Abstract
Background
Anal squamous intraepithelial lesions (ASILs) correspond to premalignant changes preceding the development of anal squamous cell carcinoma.
Objective
To describe a new endoscopic technique to detect and remove ASILs in non-anesthetized patients and compare it with standard surgical treatment.
Methods
For endoscopic treatment, high resolution (HR) flexible endoscopes with a distal attachment were used. Underwater inspection of the anal canal was performed in near-focus mode with white light and narrow-band imaging. Detected lesions were resected with a diathermia snare after local injection of xylocaine/adrenaline. We did a retrospective comparison of all patients who underwent endoscopic or standard surgical treatment for ASILs at Ersta hospital in Stockholm between 2018 and 2020. Patient files were reviewed for number of lesions, treatments until macroscopic radicality, degree of dysplasia, bleeding, pain and other complications.
Results
Endoscopic (n = 37) and surgical (n = 43) treatment displayed comparable number of lesions per patient (p = .37). The number of procedures until macroscopic radicality was higher for endoscopy than surgery (p = .04). However, in endoscopic follow up of 12 of the surgically treated patients, residual ASIL was found in 10 cases. Post-procedural bleeding requiring healthcare occurred in two endoscopy patients and one surgically treated patient.
Conclusions
Underwater resection using a HR flexible endoscope in non-anesthetized is a new, feasible and well tolerated method for ASILs treatment. Its efficacy and risk of complications seem comparable to standard surgical treatment while avoiding general anesthesia. However, minor lesions might be overlooked at surgery.
Introduction
The incidence of anal squamous cell carcinoma (ASCC) is low in the general population; however, an increased risk is found in individuals with a high number of sexual partners, human immunodeficiency virus infection or previous human papilloma virus-driven anogenital dysplasia, and in men who have sex with men [Citation1].
ASCC is preceded by a premalignant lesion called anal squamous intraepithelial lesion (ASIL), formerly designated as anal intraepithelial neoplasia (AIN). Depending on the grade of dysplasia, ASIL is classified into low-grade squamous intraepithelial lesion (LSIL), previously designated as AIN 1 and high-grade squamous intraepithelial lesion (HSIL), previously designated as AIN 2–3 where LSIL represents low grade dysplasia and HSIL represents high grade dysplasia [Citation2].
Prospective studies suggest that 9–13% of patients with HSIL progress to cancer within 5 years, with highest risk of progression among HIV-positive individuals and immunocompromised [Citation3,Citation4].
Currently, screening programs for ASCC are uncommon. High risk individuals may be hesitant to undergo regular examinations often experiencing fear and shame [Citation5]. Examinations may be cumbersome and even painful if performed inadequately. Detailed examination of the anal canal during colonoscopy is not a routine practice and has only been described in few centers [Citation6] and most procedures are carried out using rigid rectoscopy or anoscopy. In a limited number of clinics, colposcopes with an 8–25 times magnification are used to perform high resolution (HR) anoscopy [Citation7].
At present, the standard treatment modalities for ASIL are surgery or targeted ablation in anesthetized patients, with risk of relapse and local side effects. Stenosis or incontinence may also occur [Citation1].
At our hospital (a tertiary care center for patients with ASIL in the Stockholm region), we have developed a new method using underwater HR flexible endoscopy with a distal attachment to examine the anal canal in detail and endoscopically treat ASIL under local anesthesia, with mild sedation as needed.
In this article, we describe our endoscopic method and compare it with the standard surgical treatment performed at our institution.
Methods
Underwater inspection
Patients scheduled for a diagnostic procedure do not undergo any bowel preparation. For patients scheduled for therapeutic endoscopy, bowel preparation includes liquid meals the day before examination and 1 liter of polyethylene glycol (PEG) solution (Movprep, Norgine, Amsterdam, Netherlands) on the morning of the procedure.
Olympus instruments EVIS EXERA GIF-HQ190 or GIF-EZ1500 with Dual focus function (Olympus Medical System, Shinjuku, Japan) with an outer diameter of 9.9 and 9.6 mm, respectively, were used. Dual focus enables observation in normal mode and in near-focus mode that provides higher magnification. A distal attachment with an outer diameter of 11.35 mm (Reveal Distal Cap, Steris, Galway, Ireland) was secured leaving approximately 2 mm of free cap at the tip to maintain a suitable distance between the instrument and the mucosa. The CO2 was switched off.
Examinations were performed with the patient in the left lateral position. Low doses of Midazolam (Panpharma Nordic AS, Ski, Norway) and/or alfentanil (Piramal Critical Care B.V., Voorschoten, Netherlands) were given intravenously as needed or upon patient request. Both, the anal canal and the instrument tip were lubricated with xylocaine gel (lidocaine, AstraZeneca, Stockholm, Sweden, London, UK).
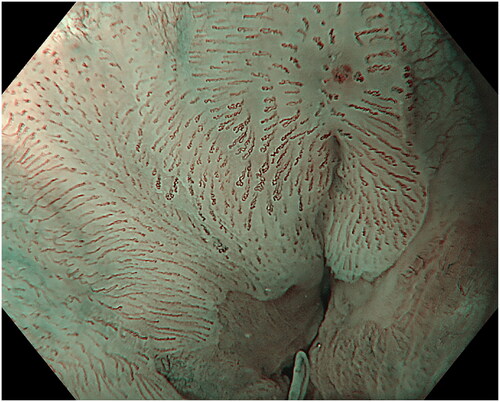
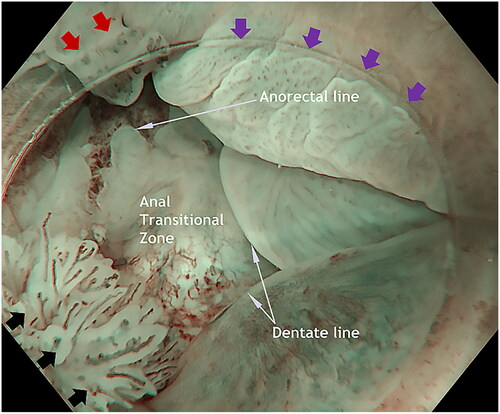
In patients scheduled for a diagnostic procedure, careful observation in near-focus mode – using both white light imaging (WLI) and narrow-band imaging (NBI) was performed while introducing the endoscope with circular movements and flushing with water as needed to keep the anal canal open until reaching the anorectal line (). Both the water flushing and the slow circumferential movements cause the folds in the anal canal to flatten out. Special attention was given to the transitional zone, a common location for ASIL (). The last part of the investigation of the anal canal is intubation of distal rectum and investigating the anorectal line and the proximal anal canal from above with a retroflected instrument. Careful inspection of the perianal tissue is done at the end of the examinations. In case of perianal lesions, the patient is referred to surgery.
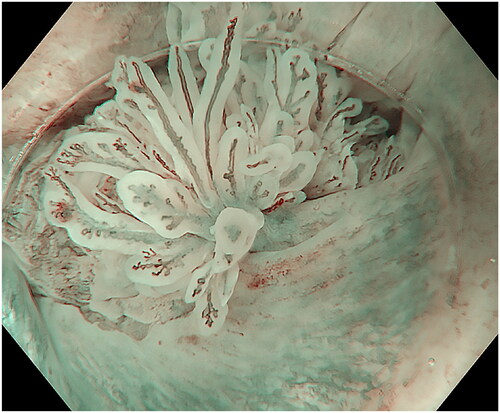
Figure 1. Landmarks in the anal canal seen under water: anorectal line, dentate line and the area between, the anal transitional zone. Purple arrowheads show a flat, slightly raised ASIL (anal squamous intraepithelial lesions) with widened and irregular IPCL (intra papillary capillary loops). Black arrowheads show an exophytic villous ASIL and the red arrowheads show an exophytic non-villous ASIL.
In patients scheduled for a therapeutic procedure, the endoscope was introduced into the anal canal and further up into the rectum. After removing liquid and air, inspection was performed as described above. In some cases, inspection with an inverted instrument can be helpful.
Underwater resection
Small amounts of lidocaine 1% with adrenaline 1:200,000 (Aspen Nordic, Ballerup, Denmark) were intermittently injected using a 4 mm long, 23-gauge injection therapy needle catheter (Boston-Scientific, Marlborough, MA) to create a submucosal cushion under the lesion also providing local anesthesia (). The first injection was applied at the rectal side above the anorectal line, since injections are generally painless there. Consecutive injections within the cushion’s edge were placed distally surpassing the anorectal line, finally creating a large cushion so that all squamous epithelium surrounding the lesion was anesthetized.
Figure 2. Creating a submucosal cushion under the lesion and also providing local anesthesia. Proximal and on the left side of the lesion is the mucosa paler after the previous injection. At 11 o’clock is an exophytic whitish non-villous exophytic ASIL.
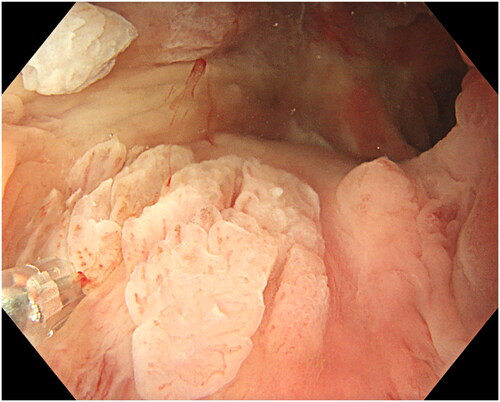
Due to the narrow space in anal canal, resection was performed using a 10 mm polypectomy snare with a 0.3 mm braided wire (SnareMaster Plus, Olympus, Tokyo, Japan) and an electrosurgical system (Erbe VIO 300D Electrosurgery Unit, Tübingen, Germany) with Endo Cut Q 3 and forced COAG 3 settings. The snare was placed around the lesion, slight pressure applied and the snare was slowly closed until tightened (). Slight suction while tightening helped to gain a better grip on the lesion. When closed and tightened, the snare was lifted to minimize the risk of deep coagulation and to avoid contact with the non-anesthetized surrounding mucosa and then the lesion is resected (). Piecemeal resection was performed for larger lesions until macroscopic radicality was achieved. When no visible lesion remains, we regarded it as a radical resection. In case of unclear resection, the edges were coagulated with the top of the snare as well as small visible or bleeding vessels. In the case of multifocal ASIL covering almost the entire circumference, resection of 40–50% was carried out during the first session. Exposed hemorrhoids were left untreated unless bleeding occurred usually from small branches in which case coagulation was performed using the tip of the snare.
Figure 3. Placing the snare with a slight pressure. At the tip of the casing is a small non-villous exophytic ASIL.
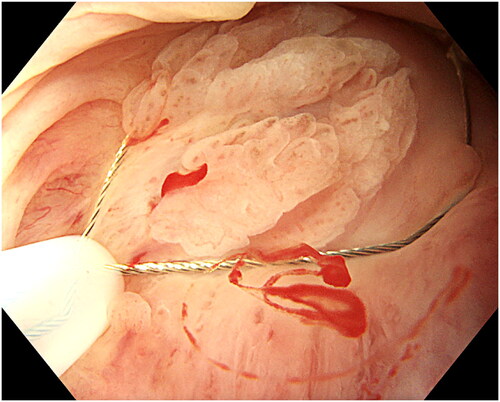
Figure 4. Resection of the lesion. The lesion is retrieved and sent for histopathology. Minor bleeding can be coagulated with the top of the snare. In case of uncertainty about radical resection, the edges can also be coagulated.
When discharged, patients were provided with xylocaine gel (lidocaine, AstraZeneca, Stockholm, Sweden, London, UK) to apply locally as needed and informed to use acetaminophen if experiencing mild pain. Laxatives were only given to those with known constipation. No special diet was required.
Patients with LSIL and single lesions were re-examined after 6 months, while those with HSIL or with multifocal lesions were scheduled for follow-up after 3 months. Those requiring repeated treatments were scheduled every 3rd month until all lesions were removed.
Surgical examination and treatment
Patients were evaluated in the lithotomy position under general anesthesia as outpatient procedures. The perianal skin was examined and suspicious areas of dysplastic skin were excised with a knife for examination (to facilitate microscopic evaluation of dysplasia). After resection, hemostasis was achieved using diathermy. Using a speculum or proctoscopy, the anal canal was examined for dysplastic mucosa or condylomas, and if found, these were resected with a knife and/or diathermy for microscopic examination. Mapping biopsies of skin or mucosa were taken on demand. Postoperatively, patients were given pain killers, laxatives and local anesthetic ointments. Some patients with extensive perianal HSIL underwent repeated perianal resections allowing sufficient time for perianal skin to heal in between procedures. Patients with no signs of remaining HSIL were monitored with clinical examination and proctoscopy every 6–12 months for 1–3 years depending on risk factors to develop anal cancer such as HIV, men who have sex with men and immunosuppression as well as the extent of disease.
Pathology
Pathologic findings were classified as AIN 1–3 and LSIL or HSIL [Citation2].
Retrospective comparison between endoscopic and surgical treatment
All patients who underwent endoscopic or surgical treatment for ASIL at Ersta hospital between 2018 and 2020 were included. Medical files were reviewed and the following data were extracted: gender, age, type of treatment (endoscopic or surgical), if the lesions were multifocal or not, degree of dysplasia, location: transitional epithelium (proximal to dentate line), squamous epithelium in the anal canal, or in the perianal area, follow up including number of procedures until macroscopic radicality, post-procedural bleeding, incontinence and anal stenosis.
The study has been approved by the Swedish Ethics Review Board (D.nr. 2020-06915).
Statistics
Data are expressed as numbers and percent or mean and SD, as appropriate.
Fisher’s exact test was used for statistical comparison between endoscopy and surgery regarding grade of dysplasia (non-dysplastic or LSIL versus HSIL or cancer), focality (focal versus multifocal) and post-procedural bleeding. Statistical analysis was carried out with GraphPad Prism, version 9 (GraphPad Software, San Diego, CA).
Results
A total of 80 patients were included, 37 undergoing endoscopic resection and 43 surgical resections ().
Table 1. Comparison between endoscopic and surgical treatment.
Endoscopic detection and resection
At endoscopy, nine of the 37 patients (24%) received either intravenously midazolam (max dose 2 mg) and/or alfentanil (max dose 0.75 mg). One patient required light sedation with propofol. The remaining 27 of 37 patients (73%) received no medication.
Underwater inspection
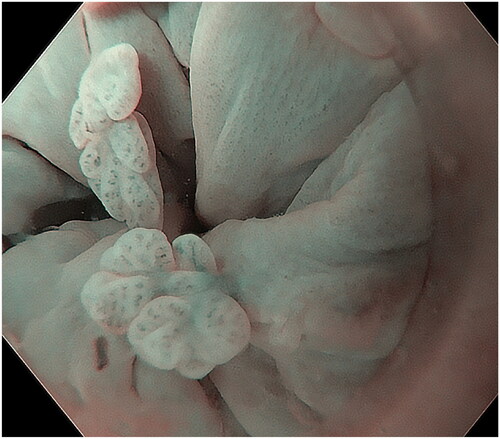
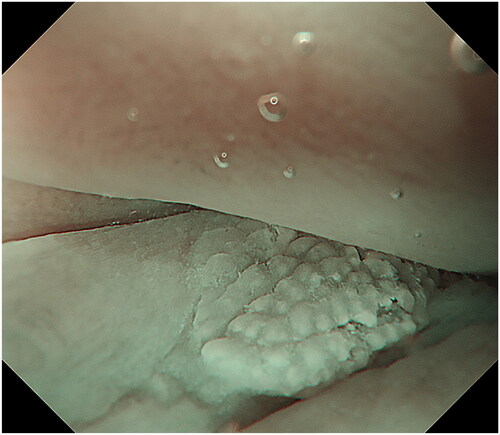
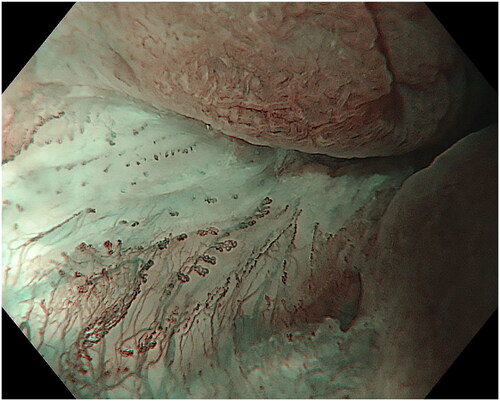
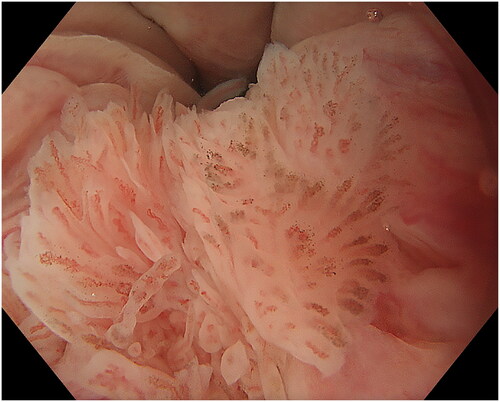
The endoscopic appearance of ASIL varies. The most common morphologies were villous exophytic with branchlike blood vessels (), non-villous exophytic (), lesions with nodular-granular configuration () and slightly raised lesions with and without intrapapillary capillary loops (IPCLs) (). We have also seen lesions with only irregular and widened IPCL (). Sometimes, a mix of different morphologies was found. In a few lesions, we found brownish epithelium and brownish dots (). The size of ASIL varies from 0.5 mm to 10 mm, few lesions are larger and extend from the proximal to the distal part of the anal canal. The color was often the same as surrounding mucosa but some lesions are paler.
Comparison between endoscopic and surgery resection
The proportion of patients with multifocal lesions (p = .37), high-grade dysplasia/cancer (p = .82) and complications was equal in the groups (). More endoscopic treatments than surgical interventions were required before macroscopic radicality occurred. Endoscopic treatment required 1–7 procedures, median 2 and surgical treatment 1–4 procedures, median 1 (). Five patients from the endoscopic cohort did not achieve macroscopic radicality at control examinations. One from the surgery cohort had recurrent HSIL in perianal area.
In 12 of the patients who underwent surgical treatment, we performed HR flexible endoscopy. These examinations were done within 11 months after the patients were classified as radically treated at surgery. In 10 of the 12 patients, ASIL was found at endoscopy.
Complications
Three patients in each group had minor post-procedural bleeding that stopped spontaneously without intervention 1–4 days after the procedures ().
Post-procedural bleeding requiring healthcare occurred in two endoscopy patients and one surgically treated patient. One endoscopically treated patient was admitted to another hospital only receiving blood transfusion. The remaining two underwent either acute endoscopy or surgery.
No patient experienced incontinence or anal stenosis after endoscopic or surgical treatments.
Discussion
This is the first published report of a case series of underwater EMR for ASIL in the anal canal in non-anesthetized patients, currently also compared to patients undergoing surgery and intra-anal mucosal/perianal skin resection in general anesthesia as being the available reference method.
Similar endoscopic treatment of ASIL and early squamous cell carcinoma has previously been described in a small number of case reports using ESD [Citation8–15], or underwater EMR of ASIL [Citation16–18]. We are not aware of any larger studies/case series of endoscopic treatment of ASIL or any pervasive description of underwater EMR of ASIL. ESD is a time-consuming method and also a difficult technique to learn and master [Citation19]. The anal canal is narrow, why EMR may be a more readily accessible method to treat ASIL.
We currently demonstrate that underwater EMR of ASIL with HR flexible endoscopes in non-anesthetized patients is a feasible and well tolerated method. Its efficacy and risk of complications seem comparable to standard surgical treatment while avoiding general anesthesia.
Underwater inspection, while providing magnification, makes lesions more accessible for resection as they float in the liquid [Citation20–22]. Treatment of ASIL during flexible endoscopy using hot biopsy for small lesions and ablation with a Gold Probe for larger lesions has been described [Citation6]. Endoscopic submucosal dissection (ESD) for ASIL treatment has also sporadically been reported [Citation8]. Although multifocal lesions were frequently found in our patients, we did not find that ESD was indicated.
Recently, a randomized study in HIV positive patients with HSIL demonstrated that office-based treatment of HSIL (n = 2237) with targeted ablation was associated with significantly less progression to invasive anal cancer compared to only active monitoring (n = 2222), indicating the importance to effectively diagnose and treat anal dysplasia in this group of patients [Citation23].
Using endoscopy, we show that lesions with ASIL often are multifocal with variable characteristics. As in other squamous epithelia, IPCL can be visualized. If we compare the IPCL pattern in the anal canal with the modified classification for esophagus [Citation24], we often find type III and type IV. In addition, findings of brownish epithelium and brownish dots seem to have significance in high-grade dysplasia of the esophagus [Citation25]. We are now performing a prospective study on endoscopy in which the macroscopic findings of morphology, microvascular appearance and location in the anal canal are compared with histopathology to evaluate whether the macroscopic findings can predict dysplasia grade in ASIL.
The two post-procedural bleeding episodes in endoscopic patients occurred at the beginning of the study period, when the method was introduced and since then no post-procedural bleeding has occurred, suggesting a rather short learning curve.
Some lesions are difficult to handle endoscopically. HR flexible endoscopy works very well in the proximal and middle parts of the anal canal, but in the most distal part of the anal canal, it is difficult to obtain a stable position, and these lesions are often better handled surgically. Furthermore, we have not treated perianal changes with endoscopy. If perianal lesions are detected during endoscopy, the patient is referred for surgery.
The high number of lesions found at endoscopy after the patients were classified as radically treated at surgery suggests that patients undergoing surgical treatment of ASIL always should be monitored with endoscopy, which now is routine in our hospital.
After resection of ASIL, it is sometimes difficult at follow-up to know if lesions represent a recurrence of ASIL or if there are new lesions. If the lesion is adjacent to or in a scar, we can suspect that it is a recurrence.
If ASIL is left for the next treatment, this should be described, as well as how the lesions look and if possible, the number of lesions or percentage of circumference remaining to be treated.
Limitations in this study include the retrospective single-center design. No systematic patient selection or randomization was applied to allocate patients to endoscopic or surgical treatment, but the lack of differences in age and sex suggests that the two groups are comparable.
Conclusions
Standard endoscopic EMR or ESD would be difficult in the narrow anal canal. We utilized underwater endoscopy to secure the field of vision in the narrow space. ASILs often recur and need repeat treatment; therefore, less-invasiveness is important. Thus, endoscopic underwater treatment being less invasive seems favorable compared to the standard surgical treatment. Slightly more endoscopic treatments than surgical interventions were required before macroscopic radicality occurred. However, in some cases where the patients were classified as macroscopic radical at surgery, ASIL was found at endoscopic follow up.
HR flexible endoscope is widely available in contrast to HR anoscopy. With our method, we can detect and resect ASIL with high accuracy and control the radicality after resection. Furthermore, snare-resection provides larger samples of appropriate size and depth for better histopathological analysis.
In conclusion, underwater endoscopic mucosal resection with HR flexible endoscopy is a feasible technique for the treatment of ASIL in non-anesthetized patients.
| Abbreviations | ||
| ASCC: | = | Anal Squamous Cell Carcinoma |
| AIN: | = | Anal Intraepithelial Neoplasia |
| ASIL: | = | Anal Squamous Intraepithelial Lesion |
| EMR: | = | Endoscopic Mucosa Resection |
| ESD: | = | Endoscopic Submucosal dissection |
| HR: | = | High Resolution |
| HSIL: | = | High-grade Squamous Intraepithelial Lesion |
| IPCL: | = | Intra Papillary Capillary Loops |
| LSIL: | = | Low-grade Squamous Intraepithelial Lesion |
| NBI: | = | Narrow Band Imaging |
| PEG: | = | Polyethlene glycol |
| WLI: | = | White Light Imaging |
Disclosure statement
Peter Borch-Johnsen is a paid lecturer for Olympus Sweden. Peter T. Schmidt has served as Advisory Board member for Gilead Nordic, Janssen Cilag and Norgine. Jonas Nygren has no conflict of interest.
Additional information
Funding
References
- Chittleborough T, Tapper R, Eglinton T, et al. Anal squamous intraepithelial lesions: an update and proposed management algorithm. Tech Coloproctol. 2020;24(2):95–103. doi: 10.1007/s10151-019-02133-4.
- Darragh TM, Colgan TJ, Cox JT, et al. The lower anogenital squamous terminology standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch Pathol Lab Med. 2012;136(10):1266–1297. doi: 10.5858/arpa.LGT200570.
- Watson AJ, Smith BB, Whitehead MR, et al. Malignant progression of anal intra-epithelial neoplasia. ANZ J Surg. 2006;76(8):715–717. doi: 10.1111/j.1445-2197.2006.03837.x.
- Scholefield JH, Castle MT, Watson NF. Malignant transformation of high-grade anal intraepithelial neoplasia. Br J Surg. 2005;92(9):1133–1136. doi: 10.1002/bjs.4994.
- Koskan AM, LeBlanc N, Rosa-Cunha I. Exploring the perceptions of anal cancer screening and behaviours among gay and bisexual men infected with HIV. Cancer Control. 2016;23(1):52–58. doi: 10.1177/107327481602300109.
- Inkster MD, Wu JS. Detection of anal dysplasia by chromoendoscopy with narrow band imaging and acetic acid (NBIA) in 182 patients. Clin Surg. 2017;2:1583.
- Cho SD, Groves E, Lao VV. History of high-resolution anoscopy. Clin Colon Rectal Surg. 2018;31(6):336–346. doi: 10.1055/s-0038-1668103.
- Tamaru Y, Oka S, Tanaka S, et al. Early squamous cell carcinoma of the anal canal resected by endoscopic submucosal dissection. Case Rep Gastroenterol. 2015;9(1):120–125. doi: 10.1159/000382074.
- Tsuji S, Doyama H, Yamada S, et al. Endoscopic submucosal dissection of a squamous cell carcinoma in situ in the anal canal diagnosed by magnifying endoscopy with narrow-band imaging. Clin J Gastroenterol. 2014;7(3):233–237. doi: 10.1007/s12328-014-0481-7.
- Uozumi T, Sumiyoshi T, Kondo H, et al. Endoscopic submucosal dissection for early squamous cell carcinoma in the anal canal and Lugol chromoendoscopy for assessment of the lateral margin. Endosc Int Open. 2018;6(9):E1130–E1133. doi: 10.1055/a-0584-7060.
- Chou YP, Saito Y, Matsuda T, et al. Novel diagnostic methods for early-stage squamous cell carcinoma of the anal canal successfully resected by endoscopic submucosal dissection. Endoscopy. 2009;41(Suppl. 2):E283–E285. doi: 10.1055/s-0029-1214942.
- Kasuga K, Saito Y, Wu SY, et al. Impact of endoscopic submucosal dissection of an anal squamous intraepithelial lesion with indistinct border. Endoscopy. 2020;52(2):E75–E77. doi: 10.1055/a-0977-2446.
- Ozawa SI, Yasuda H, Sato Y, et al. A case of depressed-type (O-IIc) squamous cell carcinoma in situ at the anal canal resected by endoscopic submucosal dissection as excisional biopsy. Gastroenterol Endosc. 2015;57:2537–2542.
- Sasaki A, Nakajima T, Egashira H, et al. Condyloma acuminatum of the anal canal, treated with endoscopic submucosal dissection. World J Gastroenterol. 2016;22(8):2636–2641. doi: 10.3748/wjg.v22.i8.2636.
- Lajin M, Othman MO, Kamyar R, et al. Endoscopic submucosal dissection to treat squamous cell carcinoma in situ of the anal canal. VideoGIE. 2022;7(6):235–239. doi: 10.1016/j.vgie.2022.02.011.
- Hirai M, Yamasaki Y, Harada K, et al. Underwater endoscopic mucosal resection for anal canal neoplasia in a patient with human immunodeficiency virus infection. Ann Gastroenterol. 2018;31:522.
- Girotra M, Friedland S. Underwater endoscopic mucosal resection of anal condyloma. VideoGIE. 2018;14(3):123–124.
- Hamada K, Uedo N, Tomita Y, et al. Underwater endoscopic mucosal resection of a condyloma acuminatum of the anal canal. Ann Gastroenterol. 2017;30:128.
- Barbour JA, O'Toole P, Suzuki N, et al. Learning endoscopic submucosal dissection in the UK: barriers, solutions and pathways for training. Frontline Gastroenterol. 2021;12(7):671–676. doi: 10.1136/flgastro-2020-101526.
- Binmoeller KF, Weilert F, Shah J, et al. “Underwater” EMR without submucosal injection for large sessile colorectal polyps (with video). Gastrointest Endosc. 2012;75(5):1086–1091. doi: 10.1016/j.gie.2011.12.022.
- Ross HE, Nawaz S. Why do objects appear enlarged under water? Arq Bras Oftalmol. 2003;66(5):69–76. doi: 10.1590/S0004-27492003000600009.
- Chaves DM, Brito HP, Chaves LT, et al. Underwater endoscopic mucosal resection of serrated adenomas. Clinics. 2018;73:E339. doi: 10.6061/clinics/2018/e339.
- Palefsky JM, Lee JY, Jay N, et al. Treatment of anal high-grade squamous intraepithelial lesions to prevent anal cancer. N Engl J Med. 2022;386(24):2273–2282. doi: 10.1056/NEJMoa2201048.
- Inoue H, Makoto K, Haruo I, et al. Magnification endoscopy in esophageal squamous cell carcinoma: a review of the intrapapillary capillary loop classification. Ann Gastroenterol. 2015;28:41–48.
- Ishihara R, Inoue T, Uedo N, et al. Significance of each narrow-band imaging finding in diagnosing squamous mucosal high-grade neoplasia of the esophagus. J Gastroenterol Hepatol. 2010;25(8):1410–1415. doi: 10.1111/j.1440-1746.2010.06378.x.