Abstract
Introduction
Since the number of medical treatment options for Ulcerative Colitis (UC) has expanded over the last decades, patients and physicians face challenges regarding decisions about the medication options. We aimed to identify patients’ preferences about their UC treatment options in the Netherlands. Furthermore, we assessed after how many failed treatment options, patients are willing to consider surgical treatment.
Methods
We conducted a web-based, multicenter, discrete choice experiment (DCE) among adult UC patients. Patients were repeatedly asked to choose between two hypothetical medicinal treatment options. The choice tasks were based on administration route, administration location, chance of symptom reduction (on short and long term) and chances on infection and other adverse events. Data were analyzed by using Hierarchical Bayes estimation.
Results
A total of 172 UC patients participated in the DCE. More than half were anti-TNF experienced (52.9%). The chance of symptom reduction after one year (relative importance (RI) 27.7 (95% CI 26.0–29.4)) was most important in choosing between medicinal treatments, followed by the chance of infection (RI 22.3 (21.4 − 23.3)) and chance of symptom reduction after eight weeks (RI 19.5 (18.3 − 20.6)). Considering surgical treatment, nineteen patients (14.3%) would not even consider surgery after failing eight treatment options without any new available therapies left. Nine patients would consider surgery before trying any treatment options.
Conclusion
We found that symptom reduction after one year was the most important attribute in choosing between treatments in UC patients. These outcomes can help understand the trade-offs and preferences of UC patients.
Key summary
Summarize the established knowledge on this subject
Since the number of medical treatment options for Ulcerative Colitis (UC) has expanded over the last decades, patients and physicians face challenges regarding decisions about the medication options.
What are the significant and/or new findings of this study?
Patients with UC preferred symptom reduction after one year over other treatment attributes such as route of administration and chance of adverse events when choosing between treatment options.
Anti-TNF and/or small molecule naïve patients found the route of administration more important compared to experienced patients.
Introduction
Ulcerative colitis (UC) is a chronic inflammation of the colon mucosa and is primarily treated with medication with the aim of achieving and maintaining clinical and endoscopic remission [Citation1]. In patients with mild and moderate disease, treatment guidelines are clear. In patients with persistence of inflammation, or with severe disease, patients and caregivers face choice options in medication, since the number of UC treatments has expanded over the last decades with biologic therapies and JAK inhibitors. Choosing the right treatment option is challenging as physicians and patients have to balance trade-offs between efficacy, potential risk of adverse events, infections or malignancies, and other considerations such as route of administration. The efficacy of biologic therapies and small molecules are all within the same range and there is a lack of head-to-head trials [Citation2, Citation3]. While awaiting prognostic biomarkers to predict which therapy will be effective in which patients, other trade-offs play an increasing role when initiating a new therapy. Although the efficacy rates are similar, pharmacological treatment options substantial differ in mechanisms of action, mode and frequency of administration, side effects and other risks.
In shared decision making, treating physicians work with patients and help them understand the trade-offs and their own preferences. When prescribing a treatment option that meets the preferences of the patient, it may improve treatment adherence, quality of life, treatment outcomes and lower healthcare costs [Citation4–6]. Also, studies suggest that patients who participate more actively in their care are more satisfied with medical services provided and may have better health outcomes [Citation7, Citation8]. Therefore, discrete choice experiments (DCE) gained more attention. A DCE is a quantitative method that can measure patients’ preferences on healthcare products and programs, but can also assess trade-offs, willingness to participate and help understanding clinical decisionmaking [Citation9–12].
No DCEs’ were performed since therapy options for UC patients has expanded in everyday clinical care with interleukin (IL)-inhibitors, sfingosine1phosfate (s1p)-modulators and JAK inhibitors. We aimed to identify patients preferences using a discrete choice experiment (DCE) about the relative risks and benefits of currently available UC treatment options in patients with UC in the Netherlands. Furthermore, we assessed after how many failed treatment options, patients are willing to consider surgical treatment.
Methods
Study design
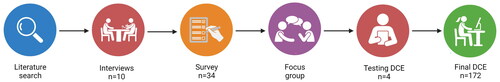
We conducted a cross-sectional, web-based, DCE survey in the Netherlands to gain insight in treatment preferences of UC patients. This method relies on the random utility theory, which posits that individuals generally choose what they prefer and value an intervention based on its attributes (e.g., route of administration) and subsequent levels of the attributes (e.g., oral, subcutaneous or intravenous). Individuals are expected to prefer the intervention with the highest relative value when offered multiple choices. The attributes and levels are processed in choice tasks, in which each choice consists of different hypothetical attribute-level combinations. For the DCE, we wanted to finish with a maximum of six attributes for the feasibility of the experiment. These six attributes were based on consecutively a literature search, patient interviews, surveys with UC patients and a focus group (see ). The DCE was presented as an online questionnaire. The International Society for Pharmacoeconomics Outcomes Research (ISPOR) best practice guidelines for DCE development and analyses were followed [Citation13].
Development of the six attributes and associated levels
The first step, the literature search, was performed with help of a librarian (JWS) of the LUMC, the Netherlands. We performed two searches in Pubmed.gov containing the following terms: Inflammatory Bowel Diseases, Drug Therapy, Patient Preference and Discrete choice experiment. The literature search used is displayed in Supplementary 1. The conducted list of 17 attributes based on the literature search is shown in Supplementary 2. The second step were the patient interviews. First, this list with 17 attributes was shown to 10 UC patients at the outpatient clinic or via video call (recruited via the newsletter of the Dutch patient association Crohn Colitis) to assess whether there were any missing attributes. No new attributed were mentioned. Then, we asked 34 patients (via the newsletter of the Dutch patient association Crohn Colitis) with UC to rank and rate these 17 attributes in a survey. This led to a list of attributes displayed in supplementary 3. Based on the multiply of the mean and the amount of votes (supplementary 3), a focus group consisting of two gastroenterologist (AEvdM and MD) and a methodologist (MEvdA), discussed the attributes to be included in the DCE. Three attributes, ‘Influence on fertility, pregnancy and lactation’ were excluded since these were probably only of interest for fertile patients. Also, the attribute ‘duration until remission’ was excluded since this was overlapping with the attribute ‘chance of clinical remission after eight weeks of treatment’. This led to six final attributes (bold in supplementary 3). Associated levels were based on literature (registration trials and meta-analysis of current available biologic therapies and JAK inhibitors for UC) [Citation3]. All included attributes and levels are displayed in Supplementary Table 1 Finally, the developed questionnaire (see below) was pretested during four verbal interviews with four patients with UC recruited at the outpatient clinic, by asking them to think out loud when filling in the questionnaire. This told us which questions were misunderstood or not clear. In this way, we could improve the layout and phrasing of the DCE and obtain an indication of the feasibility of the questionnaire
Structure questionnaire
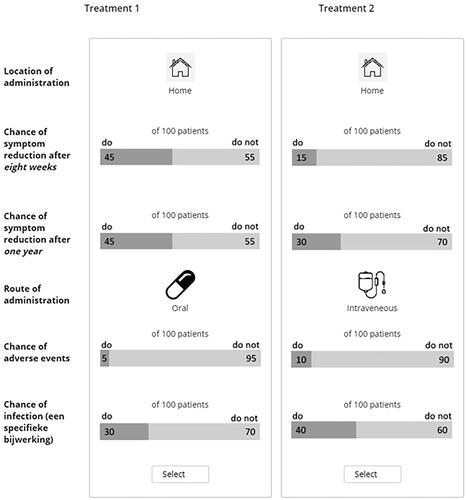
The six attributes were processed in a questionnaire with 15 choice sets. Each choice set represented two hypothetical treatment options with varying combinations of attribute levels (see for an example of a choice set). A balanced overlap design was used. One of the 15 tasks consisted of a fixed question with one less favorable option which is not expected to be chosen. For each choice set, respondents were asked to indicate their preferred treatment option.
The 15 choice sets were preceded by detailed information on the six attributes and attribute levels and included a clearly explained example of a choice set containing two treatment options consisting of only two different attributes. The overall questionnaire started with general questions regarding the respondent’s diagnosis and current and past treatment of UC (self-reported). At last, patients were asked whether they would be willing to receive surgical treatment after failing a certain number of treatment options. This question was repeated with different number of failed treatment options depending on the respondents previous answer.
The primary outcome was the importance of the attributes when choosing between treatments of ulcerative colitis. Secondary outcomes included preferred admission route, preferred location of administration and differences in preferences between patients who are anti-TNF and/or small molecule experienced and patients who are not. Also, we assessed after how many medical treatment options patients are willing to undergo surgical treatment.
For developing the internet survey and data collection, we used Sawtooth Software’s SSI Web (Sawtooth Software. Orem, UT, USA).
Study population
Patients were recruited from September 2022 till October 2022. The link to the DCE and a QR code was spread from September 2022 till October 2022 in two ways: 1. via the newsletter and social media channels of the Dutch patient association Crohn Colitis and 2. via flyers and posters at the outpatient clinics of three academic centers. Inclusion criteria for the DCE were adult (age ≥ 18 years) patients with UC. Patients who did not complete the DCE questions were excluded.
Statistical methods
Data are presented as mean ± standard deviation or median with interquartile range (IQR) for normally distributed or skewed data, respectively. Hierarchical Bayes estimation was used to calculate the relative importance (RI) of each attribute for each respondent, according to the choices made in the conjoint tasks (using the maximum difference in the average overall utility between levels of the different attributes). The relative importance was averaged over all respondents, to assess which attributes on average are most important for patients in choosing their UC treatment. Average utilities display how much a level of an attribute is preferred over the substitutes. The larger the difference between the levels, the higher the relative importance of a particular attribute. To assess whether biologic or JAK inhibitor experienced patients, assign a greater importance to specific attributes compared to non-biologic or JAK inhibitor experienced patients, the relative importance was compared between these groups. These comparisons were made using an independent t-test.
Sample size calculation for DCE depend on the weight of preferences, which are unknown upfront [Citation13, Citation14]. A rule of thumb is to include over 100 patients to provide preference data [Citation15].
All data were analyzed using the Sawtooth Software SSI Web (Sawtooth Software. Orem, UT, USA) and the statistical package SPSS for Windows 28.0 (SPSS Inc, Chicago, IL, USA). p value < 0.05 was considered statistically significant.
Ethical consideration
The protocol of ICC Registry was reviewed and approved by the Committee on Research Involving Human Subjects at the LUMC, Leiden, The Netherlands (institutional review board: N21.162).
Results
Baseline characteristics
A total of 277 patients opened the survey of which 172 patients participated in the DCE. Baseline characteristics are displayed in Most respondents were female (77.3%) and median age was 39 years (IQR 29-54). Median disease duration was 8 years (IQR 3-16) and most patients were biologic or small molecule experienced (52.9%). Almost all patients were recruited via CCNL (96.5%). Five patients were recruited in the LUMC and one patient in the Amsterdam UMC.
Table 1. Baseline characteristics.
Attributes coefficients and relative importance
Main analysis
shows the main results of the DCE, including the coefficients and relative importance in the main model. Symptom reduction after one year (RI 27.68 (95% Confidence Interval (95%CI) 25.99 − 29.37)) was the most important attribute. Symptom reduction after one year was followed by symptom reduction after eight weeks (RI 19.47 (18.30 − 20.64)) and chance of infection (22.33 (21.42 − 23.25)). After that, route of administration followed (RI 13.29 (11.95 − 14.63)). The location of administration of the treatment and the chance of adverse events were the least preferred important (8.72 (7.83 − 9.60) and 8.51 (7.96 − 9.05), respectively).
Table 2. Utilities of attributes in all patients.
Patients preferred oral administration over subcutaneous injections. Intravenous route of administration was least favorite. Patients chose administration at home over administration in the hospital.
Subgroup analysis
The average utilities of patients who are biologic therapy and/or a small molecule experienced are displayed in . Both patient groups found a high chance of reduction of symptoms after one year more important than the alternative attributes. Biologic and/or small molecule naïve patients find route of administration more important compared to biologic and/or small molecule experienced patients (p < 0.001). Naïve patients tend to found chance of adverse events less important compared to experienced patients, though this was not statistically significant. (p = 0.059).
Table 3. Utilities of attributes in biologic and/or small molecule experienced and naive patients.
Surgical treatment
Off the 172 patients who completed the DCE, 133 patients filled in the trade off questions regarding surgery. Forty-four patients (33.1%) responded with unknown. These patients did not differ in age (p = 0.502) or whether they were biologic and/or small molecule experienced (p = 0.501) compared to patients that responded these questions. Seventy patients (52.6%) would consider surgery after failing eight treatment options without any new available therapies left. Nine patients would consider surgery before trying any treatment options. Of the remaining patients, four would consider surgery after failing six treatment options, one patient after failing three treatment options, two patients after four treatment options, one after five treatment options and two after seven treatment options.
Discussion
This study is the first discrete choice experiment assessing the patient preferences regarding medication options of patients with ulcerative colitis with all currently available treatment options included in the Netherlands. We found that symptom reduction after one year was the most important attribute in choosing between treatments in UC patients, followed by symptom reduction after eight weeks and chance of infection.
A German discrete choice experiment comparable to ours, showed that in 219 patients with IBD efficacy outcomes were rated most important followed by the frequency of serious AE [Citation16]. In the United Kingdom, a discrete choice experiment in 115 patients with steroid resistant UC revealed that patient preferences were strongest for treatments with a lower chance of side effects. The second most important attribute was an improvement in maintaining remission [Citation17]. In our DCE, the chance of side effects was rated as one of the least important attributes. This difference might be explained by the definition of adverse events, as we made a distinction between the risk of infection and the remaining adverse events. In a qualitative study consisting of a scoping literature search, two focus group discussions with IBD patients and two expert panel discussions, long-term clinical remission was identified as one of the most important treatment characteristics, next to preventing surgery [Citation18]. The importance of long-term remission was also confirmed in a study showing that IBD patients were willing to accept high levels of lymphoma and serious infection risk to avoid an UC relapse [Citation19]. Compared to patients with Crohn’s disease, patients with UC were more likely to value efficacy over side effects [Citation20]. Preferring the chance of symptom reduction after one year over symptom reduction after eight weeks in our study, shows that patients understand the importance of maintaining remission. Though patients value efficacy the most, the efficacy rates of currently available biologicals and small molecules are all within the same range. Therefore, when initiating a new treatment, this decision can hardly be based on the efficacy alone.
In a systematic review assessing patients’ preferences for disease-modifying anti-rheumatic drug (DMARD) in rheumatoid arthritis, treatment benefits (disease improvement) were more important than adverse events and dosing and administration considerations in the included DCE studies [Citation21]. This is in line with our results in patients with UC. Route and frequency of administration were often more important than adverse events. However, the results varied across studies and preferences were commonly associated with sociodemographic characteristics (age, education, ethnicity, and income). In our cohort, chance of infection was more important compared to administration route. Preferences did not differ based on age or sex. We were not able to assess differences based on ethnicity and income. Chance of infection and chance of symptom reduction after eight weeks were chosen second and third important after symptom reduction after one year. In some therapeutic treatment options, it is known that it may take weeks or even months to achieve remission and others might have a higher chance of infections. We showed that it is important to discuss these attributes when considering treatment options with patients with UC, next to long term remission rates and administration characteristics. Discussing the characteristics that patients value about their treatment may lead to better treatment adherence, treatment outcomes and quality of life [Citation4–6].
In sub analysis, there was a significant difference in preferences between patients who were biologic and/or small molecule experienced and patients who were biologic and/or small molecule naïve. Naïve patients found route of administration more important compared to experienced patients. All patients preferred oral administration. However, in naïve patients, the difference in preference between oral and subcutaneous therapy was smaller compared to the difference in preference in experienced patients. This might be explained by the experiences of patients previously treated with subcutaneous formulation. Also, naïve patients found intravenous less favorable compared to experienced patients. We hypothesize that patients previously treated with intravenous therapy, found this administration route relatively acceptable.
We assessed after how many failed treatment options, patients were willing to consider surgery. One third of patients could not decide whether they would be willing to consider surgery based on the information given in the experiment and most of the remaining patients would consider surgery after failing at least four treatment options. Previous studies confirm that patients are willing to try several medical therapy before considering surgical treatment. A qualitative study consisting of literature review, focus group discussions and expert panel discussion showed that preventing surgery was the highest ranked characteristic amongst IBD patients [Citation18]. Only one study on patient preferences for surgical treatment was performed in patients with UC [Citation22]. Based on questionnaires, patients would rather choose for escalating medical therapy rather than any operation in the acute setting.
A strength of our study is the carefully selection of attributes by consecutively literature search, patient interviews and surveys. Attributes and levels included in the DCE cover all the currently available therapies for the treatment of moderate to severely active UC. For example, until the introduction of JAK inhibitors, only intravenous of subcutaneous treatment was available. We also included oral administration route to cover the JAK inhibitors and s1p-modulators as well. Furthermore, we included a representative population of both biologic and/or small molecules experienced and naïve patients. We also note some limitations. First, we included patients with self-reported UC with the potential for misclassification of IBD type, treatment history and current treatments. However a previous study showed that IBD patients are capable of accurately report their medical history [Citation23]. S, our discrete choice experiment was limited to assess six attributes as including more attributes would make it more difficult or even impossible for respondents to choose. As a result, we could not include other attributes of interest or distinguish e.g., between mild or severe adverse event. Though, since we carefully selected the six included attributes and two of these were found to be not very important, including other attributes would probably not influence the outcomes. Another limitation is that the preferences were measured in a group of patients and though this may be insightful for shared decision making, individual preferences should be taken into account in the outpatient clinic as these might differ.
In conclusion, this study demonstrates that patients with UC preferred symptom reduction after one year over other treatment attributes such as route of administration and chance of adverse events when choosing between treatment options. Anti-TNF and/or small molecule naïve patients found route of administration more important compared to experienced patients. These outcomes can help understand the trade-offs and preferences of UC patients and be integrated in clinical practice to improve quality of care of UC patients by improving information given in the outpatient clinical when initiating a new treatment.
Authors’ contributions
TS, MvdA, MD and AvdM designed the study. T.S. performed all statistical analysis and drafted the manuscript. AvdM, EvdA and MD provided input regarding data interpretation and they reviewed the article. All authors read and approved the final manuscript.
Manuscript statement
The manuscript, including figures and tables, has not been previously published and is not under consideration elsewhere.
Supplemental Material
Download MS Word (18.1 KB)Acknowledgements
Nothing to declare.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Additional information
Funding
References
- Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet. 2017;389(10080):1756–1770. doi: 10.1016/S0140-6736(16)32126-2
- Bohm M, Xu R, Zhang Y, et al. Comparative safety and effectiveness of vedolizumab to tumour necrosis factor antagonist therapy for crohn’s disease. Aliment Pharmacol Ther. 2020;52(4):669–681. doi: 10.1111/apt.15921
- Lasa JS, Olivera PA, Danese S, et al. Efficacy and safety of biologics and small molecule drugs for patients with moderate-to-severe ulcerative colitis: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2022;7(2):161–170. doi: 10.1016/S2468-1253(21)00377-0
- Kane S, Huo D, Aikens J, et al. Medication nonadherence and the outcomes of patients with quiescent ulcerative colitis. Am J Med. 2003;114(1):39–43. doi: 10.1016/s0002-9343(02)01383-9
- Kane SV, Chao J, Mulani PM. Adherence to infliximab maintenance therapy and health care utilization and costs by crohn’s disease patients. Adv Ther. 2009;26(10):936–946. doi: 10.1007/s12325-009-0069-7
- Carter CT, Leher H, Smith P, et al. Impact of persistence with infliximab on hospitalizations in ulcerative colitis. Am J Manag Care. 2011;17:385–392
- Greenfield S, Kaplan SH, Ware JE, Jr., et al. Patients’ participation in medical care: effects on blood sugar control and quality of life in diabetes. J Gen Intern Med. 1988;3(5):448–457. doi: 10.1007/BF02595921
- Street RL, Jr., Voigt B. Patient participation in deciding breast cancer treatment and subsequent quality of life. Med Decis Making. 1997;17(3):298–306. doi: 10.1177/0272989X9701700306
- Lancsar E, Louviere J. Conducting discrete choice experiments to inform healthcare decision making. Pharmacoeconomics. 2008;26(8):661–677. doi: 10.2165/00019053-200826080-00004
- Opuni M, Bishai D, Gray GE, et al. Preferences for characteristics of antiretroviral therapy provision in johannesburg, South Africa: results of a conjoint analysis. AIDS Behav. 2010;14(4):807–815. doi: 10.1007/s10461-009-9584-4
- Fraenkel L. Conjoint analysis at the individual patient level: issues to consider as we move from a research to a clinical tool. Patient. 2008;1(4):251–253. doi: 10.2165/1312067-200801040-00005
- Nathan H, Bridges JF, Schulick RD, et al. Understanding surgical decision making in early hepatocellular carcinoma. J Clin Oncol. 2011;29(6):619–625. doi: 10.1200/JCO.2010.30.8650
- Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health–a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–413. doi: 10.1016/j.jval.2010.11.013
- de Bekker-Grob EW, Donkers B, Jonker MF, et al. Sample size requirements for discrete-choice experiments in healthcare: a practical guide. Patient. 2015;8(5):373–384. doi: 10.1007/s40271-015-0118-z
- Pearmain D, Kroes EP. Stated preference techniques: a guide to practice1990
- Schubert S, Picker N, Cavlar T, et al. Inflammatory bowel disease patients’ treatment preferences using a discrete choice experiment technique: the InPuT study. Adv Ther. 2022;39(6):2889–2905. doi: 10.1007/s12325-022-02143-z
- Wickramasekera N, Coates E, Barr A, et al. Patient preferences for treatment in steroid resistant ulcerative colitis - a discrete-choice experiment. Scand J Gastroenterol. 2022;57(7):797–806. doi: 10.1080/00365521.2022.2036808
- Schoefs E, Vermeire S, Ferrante M, et al. What are the unmet needs and most relevant treatment outcomes according to patients with inflammatory bowel disease? A qualitative patient preference study. J Crohns Colitis. 2023;17(3):379–388.2022
- Bewtra M, Fairchild AO, Gilroy E, et al. Inflammatory bowel disease patients’ willingness to accept medication risk to avoid future disease relapse. Am J Gastroenterol. 2015;110(12):1675–1681. doi: 10.1038/ajg.2015.321
- Almario CV, Keller MS, Chen M, et al. Optimizing selection of biologics in inflammatory bowel disease: development of an online patient decision aid using conjoint analysis. Am J Gastroenterol. 2018;113(1):58–71. doi: 10.1038/ajg.2017.470
- Durand C, Eldoma M, Marshall DA, et al. Patient preferences for disease-modifying antirheumatic drug treatment in rheumatoid arthritis: a systematic review. J Rheumatol. 2020;47(2):176–187. doi: 10.3899/jrheum.181165
- Byrne CM, Tan KK, Young JM, et al. Patient and clinician preferences for surgical and medical treatment options in ulcerative colitis. Colorectal Dis. 2014;16(4):285–292. doi: 10.1111/codi.12538
- Kelstrup AM, Juillerat P, Korzenik J. The accuracy of self-reported medical history: a preliminary analysis of the promise of internet-based research in inflammatory bowel diseases. J Crohns Colitis. 2014;8(5):349–356. doi: 10.1016/j.crohns.2013.09.012