Abstract
Objective
The long-term outcome of thiopurine therapy in patients with ulcerative colitis (UC) enrolled in prospective trials have not been evaluated. We aimed to assess the effects of optimised thiopurine maintenance therapy for UC.
Methods
Long-term data were obtained from patients from our center enrolled in two randomised, prospective, open-label, controlled studies comprising 66 thiopurine-naïve moderate-to-severe patients with UC consisting of a low dose azathioprine (AZA)/allopurinol combination or AZA monotherapy. Following the randomised trials, treatment was adjusted according to adverse effects and metabolites. Patients requiring optimisation initially on AZA monotherapy treatment were switched to low dose AZA in combination with allopurinol, low dose 6-mercaptopurin in combination with allopurinol, or 6-mercaptopurin treatment alone, and those treated with low dose AZA in combination with allopurinol were switched to low dose 6-mercaptopurin in combination with allopurinol or 6-mercaptopurin alone.
Results
A total of 62 patients were included in the analysis; 31 were initially treated with AZA monotherapy and 31 with low dose AZA in combination with allopurinol. Initial treatment was tolerated by 67% patients (7 AZA monotherapy and 28 low dose AZA in combination with allopurinol), increasing to 94% (58 patients) post-adjustment. After a median 52-month follow-up period, 38 (93%) out of the 41 primary responding patients-maintained clinical remission without steroids, biologics or surgery. The four intolerant patients and the 17 not responding to optimisation were more likely to require colectomy (odds ratio 16.36; 95% confidence interval 3.08–87.03, p < 0.0001).
Conclusion
Optimised thiopurine therapy demonstrated effective long-term treatment for patients with ulcerative colitis.
Introduction
Immunosuppressive thiopurines are recommended as maintenance therapy for steroid-dependent ulcerative colitis (UC) patients or those with an insufficient aminosalicylates response. The main thiopurines are azathioprine (AZA) and 6-mercaptopurine (6MP). Two retrospective studies including 4968 and 373 UC patients allocated to monotherapy with AZA or 6MP found that 52.7% and 41% (5 years) achieved remission using thiopurines [Citation1,Citation2]. Another observational study including 104 patients with UC found a remission rate of 41% after 2 years [Citation3]. However, several studies suggest that combination therapy with thiopurines and allopurinol may be even more effective in achieving long-term remission [Citation4]. Accordingly, two prospective studies concluded that low-dose AZA and allopurinol (L-AZA/ALLO) treatment lasting up to one year was safe and effective in moderate-to-severe UC patients compared to AZA monotherapy [Citation5,Citation6]. Another prospective study comparing optimised 6MP treatment with placebo demonstrated that 6MP was significantly better than placebo [Citation7]. Furthermore, both a prospective and a retrospective study reported that first-line L-AZA/ALLO or low-dose 6MP in combination with allopurinol (L-6MP/ALLO) treatment was effective and safe in patients with inflammatory bowel disease and could be considered first-line for those eligible for thiopurines. L-AZA/ALLO therapy may expedite remission, reduce adverse events and decrease the need for therapeutic drug monitoring following remission [Citation8,Citation9]. Recently published long-term population data demonstrated the profound effect thiopurines have on the natural history and colectomy rates in patients with UC [Citation10].
This study aims to evaluate the long-term effectiveness of thiopurine treatment in a prospectively enrolled UC patient cohort following treatment optimisation using 6-thioguanine nucleotide (6-TGN) measurements. Therapy was changed to a combination of L-AZA/ALLO, MP and L-MP/ALLO), if patients were intolerant to initial treatment or if the 6-TGN values were below the therapeutic level.
Material and methods
Study design
This study included long-term follow-up data from patients from our center enrolled in two previously published randomised controlled open-label trials [Citation2,Citation3]. These trials used a 1:1 random allocation to assign moderate-to-severe UC thiopurine-naïve patients to two comparison groups: L-AZA/ALLO (median AZA dose, 50 mg; ALLO dose, 100 mg) and AZA monotherapy (median dose, 200 mg).
The inclusion criteria were age between 18 and 80 years, thiopurine-naïve, clinically, and histologically established UC, and normal thiopurine S-methyltransferase (TPMT) defined as a homozygous wild-type TPMT genotype or a phenotype with more than 14 U/mL erythrocytes. Additionally, patients were categorised as steroid-dependent if they could not taper the medicine after a high-dose prednisolone course or steroid-refractory if they had a poor steroid response requiring infliximab (IFX) treatment. Inclusion criteria also included endoscopy revealing active inflammation during the current disease flare-up and a negative stool test for pathogenic bacteria, including Clostridioides difficile. The exclusion criteria included kidney disease with a glomerular filtration rate (GFR) < 50 mL/min, persistently elevated alanine aminotransferase (ALT) levels, participation in other interventional clinical trials, pregnancy, breastfeeding, previous thiopurine treatment and treatment with biologics other than IFX. The first study included 25 patients, lasting 24 weeks from July 2013 to April 2015. The second study included 41 patients from our center, lasting 52 weeks from January 2016 to February 2021. Patients from the other centers in this study were not included as they were not using optimised thiopurine treatment. Patients were followed up until 1 December 2021, thiopurine discontinuation, or therapy escalation with steroids, biologics or surgery. Data were found by retrospectively reviving the patients’ medical charts after they stopped participation in the randomised trials. The decision to change the treatment was made by the attending physician based on metabolite concentrations, side effects and clinical response. According to local guidelines, the sequence for changing treatment was as follows: from AZA to L-AZA/ALLO, if this is not tolerated, switch to 6MP/ALLO. If there is suspicion of side effects to ALLO, switch to monotherapy with 6MP.
Outcomes
The primary outcome was ongoing thiopurine therapy without surgery or steroid and biologic treatment initiation. The secondary outcomes included 6-TGN levels, cancer and death.
Subgroup analysis
In this follow-up study, patients were divided into two subgroups.
Responders (n = 41) were patients that tolerated continued or optimised thiopurine treatment and remained in clinical remission without steroids, biologics or surgery for at least one year.
Non-responders (n = 21) did not tolerate the optimised thiopurine treatment or had persistent symptoms despite 6-TGN being within therapeutic levels.
Statistical analysis
We used STATA version 17 for Windows (StataCorp LLC, College Station, TX) for statistical analyses. A Kaplan–Meier plot and log-rank test were used to evaluate the colectomy risk between responders and non-responders. A p value < 0.05 was considered statistically significant.
Ethics
At the time of their written consent to participate in the original study, all patients gave permission to access their data for up to 10 years.
Results
Clinical characteristics
A total of 41 patients were responders and 21 were non-responders. The baseline characteristics of the two groups were comparable (). The disease duration before starting thiopurine treatment was similar in the two groups.
Table 1. Patient characteristics when randomised (medians and 25–75% IQR).
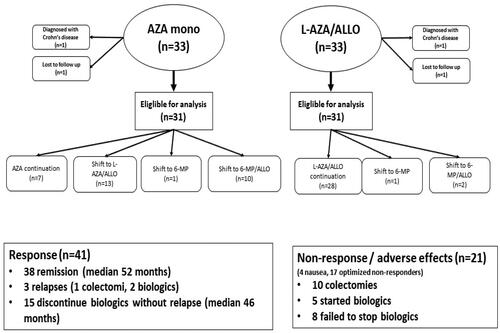
This study excluded four patients: two due to a diagnosis of Crohn’s disease and two were lost to follow-up within a year. demonstrates the patient flow chart during the thiopurine treatment optimisation. Overall, 67% patients tolerated initial treatment (seven AZA and 28 L-AZA/ALLO; ), increasing to 94% (58 patients) post-intervention. In the AZA treatment group, 23% patients initially tolerated and achieved therapeutic 6-TGN levels, compared to 90% in the L-AZA/ALLO-first treatment group. Of the 24 patients requiring optimisation in the AZA group, 21 tolerated the optimised treatment and achieved therapeutic 6-TGN levels by changing treatments to L-AZA/ALLO, 6MP or L-6MP/ALLO. Three patients in the L-AZA/ALLO group changed treatment to 6MP or L-6MP/ALLO, with two tolerating this intervention ().
Responders
Forty-one patients (66%) had a primary sustained response after 1 year. Thirty-eight patients remained in clinical remission without steroids, biologics and surgery after a 52-month median follow-up period (). Of the three patients without a sustained response, one underwent a colectomy, and two started biologic treatment.
Concomitant IFX treatment was received by 15/41 (37%) patients at inclusion. Patients in clinical remission stopped IFX treatment after 3–6 months per the randomised studies protocol. No patient reinitiated IFX treatment during the follow-up period and remained in clinical remission for a 46-month median observation period ().
Non-responders
Four patients were thiopurine intolerant during optimisation secondary to emesis. Seventeen patients had no treatment response despite therapeutic TGN levels (), with eight continued biologic treatments and five initiating treatments during the follow-up period (). In the non-responding patients who continued biologics and were not operated, three had only IFX and four treated only with vedolizumab. One patient was first treated with IFX and subsequently with vedolizumab. About 10/21 (48%) patients in the non-responder group underwent a colectomy (odds ratio 16.36; 95% confidence interval 3.08–87.03) compared to responders (). Four of the operated patients were not having a biological, three had treatment solely with IFX, three had first IFX and shifted to vedolizumab and one patient had certolizumab first and then shifted to vedolizumab. Only one patient with nausea was operated.
Figure 2. Kaplan–Meier survival curve for remaining free of colectomy comparing responders (n = 41) with non-responders (n = 21) (p < 0.0001).
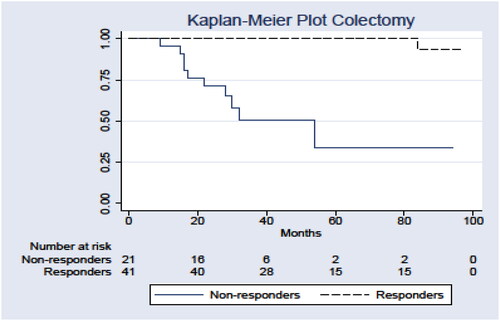
Table 2. Final 6-TGN (pmol/8 × 108 RBC) values after thiopurine optimisation – medians and 25–75 IQR, cancers and deaths.
Secondary outcomes
Responders and non-responders demonstrated no significant difference in 6-TGN levels. One older man died during the follow-up period (cardiovascular-related), while no cancers were registered ().
Discussion
In this follow-up study of UC patients from our center, previously enrolled in two randomised prospective studies, 66% patients responded to optimised thiopurine treatment. Over a 52-month median follow-up period, 61% patients remained in clinical remission without steroid, biologic and surgical treatment. These results are consistent with those of previous retrospective observations demonstrating thiopurine effectiveness in maintaining remission among UC patients [Citation1–3]. Our result was achieved by observing a median TGN value of 392 pmol/8 × 108 RBC, thus, indicating that the standard 6-TGN cut-off (>230 pmol/8 × 108 RBCs) is suboptimal [Citation11]. One prospective study demonstrated that an increased 6-TGN level, with a median level of 374 pmol/8 × 108 RBC, was associated with higher rates of clinical remission [Citation6]. We also know that very high TGN levels are seen in patients on low-dose AZA or 6MP and allopurinol co-therapy without toxicity or correlation to clinical response. This issue has been addressed and raises doubt about the significance of a therapeutic or toxic range of TGNs [Citation12]. When utilising the entire range of available options for optimising thiopurine therapy, including AZA, L-AZA/ALLO, 6MP or L-6MP/ALLO (not thioguanine due to unavailability in Denmark), 94% of patients tolerated treatment. This tolerability was greater than that previously reported for AZA, L-AZA/ALLO, L-6MP/ALLO or 6MP therapy. Previous prospective studies on AZA monotherapy had 46%-60% patients being withdrawn due to intolerance [Citation5,Citation6,Citation13]. Another study found that 52% of patients who were intolerant to AZA tolerated 6MP [Citation14]. First-line therapy using L-AZA/ALLO or L-6MP/ALLO resulted in 26% patients not tolerating the treatment [Citation10]. Another study found that 21% patients were intolerant to 6MP and about 50% of the patients changed therapy to L-6MP/ALLO in order to reach the target TGN level [Citation7]. Only four patients in this study could not tolerate optimisation attempts due to emesis as an adverse event. Patients with NUDT15 heterozygosity should be treated with low dose thiopurines which were effective and well tolerated [Citation15].
After a median 52-month observation period, the total colectomy rate was 17% in this cohort of moderate-to-severe UC patients. Similarly, colectomy rates in moderate-to-severe UC were approximately 50% after roughly five years in the pre-immunomodulation therapy period [Citation16].
Seventeen (24%) patients tolerating optimisation did not respond to thiopurine treatment despite therapeutic 6-TGN levels. Similarly, this observation was noted in line with our previous prospective study [Citation6]. Half the patients without a thiopurine response had a colectomy during the observation period and 13/17 of the non-responding patients had concomitant therapy with at least one biologic drug.
Of the 31 patients initiating treatment with L-AZA/ALLO in this study, 28 achieved therapeutic 6-TGN values without delay, tolerated the treatment and did not require optimisation. However, of the 31 patients initiated on AZA alone, seven tolerated and continued the treatment, while the remaining 24 required optimisation. This finding supports starting L-AZA/ALLO as a first-line therapy to expedite remission and decrease therapeutic drug monitoring after remission. Therefore, L-AZA/ALLO should be prescribed for patients with the wild-type genotype of TPMT.
In this study, 15 patients who responded to optimised thiopurine therapy safely discontinued concomitant IFX treatment 3–6 months following therapy and were not reinitiated on it during the follow-up period. This clinical remission was maintained for a median 46-month observation period. However, a previous systematic review and meta-analysis demonstrated an overall relapse risk in 38% UC patients following anti-TNF discontinuation after 6–24 months [Citation17].
Adverse events following thiopurine therapy initiation are thoroughly described in our previous studies [Citation5,Citation6]. Optimised patients demonstrated an excellent long-term safety profile without any serious adverse events or cancers registered; however, one death due to cardiac disease in an older man was reported.
The strength of this study is that follow-up of prospectively enrolled patients from a single center was achieved, with only two of the 64 patients lost to follow-up. The limitations of this study include the lack of data on clinical parameters, including C-reactive protein, calprotectin values, activity scores and endoscopic scores.
In conclusion, optimised thiopurine treatment in combination with L-AZA/ALLO should be considered first-line therapy rather than AZA monotherapy in UC patients, where immunomodulator therapy is indicated. The target 6-TGN concentration should be approximately 400 pmol/8 × 108 RBC. Approximately 25% of patients exhibited no treatment optimisation response and had a significantly higher colectomy risk. Further research identifying factors to predict thiopurine nonresponse is needed.
Contributors
AMN collected the data. LGG performed statistical analyses. All authors contributed to the manuscript, including the critical review and approval. All authors had full access to all data and were responsible for the decision to submit the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that all others meeting the criteria have been included.
| Abbreviations | ||
| AZA | = | azathioprine |
| 6MP | = | 6-mercaptopurine |
| UC | = | ulcerative colitis |
| L-AZA/ALLO | = | low-dose azathioprine and allopurinol |
| L-6MP/ALLO | = | low-dose 6-mercaptopurine and allopurinol |
| 6-TGN | = | 6-thioguanine |
| TPMT | = | thiopurine S-methyltransferase |
| IXF | = | infliximab |
| GFR | = | glomerular filtration rate |
| ALT | = | alanine aminotransferase |
| antiTNF | = | anti-tumor necrosis factor |
Acknowledgments
We would like to thank Editage (www.editage.com) for English language editing.
Disclosure statement
Anette Mertz Nielsen, Lise Lotte Glud, Marianne Kiszka-Kanowitz: none.
Klaus Theede received personal fees from Ferring A/S, Takeda, Tillotts and Pfizer outside of the submitted work.
Data availability statement
No data are available.
Additional information
Funding
References
- Stournaras E, Qian W, Pappas A, et al. Thiopurine monotherapy is effective in ulcerative colitis but significantly less so in crohn’s disease: long-term outcomes for 11 928 patients in the UK inflammatory bowel disease. Gut. 2021;70(4):677–686. doi: 10.1136/gutjnl-2019-320185.
- Ardabili RA, Jeuring S, Mujagic Z, et al. Classic drugs in the time of new drugs: real-world long-term outcomes of thiopurine monotherapy in 1016 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2022;56(6):1030–1043. doi: 10.1111/apt.17128.
- Rispo A, Testa A, De Palma GD, et al. Different profile of efficacy of thiopurines in ulcerative colitis and Crohn’s disease. Inflamm Bowel Dis. 2015;21(11):2570–2575. doi: 10.1097/MIB.0000000000000538.
- Turbayne AK, Sparrow MJ. Low‑dose azathioprine in combination with allopurinol: the past, present and future of this useful duo. Dig Dis Sci. 2022;67(12):5382–5391. doi: 10.1007/s10620-022-07719-x.
- Kiszka-Kanowitz M, Theede K, Mertz-Nielsen A. Randomized clinical trial: a pilot study comparing efficacy of low-dose azathioprine and allopurinol to azathioprine on clinical outcomes in inflammatory bowel disease. Scand J Gastroenterol. 2016;51(12):1470–1475. doi: 10.1080/00365521.2016.1216589.
- Kiszka-Kanowitz M, Theede K, Thomsen SB, et al. Low-dose azathioprine and allopurinol versus azathioprine monotherapy in patients with ulcerative colitis (AAUC): an investigator-initiated, open, multicenter, parallel-arm, randomised controlled trial. EClinicalMedicine. 2022;45:101332. doi: 10.1016/j.eclinm.2022.101332.
- Löwenberg M, Volkers A, van Gennep S, et al. Mercaptopurine for the treatment of ulcerative colitis: a randomized placebo-Controlled trial. J Crohns Colitis. 2023;17(7):1055–1065. doi: 10.1093/ecco-jcc/jjad022.
- Vasudevan A, Con D, De Cruz P, et al. Clinical trial: combination allopurinol-thiopurine versus standard thiopurine in patients with IBD escalating to immunomodulators (the DECIDER study). Aliment Pharmacol Ther. 2024;59(4):504–514. doi: 10.1111/apt.17831.
- van Liere ELSA, Bayoumy AB, Mulder CJJ, et al. Azathioprine with allopurinol is a promising first-line therapy for inflammatory bowel diseases. Dig Dis Sci. 2022;67(8):4008–4019. doi: 10.1007/s10620-021-07273-y.
- Eriksson C, Rundquist S, Cao Y, et al. Impact of thiopurines on the natural history and surgical outcome of ulcerative colitis: a cohort study. GUT. 2019;68(4):623–632. doi: 10.1136/gutjnl-2017-315521.
- Casteele NV, Herfarth H, Katz J, et al. American gastroenterological association institute technical review on the role of therapeutic drug monitoring in the management of inflammatory bowel diseases. Gastroenterology. 2017;153(3):835–857.e6. doi: 10.1053/j.gastro.2017.07.031.
- Duley JA, Florin T. Thiopurine therapies: problems, complexities, and progress with monitoring thioguanine nucleotides. Ther Drug Monit. 2005;27(5):647–654. doi: 10.1097/01.ftd.0000169061.52715.3e.
- Dassopoulos T, Dubinsky MC, Bentsen JL, et al. Randomised clinical trial: individualised vs. weight-based dosing of azathioprine in Crohn’s disease. Aliment Pharmacol Ther. 2014;39(2):163–175. doi: 10.1111/apt.12555.
- Hindorf U, Johansson M, Eriksson A, et al. Mercaptopurine treatment should be considered in azathioprine intolerant patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2009;29(6):654–661. doi: 10.1111/j.1365-2036.2008.03925.x.
- Maeda T, Sakuraba H, Hiraga H, et al. Long-term efficacy and tolerability of dose-adjusted thiopurine treatment in maintaining remission in inflammatory bowel disease patients with NUDT15 heterozygosity. Intest Res. 2022;20(1):90–100. doi: 10.5217/ir.2020.00133.
- Gustavsson A, Halfvarson J, Magnuson A, et al. Long-term colectomy rate after intensive intravenous corticosteroid therapy for ulcerative colitis prior to the immunosuppressive treatment era. Am J Gastroenterol. 2007;102(11):2513–2519. doi: 10.1111/j.1572-0241.2007.01435.x.
- Gisbert JP, Marín AC, Chaparro M. The risk of relapse after anti-TNF discontinuation in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol. 2016;111(5):632–647. doi: 10.1038/ajg.2016.54.