, Forbes AB, et al. Dexamethasone and surgical-site infection. N Engl J Med. 2021;384(18):1731–1741. doi: 10.1056/NEJMoa2028982.
- Nesheiwat Z, Antunes C. Zenker diverticulum. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2020, StatPearls Publishing LLC.; 2020.
- Verdonck J, Morton RP. Systematic review on treatment of Zenker’s diverticulum. Eur Arch Otorhinolaryngol. 2015;272(11):3095–3107. doi: 10.1007/s00405-014-3267-0.
- Beard K, Swanström LL. Zenker’s diverticulum: flexible versus rigid repair. J Thorac Dis. 2017;9(Suppl 2):S154–S162. doi: 10.21037/jtd.2017.03.133.
- Hackett NJ, De Oliveira GS, Jain UK, et al. ASA class is a reliable independent predictor of medical complications and mortality following surgery. Int J Surg. 2015;18:184–190. doi: 10.1016/j.ijsu.2015.04.079.
- Kay HF, Sathiyakumar V, Yoneda ZT, et al. The effects of American society of anesthesiologists physical status on length of stay and inpatient cost in the surgical treatment of isolated orthopaedic fractures. J Orthop Trauma. 2014;28(7):e153-9–e159. doi: 10.1097/01.bot.0000437568.84322.cd.
- Schoenfeld AJ, Carey PA, Cleveland AW, et al. Patient factors, comorbidities, and surgical characteristics that increase mortality and complication risk after spinal arthrodesis: a prognostic study based on 5887 patients. Spine J. 2013;13(10):1171–1179. doi: 10.1016/j.spinee.2013.02.071.
- Leong SC, Wilkie MD, Webb CJ. Endoscopic stapling of Zenker’s diverticulum: establishing national baselines for auditing clinical outcomes in the United Kingdom. Eur Arch Otorhinolaryngol. 2012;269(8):1877–1884. doi: 10.1007/s00405-012-1945-3.
- Crawley B, Dehom S, Tamares S, et al. Adverse events after rigid and flexible endoscopic repair of zenker’s diverticula: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2019;161(3):388–400. doi: 10.1177/0194599819839991.
- Bhatt NK, Mendoza J, Kallogjeri D, et al. Comparison of surgical treatments for Zenker diverticulum: a systematic review and network meta-analysis. JAMA Otolaryngol Head Neck Surg. 2021;147(2):190–196. doi: 10.1001/jamaoto.2020.4091.
- Howell RJ, Giliberto JP, Harmon J, et al. Open versus endoscopic surgery of Zenker’s diverticula: a systematic review and meta-analysis. Dysphagia. 2019;34(6):930–938. doi: 10.1007/s00455-019-09994-9.
- Gölder SK, Brückner J, Ebigbo A, et al. Differences in endoscopic techniques for symptomatic Zenker’s diverticulum. Endoscopy. 2018;50(2):183–184. doi: 10.1055/s-0043-123936.
- Weusten B, Barret M, Bredenoord AJ, et al. Endoscopic management of gastrointestinal motility disorders - part 2: European society of gastrointestinal endoscopy (ESGE) guideline. Endoscopy. 2020;52(7):600–614. doi: 10.1055/a-1171-3174.
- Wilmsen J, Baumbach R, Stüker D, et al. New flexible endoscopic controlled stapler technique for the treatment of Zenker’s diverticulum: a case series. World J Gastroenterol. 2017;23(17):3084–3091. doi: 10.3748/wjg.v23.i17.3084.
