Abstract
Objective
To explore the effects of pretreatment peripheral blood panimmune-inflammation value (PIV), systemic immune-inflammation index (SII), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) on the efficacy and prognostic value of immunotherapy in patients with inoperable advanced or locally advanced oesophageal squamous cell carcinoma (ESCC).
Methods
Clinical data of 107 inoperable advanced or locally advanced ESCC patients were retrospectively analysed between May 2019 and August 2023, the receiver operating characteristic curves (ROCs) of PIV, SII, NLR, and PLR in patients prior to immunotherapy were plotted, and their optimal cutoff values were determined. The risk factors were determined by univariate and multivariate analyses in groups based on the optimal cut-off values.
Results
Peripheral blood PIV, SII and PLR before immunotherapy had predictive value for the optimal efficacy of immunotherapy in patients with inoperable advanced or locally advanced ESCC; patients with PIV ≥415.885, SII ≥834.295 and NLR ≥3.740 had a low objective response rate (ORR), disease control rate (DCR), a short progression-free survival (PFS) and overall survival (OS) after immunotherapy (p < 0.05). Patient tumour stage, distant lymph node metastasis, lung metastasis, liver metastasis, PIV, SII, and NLR were risk factors affecting PFS and OS (p < 0.05). Tumour stage and SII were independent risk factors affecting PFS and OS (p < 0.05).
Conclusion
In patients with inoperable advanced or locally advanced ESCC, peripheral blood PIV, SII, and NLR have predictive value for immunotherapy outcome, SII is an independent risk factor affecting the survival prognosis, and SII ≥834.295 suggests a poor prognosis from immunotherapy.
Introduction
Oesophageal cancer (EC) is currently the seventh most commonly diagnosed malignancy and the sixth leading cause of cancer death worldwide [Citation1]. Squamous cell carcinoma (SCC) is the most common histological type of EC, accounting for more than 90% of cases in Asia, represented by China [Citation2, Citation3]. Oesophageal squamous cell carcinoma (ESCC) has an insidious onset, and the majority of patients are already in advanced stages at the time of diagnosis [Citation4]. Patients with advanced ESCC have a 5-year survival rate of less than 10% and a median survival of approximately 10 months [Citation5, Citation6]. Immunotherapy combined with chemotherapy prolongs the overall survival in patients with advanced ESCC and has become the standard care written into clinical guidelines [Citation7–11].
However, immunotherapy varies widely in patients with advanced ESCC, and there is a lack of effective predictors of efficacy and prognosis [Citation12]. Predictors such as programmed cell death ligand 1 (PD-L1), tumour mutational burden (TMB) and mismatch repair (MMR) proteins rely on histopathology and have disadvantages such as difficulty of access, long detection period, relatively subjective judgement methods, and limited access to materials that are not representative of the overall state [Citation13, Citation14] that limit their clinical application and predictive value.
The tumour immune microenvironment (TIM) interacts with immune cells and affects the effect of tumour immunotherapy [Citation15]. Neutrophils, lymphocytes, monocytes and platelets are important components of the TIM and participate in tumour-associated inflammatory responses [Citation16]. Peripheral blood inflammatory markers (NLR, PLR, PIV and SII, etc.) are comprehensive indicators based on inflammatory cells, and they have advantages such as being easy to obtain clinically, having low testing costs, being less affected by tumour heterogeneity and having predictive value for tumour immunotherapy [Citation17]. They have been shown to be associated with the poor prognosis in many tumours, including ESCC [Citation18–22]. However, relevant studies on the predictive value of peripheral blood inflammatory markers regarding the immunotherapeutic efficacy and prognosis of unresectable advanced or locally advanced ESCC have not yet been reported, and the differences in the predictive value of different peripheral blood inflammatory markers are also unclear. Therefore, the above questions urgently need to be answered by clinical studies.
To address these questions, we conducted a retrospective study to explore the predictive role of pretreatment peripheral blood inflammatory markers PIV, SII, NLR and PLR in immunotherapy in patients with unresectable advanced or locally advanced ESCC with an emphasis on efficacy and prognosis. We compared the above indices and screened the risk factors affecting prognosis by unifactorial and multifactorial survival analysis to discover the factors with the best predictive value regarding the efficacy and prognosis of immunotherapy in patients with ESCC. We will explore new indicators for predicting the efficacy and prognosis of immunotherapy in ESCC patients.
Materials and methods
Clinical materials
Clinical data of inoperable advanced or locally advanced ESCC patients who received immunotherapy in Department of Integrated Chinese and Western Medicine and Department of Medical Oncology, Zhongshan Hospital (Xiamen), Fudan University between May 2019 and August 2023 were retrospectively analysed. The patients in this study were refined with cervicothoracic, abdominopelvic and pelvic enhancement computed tomography (CT), cranial enhancement magnetic resonance imaging (MRI) and/or whole-body bone scanning as needed. The clinical staging of patients was based on the American Joint Committee on Cancer (AJCC) 8th edition. The inclusion criteria were as follows: 1) age greater than 18 years and complete medical records; 2) pathology of ESCC; 3) advanced or locally advanced patients (clinical stage III or IV) who were inoperable at the time of immunotherapy; and 4) completion of at least 2 cycles of immunotherapy. The exclusion criteria were as follows: 1) combination of other malignant tumours; 2) combination of infectious diseases, haematologic diseases, autoimmune diseases, and other conditions that could affect inflammatory indices within 2 weeks prior to immunotherapy; 3) recent or undergoing anti-inflammatory or immunosuppressive treatments; and 4) severe cardiac, hepatic, and renal insufficiency diseases. A total of 196 patients met the inclusion criteria. According to the above inclusion and exclusion criteria, a total of 107 patients were included in this study.
Research methods
Data Collection Basic data, clinical information, pathological and imaging data, and laboratory examinations of enrolled patients were collected through the electronic medical record system. The collected information included sex, age of onset, primary tumour location, pathological grade, local lymph node metastasis, distant metastasis, stage, HER-2 (human epidermal growth factor receptor 2) expression, PD-L1 expression, MMR, TMB, time of first use of immunotherapy, treatment line, specific drugs, treatment effect, time of progression after immunotherapy, time of death after immunotherapy, time of the last follow-up, patient status at the time of the last follow-up, and haematocrit value within 1 week before the first immunotherapy. The PIV, SII, NLR, and PLR were calculated from the haematocrit values of enrolled patients within 1 week before immunotherapy: 1) PIV = neutrophil count × platelet count × monocyte count/lymphocyte count; 2) SII = platelet count × neutrophil count/lymphocyte count; 3) NLR = neutrophil count/lymphocyte count; and 4) PLR = platelet count/lymphocyte count.
Follow-up Observation A combination of electronic medical records, telephone, internet hospital and outpatient review were used to establish the patient’s diagnosis and treatment, laboratory and imaging tests and patient survival. The follow-up period is up to September 30, 2023.
Treatment Enrolled patients received at least 2 cycles of immunotherapy (alone, in combination with chemotherapy, or in combination with targeted therapy) with programmed death-1 (PD-1) inhibitors (including pembrolizumab, nivolumab, tislelizumab, camrelizumab, sintilimab, toripalimab, etc.). The dosage was as recommended by the instruction manual and was to be administered every 3 weeks. Before each immunotherapy session, routine blood test, routine urine test, myocardial markers, electrolytes of liver and kidney function, inflammatory indices, adrenocortical function, thyroid function, tumour markers and electrocardiogram were checked, and enhanced CT scan of cervical, thoracic and abdominal area were checked every 2 cycles of treatment to evaluate the efficacy of the treatment (if the patient cannot tolerate enhanced CT, CT with scanning was performed instead). Observational indicator efficacy assessment was performed according to response evaluation criteria in solid tumours (RECIST) 1.1, and details of each efficacy assessment, optimal efficacy status after immunotherapy, PFS time after immunotherapy, and OS time after immunotherapy were recorded. PFS time was the time from the beginning of immunotherapy to the time of disease progression or death due to any cause, and OS time was the time from the start of immunotherapy to death due to any cause.
The flow chart of this study is detailed in .
Statistical analysis
Statistical analysis was performed using SPSS 23.0, and statistical tests were performed using two-sided tests, with p < 0.05 being considered statistically significant. Mean ± standard deviation was used to statistically describe the measurement data that conformed to normality and chi-square; median and quartiles were used to statistically describe the measurement data that did not conform to normality and chi-square; frequency or constitutive ratio was used to statistically describe the count data. For measurements that met normality and the Chi-square test, a two independent samples t test was used for between-group comparisons, and analysis of variance (ANOVA) was used for pretreatment and post-treatment comparisons between groups. Two independent samples Wilcoxon rank-sum test was used for between-group comparisons of rank information or measures that did not satisfy normal distribution or chi-square. The Chi-square test was used for component ratios.
The ROC curves of PIV, SII, NLR, and PLR were plotted according to whether the best efficacy was effective or not as the ending index, the maximum value of Youden index was calculated from the ROC curves, and the value corresponding to the maximum value of Youden index was the optimal truncation value (Youden index = sensitivity + specificity-1), with which the indices had the best sensitivity and specificity. According to the best cut-off value, the patients were categorized into groups: higher than the best cut-off value defined the high group, and lower than the best cut-off value defined the low group. Survival curves were plotted by the Kaplan–Meier method, and one-way analyses were performed using the log-rank method; multifactorial analyses were performed using the Cox proportional risk regression model.
Results
Baseline characteristics
A total of 107 patients with inoperable advanced or locally advanced ESCC were included in this study, and the baseline characteristics of the patients are shown in .
Table 1. Baseline characteristics of patients with inoperable advanced or locally advanced ESCC.
Optimal cut-off values for PIV, SII, NLR, and PLR
ROC curves were plotted according to whether the parameter was effective (including CR, PR and SD) as an outcome indicator ().
Figure 2. ROC curves for peripheral blood inflammatory markers PIV, SII, NLR and PLR in patients with inoperable advanced or locally advanced ESCC prior to immunotherapy: The areas under the curve (AUC) for PIV, SII, NLR, and PLR were 0.833, 0.813, 0.810 and 0.535, the optimal cut-off values for PIV, SII, NLR, and PLR were 415.885, 834.295, 3.740 and 151.250.
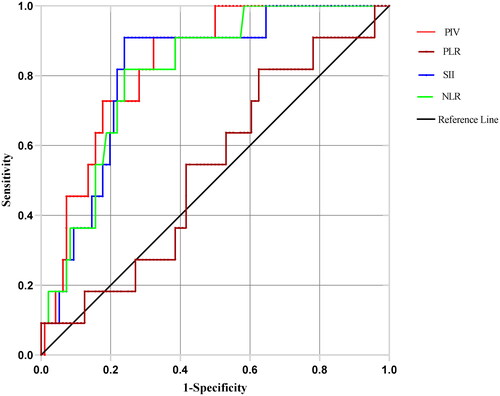
The optimal cutoff value in each group was determined by calculating the maximum value of the Youden index (). Based on the ROC curves and area under the curve of each group, it was suggested that PIV, SII and NLR had good predictive value regarding efficacy.
Table 2. Optimal cut-off values of PIV, SII, NLR, and PLR.
Effect of PIV, SII, NLR, and PLR on ORR and DCR of immunotherapy in patients with inoperable advanced or locally advanced ESCC
Grouped according to the optimal cutoff value, patients with higher values than the optimal cut-off value were defined as constituting the high group, and patients with lower values than the optimal cutoff value were defined as constituting the low group. The effects of PIV, SII, NLR, and PLR on the ORR and DCR of immunotherapy for inoperable advanced or locally advanced ESCC patients were evaluated by using the Chi-square test (). For inoperable advanced or locally advanced ESCC patients receiving immunotherapy, the SII and NLR low group had higher ORR, and the PIV, SII and NLR low group had higher DCR, and the difference was statistically significant.
Table 3. Effect of PIV, SII, NLR, and PLR on ORR and DCR of immunotherapy in patients with inoperable advanced or locally advanced ESCC.
Relationship between PIV, SII, NLR, and PLR and the prognosis of immunotherapy in patients with inoperable advanced or locally advanced ESCC
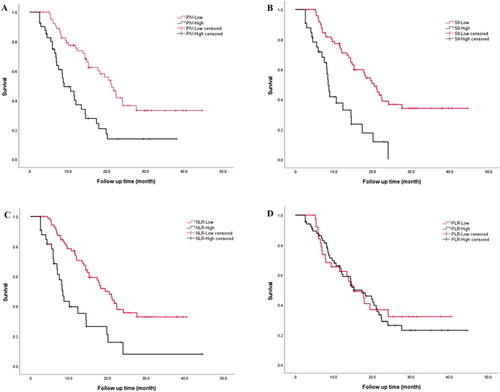
Based on the optimal cutoff values, PIV, SII, NLR, and PLR were categorized into high and low groups, and the survival analysis curves were plotted according to OS time and status at the last follow-up (). The patients in the low PIV, SII, and NLR groups had longer median OS times, with statistically significant differences (p < 0.01), and the patients in the high and low PLR groups had a similar median OS time, with no statistically significant differences (p = 0.83).
Figure 3. Kaplan–Meier estimates of overall survival (OS) according to PIV (A), SII (B), NLR (C) and PLR (D).
Univariate analysis using the log-rank method suggested that tumour stage, distant lymph node metastasis, lung metastasis, liver metastasis, PIV, SII, and NLR were risk factors affecting PFS and OS in immunotherapy of inoperable advanced or locally advanced ESCC (). The analysis of the above indicators in the multivariate Cox proportional risk model suggested that tumour stage and SII (best cutoff value 834.295) were independent risk factors for PFS and OS in immunotherapy of inoperable advanced or locally advanced ESCC ().
Table 4. Univariate analysis of factors affecting PFS and OS in immunotherapy for inoperable advanced or locally advanced ESCC.
Table 5. Multifactorial analysis of factors affecting PFS and OS in immunotherapy of inoperable advanced or locally advanced ESCC.
Discussion
Immunotherapy varies widely in patients with advanced ESCC, and clear predictors of efficacy and prognosis are lacking [Citation12]. Inflammatory response is involved in immunotherapy of tumours, and studies have confirmed that systemic inflammatory response is associated with poor prognosis of tumours [Citation16]. In this study, we retrospectively analysed the clinical data of 107 patients with unresectable advanced or locally advanced ESCC undergoing immunotherapy to reveal for the first time the efficacy and prognostic predictive value of peripheral blood inflammatory markers and to screen for independent risk factors affecting prognosis by survival analysis. The study results indicated that peripheral blood inflammatory markers PIV, SII and NLR had efficacy and prognostic value in immunotherapy for patients with unresectable advanced or locally advanced ESCC, while PLR did not; according to the area under the ROC curve, the predictive value regarding the efficacy of immunotherapy was ranked as follows: PIV, SII, and NLR exhibited areas under the curve of 0.833, 0.813 and 0.813, respectively; peripheral blood inflammatory markers were classified into low and high groups according to the optimal cutoff value determined by the ROC curve; subgroup analysis indicated that low PIV, SII and NLR suggested that patients with unresectable advanced or locally advanced ESCC would have a longer PFS and OS after immunotherapy; univariate analysis suggested that tumour stage, distant lymph node metastasis, lung metastasis, liver metastasis, PIV, SII, and NLR were risk factors affecting PFS and OS in patients receiving immunotherapy for unresectable advanced or locally advanced ESCC, and multivariate Cox proportional risk model analysis suggested that tumour stage and SII (optimal cut-off value of 834.295) were independent risk factors.
TIM plays an important role in tumorigenesis and progression and is a potential marker of tumour immunotherapy response [Citation23, Citation24]. Inflammatory cells and inflammatory mediators are important components of TIM [Citation25, Citation26], and neutrophils, lymphocytes, monocytes, and platelets are involved in tumour-associated inflammatory responses and play an important role in tumorigenesis and progression [Citation16]. Neutrophils are involved in the inflammatory response and thus tissue destruction to promote cancer development; lymphocytes are involved in the regulation of the immune system and thus influence cancer development and progression [Citation27]; and platelets act as a source of transforming growth factor β and thus regulate the complex interplay of effector immune cell activation, extravasation, and systemic inflammation to play a pleiotropic role in tumour progression and immune escape [Citation28]. The NLR and PLR, based on the ratio of neutrophils, platelets to lymphocytes, became the first peripheral blood inflammatory markers to be investigated, and have been shown to correlate with the prognosis of a variety of tumours, including lung, gastric, and colorectal cancers [Citation25, Citation26]. The ORIENT-2 study explored the predictive value of NLR regarding efficacy of combining immuno-chemotherapy in the treatment of advanced or metastatic ESCC and demonstrated that an NLR of ≥3 was associated with poor survival prognosis [Citation15]; patients with NLR ≥3.74 had significantly lower ORR and DCR than those with NLR <3.74, patients in the high NLR group had significantly lower median PFS and OS after immunotherapy than those in the low NLR group, and NLR ≥3.740 was one of the risk factors affecting the prognosis of patients with inoperable advanced or locally advanced ESCC treated with immunotherapy in the univariate analysis, further confirming the value of NLR in predicting the efficacy of immunotherapy for ESCC. PLR ≥133 was confirmed to be associated with an independent risk factor for poor prognosis in postoperative chemotherapy-treated patients with nonmetastatic ESCC [Citation29]. However, the results of the present study showed that PLR was not significantly correlated with the prognosis of immunotherapy in patients with unresectable advanced or locally advanced ESCC and did not have value in predicting the efficacy and prognosis. This suggests that PLR may have different values according to differences in stage of treatment and therapeutic approach in ESCC patients, and the possible reasons deserve further study. As emerging peripheral blood inflammatory markers in recent years, PIV and SII reflect inflammatory protumorigenic cells (i.e., neutrophils, platelets, and monocytes) and anticancer cells (i.e., lymphocytes), and a high value of PIV and SII reflects a state of systemic immunosuppression, with a greater likelihood of tumour progression and metastasis. Therefore, PIV and SII reflect host inflammation and the immune response more comprehensively than NLR and PLR [Citation30, Citation31]. Studies on the efficacy and prognosis of PIV and SII in the treatment of ESCC patients have focused on surgery, preoperative neoadjuvant chemotherapy, and postoperative adjuvant chemotherapy and radiotherapy [Citation32, Citation33], but no studies have been conducted to determine the efficacy and prognosis of immunotherapy in ESCC patients who are not operable. The present study confirmed that PIV and SII had efficacy and prognostic value in immunotherapy for patients with unresectable advanced or locally advanced ESCC and were superior to NLR and PLR; unifactorial and multifactorial regression staging further confirmed that a high SII (SII ≥834.295) was an independent risk factor for the duration of PFS and OS in immunotherapy.
In the present study, we explored the predictive value of peripheral blood inflammatory markers regarding the efficacy and prognosis of immunotherapy in patients with unresectable advanced or locally advanced ESCC, compared and screened the best predictive indicators and determined the indicator cutoff values for future use in immunotherapy. However, there are some shortcomings in this study. First, this study is a single-centre retrospective analysis, which may have sample selection bias and experimental error, and prospective studies need to be carried out in the later stages regarding the above predictive indices to further confirm their clinical value. Second, the optimal truncation values of PIV, SII, NLR, and PLR reported by a number of studies, including the present one, do not agree with each other, which is troubling in terms of clinical application, and the clinical application of these indices needs to be further explored in later stages. The follow-up study can further explore the relatively certain reference value range for clinical use. Finally, this study only explored the efficacy and prognosis of immunotherapy from the perspective of inflammatory response and TIM and did not incorporate related factors such as immune cells and even tumour cells, so the predictive value is relatively limited. Future studies can be considered for the establishment of a multidimensional comprehensive efficacy and prognosis prediction system that incorporates peripheral blood inflammatory markers along with PD-L1, TMB and MMR for greater comprehensiveness and accuracy.
Conclusions
Peripheral blood PIV, SII, and NLR before immunotherapy had predictive value regarding immunotherapy outcome in patients with inoperable advanced or locally advanced ESCC, and SII was an independent risk factor affecting the survival prognosis of patients, with SII ≥834.295 suggesting a poor prognosis.
Ethical approval
This study was approved by Ethics Committee of Zhongshan Hospital, Fudan University (Xiamen Branch) (No. B2023-137).
| Abbreviations | ||
| PIV | = | panimmune-inflammation value |
| SII | = | systemic immune-inflammation index |
| NLR | = | neutrophil-to-lymphocyte ratio |
| PLR | = | platelet-to-lymphocyte ratio |
| ESCC | = | oesophageal squamous cell carcinoma |
| ROCs | = | receiver operating characteristic curves |
| ORR | = | objective response rate |
| DCR | = | disease control rate |
| PFS | = | progression-free survival |
| OS | = | overall survival |
| PS | = | performance status |
| EC | = | oesophageal cancer |
| SCC | = | squamous cell carcinoma |
| PD-L1 | = | programmed cell death ligand 1 |
| TMB | = | tumour mutational burden |
| MMR | = | mismatch repair |
| TIM | = | tumour immune microenvironment |
| CT | = | computed tomography |
| MRI | = | magnetic resonance imaging |
| RECIST | = | response evaluation criteria in solid tumours |
| AJCC | = | American Joint Committee on Cancer |
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The data that support the findings of this study are available from the corresponding author XP Z upon reasonable request.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660.
- Arnold M, Soerjomataram I, Ferlay J, et al. Global incidence of oesophageal cancer by histological subtype in 2012. Gut. 2015;64(3):381–387. doi: 10.1136/gutjnl-2014-308124.
- Sheikh M, Poustchi H, Pourshams A, et al. Individual and combined effects of environmental risk factors for esophageal cancer based on results from the golestan cohort study. Gastroenterology. 2019;156(5):1416–1427. doi: 10.1053/j.gastro.2018.12.024.
- Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3.
- Huang FL, Yu SJ. Esophageal cancer: risk factors, genetic association, and treatment. Asian J Surg. 2018;41(3):210–215. doi: 10.1016/j.asjsur.2016.10.005.
- Wang Z, Hu M, Hu Y, et al. Paclitaxel plus cisplatin and 5-fluorouracil induction chemotherapy for locally advanced borderline-resectable esophageal squamous cell carcinoma: a phase II clinical trial. Esophagus. 2022;19(1):120–128. doi: 10.1007/s10388-021-00864-8.
- Sun JM, Shen L, Shah MA, et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. Lancet. 2021;398(10302):759–771. doi: 10.1016/S0140-6736(21)01234-4.
- Luo H, Lu J, Bai Y, et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-Free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: the ESCORT-1st randomized clinical trial. JAMA. 2021;326(10):916–925. doi: 10.1001/jama.2021.12836.
- Doki Y, Ajani JA, Kato K, et al. Nivolumab combination therapy in advanced esophageal squamous-cell carcinoma. N Engl J Med. 2022;386(5):449–462. doi: 10.1056/NEJMoa2111380.
- Obermannová R, Alsina M, Cervantes A, et al. Oesophageal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33(10):992–1004. doi: 10.1016/j.annonc.2022.07.003.
- Ajani JA, D’Amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, version 2.2023, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2023;21(4):393–422. doi: 10.6004/jnccn.2023.0019.
- Zhang RX, Kang XZ, Zheng QF[, et al. Advances of immunotherapy-related biomarker in esophageal carcinoma]. Zhonghua Wei Chang Wai Ke Za Zhi. 2023;26:396–400.
- Salem ME, Puccini A, Xiu J, et al. Comparative molecular analyses of esophageal squamous cell carcinoma, esophageal adenocarcinoma, and gastric adenocarcinoma. Oncologist. 2018;23(11):1319–1327. doi: 10.1634/theoncologist.2018-0143.
- Tsao MS, Kerr KM, Kockx M, et al. PD-L1 immunohistochemistry comparability study in Real-Life clinical samples: results of blueprint phase 2 project. J Thorac Oncol. 2018;13(9):1302–1311. doi: 10.1016/j.jtho.2018.05.013.
- Xu J, Li Y, Fan Q, et al. Clinical and biomarker analyses of sintilimab versus chemotherapy as second-line therapy for advanced or metastatic esophageal squamous cell carcinoma: a randomized, open-label phase 2 study (ORIENT-2). Nat Commun. 2022;13(1):857. doi: 10.1038/s41467-022-28408-3.
- Sun D, Liu J, Zhang L. Establishment of tumor immune microenvironment classification model to select patients sensitive to immunotherapy. J Thorac Oncol. 2023;18(10):e111–111e112. doi: 10.1016/j.jtho.2023.07.003.
- Marazziti D, Torrigiani S, Carbone MG, et al. Neutrophil/lymphocyte, platelet/lymphocyte, and monocyte/lymphocyte ratios in mood disorders. Curr Med Chem. 2022;29(36):5758–5781. doi: 10.2174/0929867328666210922160116.
- Feng J, Wang L, Yang X, et al. Pretreatment Pan-Immune-inflammation value (PIV) in predicting therapeutic response and clinical outcomes of neoadjuvant immunochemotherapy for esophageal squamous cell carcinoma. Ann Surg Oncol. 2023;31(1):272–283. doi: 10.1245/s10434-023-14430-2.
- Kam AE, Masood A. The prognostic role of the neutrophil to lymphocyte ratio at recurrence in esophageal squamous cell carcinoma: challenges and future directions. Ann Surg Oncol. 2021;28(6):2939–2940. doi: 10.1245/s10434-021-09640-5.
- Sasahara M, Kanda M, Shimizu D, et al. High preoperative platelet to lymphocyte ratio is associated with a greater risk of postoperative complications and hematogenous recurrences in esophageal squamous cell carcinoma patients receiving neoadjuvant treatment. Dig Surg. 2023;40(1-2):48–57. doi: 10.1159/000530018.
- Guo W, Cai S, Zhang F, et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected non-small cell lung cancer. Thorac Cancer. 2019;10(4):761–768. doi: 10.1111/1759-7714.12995.
- Wang Y, Lyu J, Jia H, et al. Clinical utility of the systemic immune-inflammation index for predicting survival in esophageal squamous cell carcinoma after radical radiotherapy. Future Oncol. 2021;17(20):2647–2657. doi: 10.2217/fon-2021-0304.
- Adegoke NA, Gide TN, Mao Y, et al. Classification of the tumor immune microenvironment and associations with outcomes in patients with metastatic melanoma treated with immunotherapies. J Immunother Cancer. 2023;11(10):e007144. doi: 10.1136/jitc-2023-007144.
- Hirahara N, Matsubara T, Fujii Y, et al. Comparison of the prognostic value of immunoinflammation-based biomarkers in patients with gastric cancer. Oncotarget. 2020;11(27):2625–2635. doi: 10.18632/oncotarget.27653.
- Hara K, Aoyama T, Yamada T, et al. The prognostic value of the perioperative systemic inflammation score for patients with advanced gastric cancer. Anticancer Res. 2020;40(3):1503–1512. doi: 10.21873/anticanres.14095.
- Huang H, Liu Q, Zhu L, et al. Prognostic value of preoperative systemic immune-inflammation index in patients with cervical cancer. Sci Rep. 2019;9(1):3284. doi: 10.1038/s41598-019-39150-0.
- Binnewies M, Roberts EW, Kersten K, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med. 2018;24(5):541–550. doi: 10.1038/s41591-018-0014-x.
- Faria A, Andrade SS, Peppelenbosch MP, et al. Platelets in aging and cancer-"double-edged sword. Cancer Metastasis Rev. 2020;39(4):1205–1221. doi: 10.1007/s10555-020-09926-2.
- Yang Y, Xu H, Zhou L, et al. Platelet to lymphocyte ratio is a predictive marker of prognosis and therapeutic effect of postoperative chemotherapy in non-metastatic esophageal squamous cell carcinoma. Clin Chim Acta. 2018;479:160–165. doi: 10.1016/j.cca.2018.01.013.
- Gao Y, Guo W, Cai S, et al. Systemic immune-inflammation index (SII) is useful to predict survival outcomes in patients with surgically resected esophageal squamous cell carcinoma. J Cancer. 2019;10(14):3188–3196. doi: 10.7150/jca.30281.
- Fucà G, Guarini V, Antoniotti C, et al. The Pan-Immune-inflammation value is a new prognostic biomarker in metastatic colorectal cancer: results from a pooled-analysis of the valentino and TRIBE first-line trials. Br J Cancer. 2020;123(3):403–409. doi: 10.1038/s41416-020-0894-7.
- Feng J, Wang L, Yang X, et al. Clinical utility of preoperative pan-immune-inflammation value (PIV) for prognostication in patients with esophageal squamous cell carcinoma. Int Immunopharmacol. 2023;123:110805. doi: 10.1016/j.intimp.2023.110805.
- Zhang H, Shang X, Ren P, et al. The predictive value of a preoperative systemic immune-inflammation index and prognostic nutritional index in patients with esophageal squamous cell carcinoma. J Cell Physiol. 2019;234(2):1794–1802. doi: 10.1002/jcp.27052.