Abstract
Objective: Endoscopic ultrasound-guided fine-needle aspiration/biopsy (EUS FNA/FNB) and potential endoscopic retrograde cholangiopancreatography (ERCP) for biliary decompression are indicated in patients with pancreatic cancer before initation of primary chemotherapy. This study aims to investigate the performance and safety of these two procedures in patients with borderline resectable (BRPC) or locally advanced pancreatic cancer (LAPC). Methods: Endoscopy and pathology reports, and hospital records of consecutive patients with a radiological diagnosis of BRPC/LAPC included in a population based, protocol-driven study (NORPACT-2) were reviewed. Results: Of 251 patients, 223 (88.9%) underwent EUS-FNA/FNB, and 133 (53%) underwent ERCP. Repeated EUS attempts were performed in 33 (14.8%), eight (3.6%), and four (1.8%) patients. FNA was performed in 155 procedures, FNB in 30, and combined EUS-FNA/FNB in 83. Diagnostic accuracy was 86.1% for first EUS-FNA/FNB. The cumulative diagnostic accuracy for all attempts was 96%. False positive rate for malignancy was 0.9%. Of a total of 149 ERCP procedures, 122 (81.9%) were successful, and 27 (18.1%) were unsuccessful. Success rate of first ERCP attempt was 80.5% (107/133). Sixteen patients (12%) underwent a second attempt with a success rate of 93.8% (15 of 16). Combined EUS and ERCP was performed in 41 patients. Complications occurred in eight procedures (3%) after EUS-FNA/FNB, 23 procedures (15.3%) after ERCP, and four (9.8%) patients after combined EUS-FNA/FNB and ERCP. Conclusion: EUS-FNA/FNB and ERCP with biliary stenting in patients with BRPC/LAPC demonstrated acceptable performance and safety. Repeat procedures were performed with high success rates. Same session EUS-FNA/FNB and ERCP for biliary decompression is safe.
Introduction
Pancreatic cancer is expected to be the second-leading cause of cancer-related death by 2030 [Citation1]. Clinical presentation varies according to the size and location of the tumor, and more than 50% of the patients present with metastatic disease. Resection is the only curative treatment. Multidetector computed tomography (CT) angiography is considered the gold standard imaging tool for staging, whereas magnetic resonance imaging (MRI) can be a helpful supplement for characterization of CT-indeterminate liver lesions to rule out potential metastases [Citation2].
For patients with radiologically suspected borderline resectable (BRPC) or locally advanced pancreatic cancer (LAPC) a prompt tissue diagnosis is needed to commence primary chemotherapy. The primary role of endoscopic ultrasound (EUS) is to procure tissue for cytological or histological diagnosis, but EUS may also increase the sensitivity in detecting pancreatic neoplasms [Citation3]. In obtaining a definite diagnosis of pancreatic cancer, uncertainty remains in terms of potential benefits of fine-needle aspiration (FNA), biopsy (FNB) or combined FNA/FNB in terms of accuracy and sensitivity/specificity. EUS-FNB has the advantage of procuring adequate tissue for genomic testing [Citation4]. In addition, many patients with BRPC and LAPC require biliary drainage prior to commencing initial chemotherapy, for which endoscopic retrograde cholangiopancreatography (ERCP) is the preferred approach [Citation5].
This study aims to investigate the diagnostic performance of EUS FNA/FNB and incidence of adverse advents in a population-based, consecutive cohort of patients with BRPC or LAPC. The secondary aim was to assess the technical success rate of ERCP for stent insertion and the incidence of complications after this procedure.
Material and methods
Study population
Norway has four independent regional health care authorities, which cover the total population of 5.4 million inhabitants. Oslo University Hospital (OUH) Rikshospitalet is the largest Hepato-biliary-pancreatic (HPB) center in South-Eastern Norway, covering 3.1 million people, and it is the only hospital providing HPB surgery in this region. NORPACT-2 is a prospective, observational protocol-driven study, following national guidelines, investigating the role of neoadjuvant and primary chemotherapy for BRPC and LAPC prior to potential surgery. From January 2018 to December 2020, all patients with BRPC or LAPC in the South-Eastern Norway Regional Health Authority were offered participation in the study and included upon written informed consent. Patients were followed up until date of death or until 15 September 2022. The study protocol was approved by the Regional Ethical Committee, Norway (REC Nord 2017/1382, Norwegian Pancreatic Cancer Trial-2; NORPACT-2) in August 2017. Primary end-point of the trial was resection rates and survival. Performance and safety of diagnostic EUS-FNA/FNB and therapeutic ERCP were among the secondary end-points. Clinical data were collected prospectively in eReg database, approved by and maintained according to OUH regulation, and supervised by the Data Protection officer for Research.
Before study inclusion, distant metastases were ruled out by contrast-enhanced dual-phase, multi-slice computer tomography (CT) scans of the abdomen and chest. An experienced abdominal radiologist evaluated vascular involvement of the coeliac trunk, superior mesenteric artery, hepatic artery and portomesenteric veins. All patients were discussed at the multidisciplinary team meeting. The protocol for assignment, diagnostic work-up, treatment sequence and medical and surgical interventions followed national Norwegian guidelines [Citation6]. Performance status (Eastern Cooperative Oncology Group, ECOG) and Charlson comorbidity index (CCI) (https://www.mdcalc.com/charlson-comorbidity-index-cci) were recorded at the time of diagnosis. The points given in CCI for the diagnosis of a solid tumor and for age were excluded from the final score.
Endoscopic procedures
As part of the diagnostic work-up, all patients were required to have cytological or histological confirmation of pancreatic ductal adenocarcinoma (PDAC) or variants prior to initiation of chemotherapy, in addition to CT or MRI verification of a solid, likely malignant tumor [Citation7]. Tissue diagnosis was obtained by EUS-FNA or FNB, either as a day procedure or in-hospital procedure. In addition, patients with biliary obstruction requiring biliary drainage underwent ERCP and stent placement. The vast majority of endoscopic procedures were performed at OUH Rikshospitalet.
The acquired tissue through FNA or FNB was analyzed by cytopathologists and histopathologists respectively. In most cases, an attending cytopathologist throughout the study period provided rapid on-site evaluation (ROSE) of the cytological specimen on direct smears while the patient remained sedated. Hence, in case of inconclusive results, a repeat FNA or FNB could be carried out during the same procedure. In lack of an attending cytopathologist and where FNA proved inconclusive or negative, patients had to be recalled for a subsequent EUS and FNA/FNB attempt.
All patients were observed for potential complications throughout the day, and the records from the patients’ local hospitals were reviewed to identify complications diagnosed after hospital discharge. The number of EUS procedures needed to reach a final diagnosis was recorded for each patient. The final cytology and histology diagnosis of pancreatic samples was assigned to one of the following categories; adenocarcinoma, malignant tissue, suspicion of malignancy/adenocarcinoma, atypical not otherwise specified, neuroendocrine tumor (NET), intraductal papillary mucinous neoplasm (IPMN), acinar cell carcinoma, chronic pancreatitis, benign, or inadequate. Patients with jaundice were eligible for ERCP with direct stent insertion during the same procedure, or as a separate procedure prior to initiating chemotherapy. Fully covered self-expanding metal stents (c-SEMS) were preferred. If ERCP was not possible, percutaneous transhepatic biliary drainage (PTBD) was performed. The number of patients receiving metal or plastic stents, the number of patients undergoing PTBD, and the rate and nature of complications were recorded.
Diagnostic accuracy of EUS-FNA/FNB was defined for both the first and for all EUS FNA/FNB procedures, as the percentage of sampled lesions for which a final diagnosis could be reached prior to treatment initiation. ‘Suspicion of malignancy’ and ‘malignant’ were considered positive samples. The definitive diagnosis was defined by histopathologic evaluation of the surgical specimen or, in non-resected patients, by the evolution of the disease assessed until the date of last follow-up based on a combination of clinical course, imaging studies, and/or additional tissue sampling from metastasis [Citation8].
The definition of adverse events were recorded according to the American Society for Gastrointestinal Endoscopy lexicon for adverse events [Citation9].
Results
EUS FNA/FNB
In total 250 patients with a radiological diagnosis of BRPC or LAPC were included, of whom 223 (89.2%) underwent EUS-FNA/FNB (). Characteristics of patients undergoing EUS FNA/FNB and their tumors are presented in . In four patients, a percutaneous biopsy was taken without prior EUS attempts for the following reasons: post Roux-en-Y gastric bypass (n = 1), patient preference (n = 1), at treating physician`s decision (n = 2). Three patients underwent primary EUS procedure without FNA/FNB (Roux-en-Y gastric bypass and decision for upfront surgery, n = 1; best supportive care due to old age and frailty, n = 2). The remaining 20 patients received best supportive care only due to advanced age or low performance status, such that pathology-based diagnostic confirmation was not considered necessary. One hundred and eleven patients were classified as BRPC, and 112 as LAPC. In 183 cases the tumor was located in the head of the pancreas (head, neck, uncinate process), while the remaining 40 tumors were located in the body or tail. Median tumor diameter was 35.5 mm. A second, third or fourth EUS attempt was performed in 33 (14.8%), eight (3.6%), and four (1.8%) patients, respectively (). One of the four patients with four FNA/FNB attempts had a definitive diagnosis of chronic pancreatitis. One patient with BRPC undergoing four attempts had more than three months delay in initiation of chemotherapy due to difficulty in obtaining a conclusive diagnosis, which contributed to derailment from a surgical pathway, as the patient became unresectable due to tumor progression. A total of 268 EUS FNA/FNB procedures were performed. Combined FNA/FNB was performed in 83 cases, whereas EUS-FNA and EUS-FNB were performed separately in 155 and 30 cases, respectively ().
Figure 1. Pathologic findings of samples collected during 268 EUS FNA and/or FNB procedures in 223 patients with a radiological diagnosis of BRPC or LAPC.
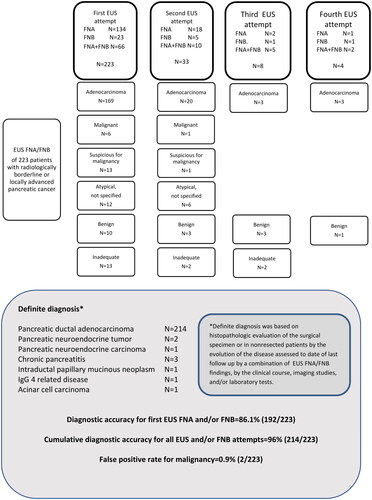
Table 1. Patient characteristics and tumor details for 223 patients undergoing EUS FNA/FNB.
The diagnostic accuracy for the first EUS-FNA/FNB and the cumulative diagnostic accuracy for all EUS-FNA/FNB attempts are presented in . In 195 samples EUS FNA/FNB described a diagnosis of PDAC, seven samples were described as malignant, and 14 samples were described as suspicious of malignancy. Thirteen samples described other diagnosis; NET (n = 3), IPMN (n = 1), pancreatitis (n = 4), other benign/atypical (n = 3), and high grade dysplasia (n = 2). The definitive diagnosis was adenocarcinomas in 214, pancreatitis in 3, NET in 2, neuroendocrine carcinoma (NEC) in 1, IPMN in 1, IgG4-related disease in 1, and acinar cell carcinoma in 1. Of 61 patients undergoing resection, histological examination of the surgical specimen showed PDAC (n = 56), IPMN (n = 1), NEC (n = 1), IgG4 related pancreatitis (n = 1), groove pancreatitis (n = 1), and complete tumor regression (n = 1). In the latter five patients, the diagnosis based on EUS FNA/FNB was IPMN (FNA), adenocarcinoma (FNA), adenocarcinoma (FNA), inflammation (FNA + FNB), and acinar cell carcinoma (FNA), respectively.
Diagnostic accuracy of EUS-FNA/FNB was 86.1% (192/223) at the first attempt, and the cumulative diagnostic accuracy for all attempts was 96% (214/223). The false positive rate for carcinoma was 0.9% (2/224 patients) (definitive diagnosis: chronic pancreatitis n = 1, IgG4 related pancreatitis n = 1).
ERCP
Patient and tumor characteristics of 133 patients undergoing primary ERCP are presented in . Of a total 149 ERCP procedures, of which 16 were re-attempts, 122 (81.9%) were successful. 107 of 133 had a successful first attempt (success rate 80.5%), and 15 out of 16 had a successful second attempt (success rate 93.8%). 27 were unsuccessful (18.1%). A plastic stent was inserted in fifteen patients while c-SEMS was inserted in 107 patients. Sixteen patients underwent PTBD, of whom 11 had undergone a prior ERCP attempt. Five patients had direct PTBD without prior ERCP attempt for unknown reasons. There were 41 procedures of combined EUS and ERCP.
Table 2. Patient and treatment characteristics for 133 patients undergoing primary ERCP with placement of a biliary stent.
Adverse events
Post-procedural adverse events are presented in . Eight adverse events (3%) were recorded for a total of 271 EUS-FNA/FNB procedures (pancreatitis n = 7, cholangitis n = 1). There were 23 adverse events (15,4%) recorded following ERCP (pancreatitis, n = 12; cholangitis, n = 4; perforation, n = 1; bleeding, n = 1; stent failure, n = 1; other, n = 4). Four adverse events (25%) were recorded after PTBD (cholangitis, n = 2; immediate bile leakage, n = 1; dislocated stent, n = 1). For a total of 41 procedures of combined EUS and ERCP, four adverse events (9,8%) were recorded (pancreatitis, n = 3; renal failure requiring hospitalization following dehydration in frail patients undergoing prolonged procedure, n = 1). One patient with pancreatitis and one patient with procedure-related bleeding experienced a course of moderate adverse event requiring intensive care unit admission. The remaining adverse events recorded were classified as mild [Citation9].
Table 3. Postprocedural adverse events after ERCP, PTC or EUS FNA/FNB.
Discussion
Practice guidelines recommend systemic chemotherapy as the primary treatment in patients diagnosed with BRPC and LAPC. Swift cytological or histopathological diagnosis and relief of biliary obstruction enable the timely initiation of chemotherapy. The population-based cohort examined in this study represents a patient group that requires the involvement of surgeons, oncologists, endoscopists, and pathologists. EUS FNA/FNB had an overall diagnostic accuracy of 96%, and a false positive rate of carcinoma of 0.9%. Therapeutic ERCP had a success rate of 80.5% for the first attempt. Repeat procedure was performed in 14.8% of the patients after EUS FNA/FNB and 12% after ERCP, with a diagnostic accuracy of 66.7% and a success rate of 93.8% respectively. The complication rates of EUS-FNA/FNB and ERCP were 3% and 15.4%, respectively.
Due to the extensive tumor stroma and, consequently, the low cancer cell density, obtaining representative cytological or histological samples from PDAC is challenging. EUS has been the gold standard and EUS-FNA has been the standard examination, however the diagnostic use of FNA samples is more limited compared to FNB due to the lack of histological architecture and a usually more restricted antibody panel for immunocytochemical evaluation. EUS-FNB with a larger biopsy needle was developed to overcome these obstacles [Citation10]. This study demonstrates the diagnostic performance and safety of common endoscopic procedures, and identifies the specific challenges in achieving a correct diagnosis. However, the study was not designed to compare the diagnostic accuracy of FNA versus FNB. The decision to sample an FNB in addition to an FNA during the same procedure was made by the on-site cytopathologist and the endoscopist. Both the safety profile and technical feasibility of EUS-FNA prove to be similar to those of EUS-FNB [Citation11,Citation12]. The complication rate of EUS-FNA/FNB observed in this study amounts to 3%, which is in line with the literature [Citation13]. EUS-FNB offers the possibility of examination with a more extensive immunohistochemical panel, which is key to the differential diagnosis that is relevant to solid pancreatic lesions and includes autoimmune pancreatitis, lymphoma and metastasis. When it comes to the number of passes that are required for a final diagnosis, a first randomized controlled trial comparing EUS-FNA 22 G and EUS-FNB 22 G with rapid on-site evaluation (ROSE) showed no significant difference. An overall similar rate of diagnosis was achieved with up to three passes (100% EUS-FNA, 89% EUS-FNB) [Citation14]. Two subsequent studies have supported similar accuracy in diagnostics, but a need for fewer passes with EUS-FNB compared to EUS-FNA [Citation15,Citation16]. A Danish study showed similar results and also a significantly higher number of microcores procured per pass by FNB compared to FNA upon the introduction of the Franseen type needle for FNB [Citation17]. This study shows a cumulative diagnostic accuracy of 96% for all EUS attempts and confirms that EUS-FNA/FNB should be repeated in cases of initial inadequate sample, or inconclusive or atypical/benign diagnosis. The rate of repeat EUS-FNA/FNB is in line with a recent report from the Dutch PREOPANC-1 and 2 trials in patients with resectable pancreatic cancer and BRPC which had a second and third endoscopic tissue acquisition rate of 11% and 1%, respectively [Citation18].
During the study period, the use of EUS-FNB gradually increased. Currently, NCCN and ESMO guidelines recommend EUS-FNB in BRPC and LAPC at the time of diagnosis to obtain adequate tissue for molecular analysis, the results of which may guide treatment decisions and inclusion in ancillary studies [Citation19]. In addition, molecular analysis is more straightforward on EUS-FNB samples. While panel sequencing from diagnostic EUS-FNA is feasible, the failure rate is reported to be high [Citation20]. Recent reports show that a EUS-FNB protocol tailored towards both diagnosis and next-generation sequencing purposes, targeted capture sequencing can be performed with excellent success rates [Citation21].
False positive and false negative results after EUS-FNA-FNB are challenging. False positive EUS-FNA/FNB carcinoma occurred in two patients (0.9%), in whom the definitive diagnosis was IgG4 related pancreatitis and chronic pancreatitis, respectively. Both patients received primary chemotherapy, and one of the patients underwent a pancreatoduodenectomy. The false positive rate of carcinoma of 0.9% in this study aligns with other reports [Citation18]. Difficulty in reaching a pathology-based confirmation of carcinoma occurred in one patient, who radiologically had BRPC with a small thrombus in the portal vein. After three EUS-FNA/FNB attempts showing no evidence of malignancy, medical treatment was initiated for suspected autoimmune pancreatitis due to slightly elevated serum Ig4. The patient had rapid radiological progression and developed major vascular involvement precluding resection. Only at the fourth attempt, EUS-FNB showed adenocarcinoma. Finally, one patient with radiologically LAPC in which cytology showed acinar cell carcinoma was treated with FOLFIRINOX followed by pancreatoduodenectomy. Histological examination of the surgical specimen showed complete tumor regression and inflammatory changes. Complete histopathological response after neoadjuvant FOLFIRINOX in acinar cell carcinoma has been described in the literature, and the primary diagnosis was maintained although some uncertainty exists regarding the primary diagnosis based on EUS-FNA [Citation22].
Many pancreatic cancer patients present with obstructive jaundice and require biliary drainage before starting primary chemotherapy. Practice guidelines recommend ERCP with insertion of SEMS for preoperative biliary drainage of extrahepatic malignant biliary obstruction in patients scheduled for neoadjuvant or primary chemotherapy [Citation5]. A success rate of 80.5% at the first attempt, and 93.8% after two attempts is in line with the literature [Citation5]. In our study, most patients received c-SEMS, but 14 received initially a plastic stent that later was replaced by a c-SEMS. Metal stents offer better patency, reduce the risk of complications, and decrease the need for reintervention [Citation23]. Plastic stents require exchange every 2-3 months, while SEMS show longevity of 6-12 months [Citation24]. The 15.4% complication rate observed in this study is in accordance with existing literature [Citation5]. For combined EUS-FNA/FNB and ERCP procedures performed in 41 patients, the complication rate was 12.2%. The study was not designed to evaluate the performance of combined or separate EUS-FNA-FNB and ERCP procedures, however, the success and complication rates were shown to be comparable for both approaches [Citation25]. Current practice guidelines suggests that EUS-guided sampling and ERCP can safely be performed in a single session [Citation26]
Primary chemotherapy is standard of care for BRPC and LAPC, and the resection rate following primary chemotherapy is reported to be 60.6% and 20.2%, respectively [Citation27]. Neoadjuvant treatment is increasingly used in primary resectable PDAC, but evidence of higher quality is required to determine whether neoadjuvant therapy has potential benefits and improves survival compared to upfront surgery in this patient group [Citation28,Citation29]. Limited data are available regarding the potential delay in the commencement of treatment caused by diagnostic EUS FNA/FNB and therapeutic ERCP in resectable PDAC. However, the results of this study in patients with BRPC and LAPC, and from the Dutch Preopanc 1 and 2 trials in patients with resectable and BRPC show that repeat endoscopic procedures are required in 13.3–14.8% of patients to achieve an adequate diagnostic sample, with a false positive rate of malignancy of 0.9–2% [Citation18]. Moreover, in a neoadjuvant pathway every jaundiced patient will need ERCP for biliary drainage, which has a complication rate of about 15% and requires a second attempt in about 10% of the patients. These diagnostic and therapeutic challenges are important to consider when evaluating the treatment sequencing in resectable PDAC [Citation28,Citation30].
This study has some limitations. First, this is a single-center study, and external validity may be limited. However, the population-based non-selected cohort evaluated and treated in a universal health care system represents a patient group that potentially encompasses the practices of surgeons, oncologists, endoscopists, and pathologists. Second, the study was not designed to evaluate the diagnostic accuracy and safety of FNA versus FNB. However, this has been thoroughly described in the literature. FNB was performed based on the evaluation of the on-site cytopathologist or at the discretion of the endoscopist. Third, no systematic follow-up was performed after the endoscopic procedures to identify potential complications. Records from the local hospitals were available for review, but information about possible consultation of a general practitioner was not available. Thus, minor complications not requiring hospitalization may have been missed.
In conclusion, EUS-FNA/FNB and ERCP with biliary stenting in a population-based cohort of patients with BRPC/LAPC demonstrated acceptable performance and safety. Same session EUS-FNA/FNB and ERCP for biliary decompression was safe, but not carried out systematically in this study and needs further evaluation in terms of optimal methods, timing of procedures, and patient selection.
References
Acknowledgements
The authors acknowledge the financial support provided by grants from the Norwegian Cancer Society (grant numbers 198039-2018 and 212734-2019 (The Norwegian Cancer Society’s National Group of Expertise on Pancreatic Cancer Research)) and the South-Eastern Norway Regional Health Authority (grant numbers 2018088 and 2019029). The authors are grateful to the study nurses Asle Sandved Rudjord, Eline Angard Ulateig, Ann Charlott Moèll, and Gyda Grodås Christiansen for their excellent work in the project office and with patients.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data access statement
The data presented in this study are available upon reasonable request from the corresponding author. The data are not publicly available due to privacy regulations.
Additional information
Funding
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74(11):2913–2921. doi: 10.1158/0008-5472.CAN-14-0155
- Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(4):439–457. doi: 10.6004/jnccn.2021.0017.
- Li H, Hu Z, Chen J, et al. Comparison of ERCP, EUS, and ERCP combined with EUS in diagnosing pancreatic neoplasms: a systematic review and meta-analysis. Tumour Biol. 2014;35(9):8867–8874. doi: 10.1007/s13277-014-2154-z.
- Elhanafi S, Mahmud N, Vergara N, et al. Comparison of endoscopic ultrasound tissue acquisition methods for genomic analysis of pancreatic cancer. J Gastroenterol Hepatol. 2019;34(5):907–913. doi: 10.1111/jgh.14540.
- Dumonceau JM, Tringali A, Papanikolaou IS, et al. Endoscopic biliary stenting: indications, choice of stents, and results: European society of gastrointestinal endoscopy (ESGE) clinical guideline - updated october 2017. Endoscopy. 2018;50(9):910–930. doi: 10.1055/a-0659-9864.
- Nasjonalt handlingsprogram med retningslinjer for diagnostikk, behandling og oppfølging av pasienter med pancreaskreft: Helsedirektoratet. 2017 Available from: https://www.helsedirektoratet.no/retningslinjer/pancreaskreft-bukspyttkjertelkreft-handlingsprogram#referere.
- Board TEbtWCoTE. Digestive system tumours: WHO classification of tumours. 5th ed. Vol 1. World Health Organization; 2019. p. 295–370.
- Wani S, Muthusamy VR, McGrath CM, et al. AGA white paper: optimizing endoscopic ultrasound–guided tissue acquisition and future directions. Clin Gastroenterol Hepatol. 2018;16(3):318–327. doi: 10.1016/j.cgh.2017.10.020.
- Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71(3):446–454. doi: 10.1016/j.gie.2009.10.027.
- Levine I, Trindade AJ. Endoscopic ultrasound fine needle aspiration vs fine needle biopsy for pancreatic masses, subepithelial lesions, and lymph nodes. World J Gastroenterol. 2021;27(26):4194–4207. doi: 10.3748/wjg.v27.i26.4194.
- Yousri M, Abusinna E, Tahoun N, et al. A comparative study of the diagnostic utility of endoscopic ultrasound-guided fine needle aspiration cytology (EUS-FNA) versus endoscopic ultrasound-guided fine needle biopsy (EUS-FNB) in pancreatic and non-pancreatic lesions. Asian Pac J Cancer Prev. 2022;23(6):2151–2158. doi: 10.31557/APJCP.2022.23.6.2151.
- Yousaf MN, Chaudhary FS, Ehsan A, et al. Endoscopic ultrasound (EUS) and the management of pancreatic cancer. BMJ Open Gastroenterol. 2020;7(1):e000408. doi: 10.1136/bmjgast-2020-000408.
- Dumonceau JM, Deprez PH, Jenssen C, et al. Indications, results, and clinical impact of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European society of gastrointestinal endoscopy (ESGE) clinical guideline - updated january 2017. Endoscopy. 2017;49(7):695–714. doi: 10.1055/s-0043-109021.
- Bang JY, Hebert-Magee S, Trevino J, et al. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2012;76(2):321–327. doi: 10.1016/j.gie.2012.03.1392.
- Tian L, Tang A-L, Zhang L, et al. Evaluation of 22G fine-needle aspiration (FNA) versus fine-needle biopsy (FNB) for endoscopic ultrasound-guided sampling of pancreatic lesions: a prospective comparison study. Surg Endosc. 2018;32(8):3533–3539. doi: 10.1007/s00464-018-6075-6.
- Chen Y-I, Chatterjee A, Berger R, et al. Endoscopic ultrasound (EUS)-guided fine needle biopsy alone vs. EUS-guided fine needle aspiration with rapid onsite evaluation in pancreatic lesions: a multicenter randomized trial. Endoscopy. 2022;54(1):4–12. doi: 10.1055/a-1375-9775.
- Kovacevic B, Toxværd A, Klausen P, et al. Tissue amount and diagnostic yield of a novel franseen EUS-FNB and a standard EUS-FNA needle-a randomized controlled study in solid pancreatic lesions. Endosc Ultrasound. 2023;12(3):319–325. doi: 10.1097/eus.0000000000000007.
- Janssen QP, Quispel R, Besselink MG, et al. Diagnostic performance of endoscopic tissue acquisition for pancreatic ductal adenocarcinoma in the PREOPANC and PREOPANC-2 trials. HPB. 2023;25(10):1161–1168. doi: 10.1016/j.hpb.2023.04.018.
- Mosele F, Remon J, Mateo J, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO precision medicine working group. Ann Oncol. 2020;31(11):1491–1505. doi: 10.1016/j.annonc.2020.07.014.
- Gleeson FC, Kerr SE, Kipp BR, et al. Targeted next generation sequencing of endoscopic ultrasound acquired cytology from ampullary and pancreatic adenocarcinoma has the potential to aid patient stratification for optimal therapy selection. Oncotarget. 2016;7(34):54526–54536. doi: 10.18632/oncotarget.9440.
- Dreyer SB, Jamieson NB, Evers L, et al. Feasibility and clinical utility of endoscopic ultrasound guided biopsy of pancreatic cancer for next-generation molecular profiling. Chin Clin Oncol. 2019;8(2):16–16. doi: 10.21037/cco.2019.04.06.
- Izumo W, Higuchi R, Furukawa T, et al. A case of pathologically complete response after preoperative chemotherapy in a pancreatic acinar cell carcinoma patient with portal vein tumor thrombosis. Clin J Gastroenterol. 2022;15(3):642–648. doi: 10.1007/s12328-021-01571-8.
- Almadi MA, Barkun A, Martel M. Plastic vs. self-expandable metal stents for palliation in malignant biliary obstruction: a series of meta-analyses. Am J Gastroenterol. 2017;112(2):260–273. doi: 10.1038/ajg.2016.512.
- Uppal DS, Wang AY. Advances in endoscopic retrograde cholangiopancreatography for the treatment of cholangiocarcinoma. World J Gastrointest Endosc. 2015;7(7):675–687. doi: 10.4253/wjge.v7.i7.675.
- Purnak T, El H, II, Sherman S, et al. Combined versus separate sessions of endoscopic ultrasound and endoscopic retrograde cholangiopancreatography for the diagnosis and management of pancreatic ductal adenocarcinoma with biliary obstruction. Dig Dis Sci. 2021;66(8):2786–2794. doi: 10.1007/s10620-020-06564-0.
- Jenssen C, Hocke M, Fusaroli P, et al. EFSUMB guidelines on interventional ultrasound (INVUS), part IV – EUS-guided interventions: general aspects and EUS-guided sampling (long version). Ultraschall Med. 2016;37(2):E33–E76. doi: 10.1055/s-0035-1553785.
- Brown ZJ, Heh V, Labiner HE, et al. Surgical resection rates after neoadjuvant therapy for localized pancreatic ductal adenocarcinoma: meta-analysis. Br J Surg. 2022;110(1):34–42. doi: 10.1093/bjs/znac354.
- Springfeld C, Ferrone CR, Katz MHG, et al. Neoadjuvant therapy for pancreatic cancer. Nat Rev Clin Oncol. 2023;20(5):318–337. doi: 10.1038/s41571-023-00746-1.
- Andersson R, Haglund C, Seppanen H, et al. Pancreatic cancer - the past, the present, and the future. Scand J Gastroenterol. 2022;57(10):1169–1177. doi: 10.1080/00365521.2022.2067786.
- Ratnayake CBB, Roberts KJ, Pandanaboyana S. Upfront surgery vs. neoadjuvant therapy for resectable pancreatic cancer: a narrative review of available evidence. Chin Clin Oncol. 2022;11(1):2–2. doi: 10.21037/cco-21-161.

