Abstract
Background
Imaging is used to monitor disease activity in small bowel Crohn’s disease (CD). Magnetic Resonance Enterography is often employed as a first modality in the United Kingdom for assessment and monitoring; however, waiting times, cost, patient burden and limited access are significant. It is as yet uncertain if small bowel intestinal ultrasound (IUS) may be a quicker, more acceptable, and cheaper alternative for monitoring patients with CD.
Methods
A clinical service evaluation of imaging pathways was undertaken at a single NHS site in England, United Kingdom. Data were collected about patients who were referred and underwent an imaging analysis for their IBD. Only patients who underwent a therapy change were included in the analysis. Data were collected from care episodes between 01 January 2021–30 March 2022.
Results
A combined total of 193 patient care episodes were reviewed, 107 from the IUS pathway and 86 from the MRE pathway. Estimated costs per patient in the IUS pathway was £78.86, and £375.35 per patient in the MRE pathway. The MRE pathway had an average time from referral to treatment initiation of 91 days (SD= ±61) with patients in the IUS pathway waiting an average of 46 days (SD= ±17).
Conclusions
Findings from this work indicate that IUS is a potential cost-saving option when compared to MRE when used in the management of CD. This is in addition to the cost difference of the radiological modalities. A large, multicentre, prospective study is needed to validate these initial findings.
KEY MESSAGES
What is already known on this topic – Ultrasound is a quick and accurate imaging investigation for patients living with Crohn’s disease. Its effect on the cost utility of an Inflammatory Bowel Disease service is unknown.
What this study adds – This work provides initial data suggesting that an ultrasound-based service may provide significant cost savings when compared to a magnetic resonance imaging-based service.
How this study might affect research, practice, or policy – This work is part of a larger programme of work to investigate the barriers to wider ultrasound implementation in UK IBD services. This work will contribute to the design of an implementation and training package for intestinal ultrasound in the UK.
Introduction
Disease distribution in Crohn’s Disease (CD) varies with up to 70% of patients having small bowel involvement [Citation1]. To ensure optimal long-term clinical outcomes, current recommendations based on the Selecting Therapeutic targets in inflammatory bowel disease (STRIDE-II) [Citation2] suggest using objective measures as treatment targets, rather than symptom resolution [Citation3].
Cross-sectional imaging is used to diagnose and monitor disease activity in small bowel CD [Citation4]. Magnetic Resonance Enterography (MRE) is often employed as a first modality in the United Kingdom for assessment and monitoring of small bowel CD [Citation4]. Waiting times for an national health service (NHS) MRE may be up to 4 weeks or in some instances longer and have increased due to the impact of the Covid-19 pandemic [Citation5–7]. Radiological reporting is then undertaken at a later date and may also add to delays. There is a clinical need to find quicker and cheaper alternatives for monitoring patients with CD.
Small bowel intestinal ultrasound (IUS) is an alternative to MRE, and has the potential to significantly reduce waiting times, speed up clinical decision making and improve patient experience and outcomes [Citation8]. Internationally, IUS is widely used for assessing and monitoring CD, and the METRIC trial has demonstrated its relative diagnostic accuracy in comparison to MRE [Citation9,Citation10], with levels of accuracy correlated with the volume of IUS reporting. There is an eagerness for the introduction of IUS into UK CD clinical practice [Citation11]. However, there remains questions regarding reasons why IUS is not more widely utilised in the United Kingdom. In a UK-wide survey of British Society of Gastroenterology members (Gastroenterologists, specialist medical trainees and IBD Nurse specialists), 103 responses were included in the data analysis. Responses came from 66 different NHS trusts from 14 different regions of the United Kingdom. All respondents reported that they currently have an MRI service for CD, whereas only 31 had an IUS service. Only 6 sites reported that they regularly use IUS as part of their IBD services [Citation12]. The same survey showed that clinicians felt less confident in decision--making when this was based on the IUS rather than MRE [Citation13–16].
This work explores and analyses the cost implications of the introduction of IUS into the IBD service at one NHS site in England and compares and contrasts those with the costs accumulated in the standard of care MRE pathway.
Methods
Data were collected from a clinical service evaluation of imaging pathways at a single NHS site in England, UK; Nottingham University Hospitals NHS trust (NUH). NUH is a large teaching hospital with an active clinical CD research activity. The ultrasound service was introduced in October 2020, with a single radiology consultant performing the ultrasound examinations. Analysis of patient care episodes and flow through the established imaging and IBD care pathways was undertaken. Data relating to patient flow, waiting times, resource use and healthcare engagement of patients were collected from care episodes between 01 January 2021–30 March 2022. We have used internal auditing data regarding pathway time points. Only data able to be obtained through electronic medical records were included in the analysis. Costs were calculated per care episode utilising data derived from the 2022/2023 NHS tariff and local service delivery costs [Citation17]. The time horizon was defined as the length of the episode of care from consultation where the patient was referred for imaging investigations to determine disease activity levels, to the time point where a treatment decision was acted upon, either by starting (or restarting) a medication, deescalating treatment by stopping one or more medical therapies, or if the patient underwent surgical intervention. The decision regarding which imaging investigation the patient was referred for was at the discretion of the referring clinician, no data regarding this decision process were gathered for this analysis. Similarly, it was impossible to determine a timeline where the clinical decision to continue current therapies was made, therefore only patients who underwent a therapy change were included in the analysis. This time horizon was chosen as it was expected that within this period all patients would be equally likely to be assessed by clinicians and care decisions made. During analysis, implications on CD burden were made based on disease phenotype, exposure to advanced therapies, history of surgery and disease duration [Citation18].
To simplify the cost analysis, it was assumed that all corticosteroid prescriptions were for the same duration and dose of prednisolone. At the time of the analysis, for treatment of relapses in CD, typically the standard prescription was 40 mg of prednisolone orally, once daily for a week then reducing by 5 mg weekly thereafter, in a reducing course for a total of 8 weeks [Citation19].
Costs of healthcare interactions are calculated using the number of interactions between the dates of referral for medical imaging and the date of treatment initiation and the cost to the service for each type of healthcare professional with which the interaction encounters. Item costs are taken from the annual costs of NUH NHS trust outpatient costs in 2021 and requirements of outpatient treatment recommendations (i.e. length of time of appointments) from NICE and NHS England across all clinical specialties [Citation20–22].
Costs are based on all interactions being outpatient appointments or telephone-based helpline interactions. I was estimated that helpline interactions were approximately 20 min of Band 6 NHS Nurse specialist time from allocated office space, Nurse appointments were 30 min of nurse time from an allocated clinic room, and medical appointments were 30 min of consultant time from an allocated clinic room [Citation23].
Ethical approval
Favourable ethical opinion was given to this study by the Nottingham research ethics committee and overall study approval was granted through the Health Research Authority on the 26th of March 2021.
Statistical analysis
There was no a priori hypothesis, therefore no attempt at statistical comparison has been undertaken. The results are descriptive and hypothesis-generating. A variety of data sources were used to acquire information about resource use as several patient care episodes had incomplete or imperfect data. Those patient episodes where the data were not able to be reconciled across records were removed from the final analysis. Consecutive patient cases were considered, for patients who underwent both imaging tests only the first test was considered as part of this analysis. Mean costs for each item of resource use were calculated and then aggregated to estimate the total cost per patient. Statistical testing was therefore not possible at the level of total resource use per patient.
Results
Group demographics
Variance of disease severity across the two groups was equal.
A combined total of 193 patient care episodes were reviewed, 107 from the IUS pathway and 86 from the MRE pathway. displays demographics of the patients whose cases were reviewed in the care pathway analysis. There were more males than females in the MRE pathway, and more females than males in the IUS pathway. Those in the IUS pathway had a slightly longer disease duration than those in the MRE pathway; however, the patients in each pathway were nearly equal in percentage for disease location and behaviour. Similarly, the two patient cohorts where similar for previous thiopurine and biological therapies exposure, with a slight increase in previous surgery rates in the MRE pathway. This demonstrates that the two cohorts were similar for factors relating to disease severity.
Table 1. Demographics of patients from care pathway analysis.
Care pathway model
A combined total of 193 patient care episodes were reviewed, 107 from the IUS pathway and 86 from the MRE pathway between 01 January 2021 and 30 March 2022.
displays the average waiting times; MRE pathway patients waited an average of 59 days (SD: ±57) from referral to test, with an additional average of 13 days (SD: ±12) before the report was available for review by the Gastroenterologist. The average time from referral to report for patients in the MRE pathway was 72 days (SD: ±62). Patients in the IUS pathway waited an average of 28 days (SD: ±16) from referral to test, with an additional 4 h (±17 h) from test to available report. The average time from referral to report was 29 days (±16).
Figure 1. Average waiting times.
Legend: MRE: magnetic resonance enterography; IUS: intestinal ultrasound; SD: standard deviation
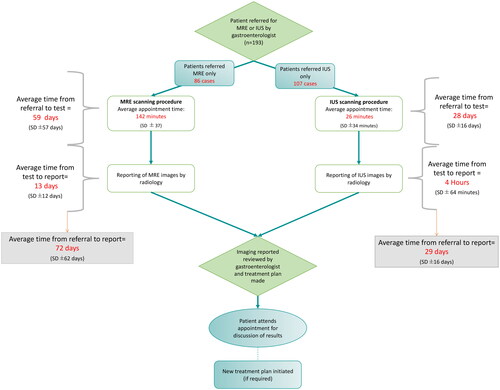
Patients in the IUS pathway-initiated treatments in an average of 46 days (SD: ±17) from the date of referrals for imaging, with patients from the MRE pathway starting their new treatment plans in an average of 91 days (SD: ±61) from the date of referrals for imaging. There was little difference between the IUS and MRE pathways for the time from report to treatment initiation.
Healthcare interactions
Patients in the MRE pathway had a total of 104 IBD helpline interactions, patients in the IUS pathway had a total of 80 helpline interactions. Patients in the MRE pathway had a total of 159 Nurse appointments, with patients in the IUS pathway having a total of 78 Nurse appointments. Patients in the MRE pathway had a total of 66 medical appointments, with patients in the IUS pathway having a total of 26 medical appointments.
Medications and treatment initiation
displays the proportion of patients where there was a treatment initiation or change. Details included the type of medication (corticosteroids, thiopurine or biological) escalation or de-escalation, or if the patient underwent CD resectional surgery during the study period.
Table 2. Proportion and number of patients with treatment changes.
There were 65 patients from the MRE group that received corticosteroid treatment, 57 received one prescription, 14 received two prescriptions and five patients received three corticosteroid courses. Patients in the MRE pathway received a total of 101 prescriptions for corticosteroid treatment, that is, 0.76 prescriptions per patient.
There were 60 patients from the IUS group that received one or more courses of corticosteroid treatment, 43 received one prescription, 15 patients received two prescriptions and four patients received three prescriptions for corticosteroids during this timeframe. A total of 85 corticosteroid prescriptions were issued for patient in the in IUS pathway, i.e. 0.56 prescriptions per patient. reports all the other treatment decisions in the cohort with this beingly broadly similar amongst the two groups which reflects the similar disease burden with numerically slightly larger number of patients needing surgery and biological treatment de-escalation in the MRE pathway.
Cumulative costs and potential benefits
displays costs for both IUS and MRE pathways. Patients from the IUS pathway had fewer healthcare interactions across all three categories. Patients from the IUS group had 80 helpline calls (£752.80), 78 Nurse appointments (£1163.76) and 26 medical appointments (£1063.66). In contrast patients from the MRE group had 104 helpline calls (£978.64), 159 Nurse appointments (£2372.28) and 66 medical appointments (£2700.06). displays the estimated mean unit costs per patient for both the IUS and MRE pathway. Estimated costs per patient in the IUS pathway was £78.86, and £375.35 per patient in the MRE pathway.
Table 3. Healthcare interaction costs.
Table 4. Estimated costs per patient (£, GBP).
Discussion
This work was undertaken to explore the cost implications of the introduction of IUS into the Inflammatory bowel disease service at one NHS site in England and compare and contrast those with the costs accumulated in the standard of care MRE pathway.
Behavioural and cultural barriers are often cited as major reasons for the lack of innovation adoption in the NHS [Citation24,Citation25]. The risk is that unnecessary, expensive and out-of-date care could be provided when there is resistance to moving away from established pathways, even if they are no longer efficient or effective. These inefficient clinical actions have considerable consequences in terms of personal and societal costs to patients, health care professionals and NHS services [Citation26]. The use of IUS is well established in central Europe, where gastroenterologists perform point of care assessments [Citation8,Citation27]. The use of IUS is not yet established enough in the UK for assessment of IBD to be performed in clinics. There is an identified lack of ultrasounds training for clinicians in the United Kingdom [Citation28]. Gastroenterology training in the UK does not encompass the use of IUS, though there are specialist groups working on establishing training programmes for radiologists and gastroenterologists in the United Kingdom.
This analysis has shown that compared to IUS, MRE waiting times are around double the time, with costs also being around double across the cohort examined. These waiting times are consistent with those reported across the United Kingdom [Citation12]. The longer wait for treatment in CD may be associated with an overuse in corticosteroids, over-reliance on already stretched IBD services and possible an increase in surgical exposure, although this study was not designed to look at such outcomes. The findings here are purely observational and hypothesis-generating [Citation29]. Earlier detection of active disease in IBD may avoid the need for subsequent, more invasive tests, or delays in implementing the best possible therapy [Citation30–32]. Timely therapeutic interventions can be applied, reducing the risk of disease progression and the long-term costs of poor disease management [Citation33]. The waiting times shown in the results of this work indicate that almost all of those patients with active CD were initiated on Corticosteroid therapy whilst awaiting imaging assessment or treatment decisions. Besides the cost-saving potential of the IUS pathway, there is the potential to reduce waiting times, leading to less corticosteroid use, ultimately reducing the risk of complications of untreated CD. Earlier diagnosis may provide psychological benefit by dispelling anxiety or providing earlier reassurance, as well as facilitating a faster return to normal daily activities [Citation34–36]. Timely access to healthcare has long been recognised as essential to improving patient outcomes [Citation37]. IBD remains a costly condition with modest potential cost savings [Citation10,Citation38,Citation39].
The cost per patient through this pathway for MRE is significantly more expensive than for IUS, this is despite the cohort being analysed showing similar disease severity and proportion of changes being comparable. There were a higher healthcare interactions and incidence of surgery in the MRE pathway. As the disease severity was similar across both groups it could be inferred that longer waiting time for investigations and to initiate treatments may be responsible for suboptimal management and failure to achieve adequate disease control. Failure to reach adequate disease control can lead to complications and increased incidence of surgical resection [Citation40].
When compared with biologic therapy and immunomodulators, prolonged use of corticosteroids had an increased risk of both morbidity and mortality in IBD patients [Citation41]. These data indicate that there was an increased risk to these patient cohorts through a higher corticosteroid exposure. Initiatives to reduce corticosteroid use are taking place in the UK and across Europe. In the UK, the guidelines for standard of care among IBD patients state that steroid use be monitored and that patients with chronic steroid use be discussed on a multidisciplinary level to improve patient care and quality of life [Citation42,Citation43].
It has been shown in the METRIC study that the relative cost-effectiveness of IUS versus MRE is not driven by the impact that it has on the quality-adjusted life years of the patients, but the cost of the test itself [Citation9]. IUS is significantly less costly than MRE per scan as shown in this work [Citation11]. MRE is the first-line imaging modality used to accurately stage small bowel disease location, complexity and activity in newly diagnosed CD in UK NHS IBD service [Citation2,Citation44–48]. Conversely, once disease location and phenotype are established there is an equipoise between MRE and IUS in subsequent disease follow-up and monitoring. IUS has been shown to be the preferred imaging assessment method of patients living with IBD due to the less burdensome nature of the investigation [Citation9]. Our study adds further insights to this. The cost-saving rather than just purely driven to expense related to the investigative modality, may be related to increased interaction to expensive clinical services.
Limitations
Results from this work are limited due to their lack of generalisability and retrospective nature. These results are from a UK-based single NHS site. Ultrasound assessments were undertaken by a single consultant radiologist, within a relatively newly established service. This analysis does not reflect everyday clinical practice where there are multiple kinds of medications which may be given at the discretion of the clinical prescriber. More robust, prospective, real-world multicentre data is required to conduct a comprehensive analysis to provide generalise data for the whole of NHS based IBD care. Data were collected retrospectively and therefore there are limitations in the depth of information available from medical records, data were reconciled from medical records only and therefore it was not possible to elucidate further information regarding the decisions or outcomes of clinical interactions outside of medical notations.
Alongside the measurable costs reported above, there are other cost considerations that are not included in this analysis. The cost of prescribed medicines in clinical IBD care is complex and difficult to map without considerable effort and planning, it was therefore not undertaken during this analysis due to limited resources and time, though this might have exposed more cost-savings. The initial set up costs of a new IUS service can be costly inclusive of equipment, training, and service costs such as clinic space and admin support. Expenses outside of the NHS service were not considered as part of this analysis, these include items such as patient travel costs, loss to workplace productivity through appointments and/or ill health. There was no straightforward way to calculate the full expenses incurred due to the delays due to COVID-19. A lack of economic data is often cited as a barrier to implementation, especially when decision makers are asked to allocate finite resources and face competing demands [Citation49]. Such knowledge is necessary if there is a desire to spread and replicate this work in other systems [Citation50]. It is therefore imperative that future research in this area encompass an economic analysis of the impact of IUS on NHS services.
Conclusions
This work poses that the use of IUS is a potential cost-saving option when compared to MRE use for adult patients with SBCD. There was also a difference between the IUS and MRE pathways in the waiting times for the medical imaging scans, the reports of the scans and the initiation of an appropriate treatment plan. It is important to note that existing evidence suggests there is no significant difference in the inter-rater reliability of MRE and IUS for diagnosing disease presence and extent in small bowel Crohn’s disease. IUS waiting times were shorter in all aspects except for the time between scanning report and the treatment initiation, indicating that it is the waiting times for the scans and the respective reporting that cause delays in treatment initiation rather than any inherent differences between the two patient groups which were evenly matched in this analysis. A large, multicentre, RCT with prospective data collection to conduct a comprehensive analysis to provide generalisable data for the whole of NHS-based IBD care is required in order to facilitate wider implementation of IUS in the NHS.
Author contributions
SJR: Conceptual input and design of service design, data collection and analysis guidance, whole manuscript generation.
CT: Statistical and analysis guidance, whole manuscript review.
PL: Data collection planning and guidance, whole manuscript review
JC: Whole manuscript review.
GWM: Conceptual input and design of service design, data collection and analysis guidance, whole manuscript review.
Disclosure statement
Professor Gordon Moran- Received grant funding from Astra Zeneca, Alimentiv Inc, Jansen, Bristol Myers Squibb. He has attended advisory boards for Pfizer and Abbvie. He is a consultant for Alimentiv Inc.
None of the other authors have any conflicts of interest to report.
Additional information
Funding
References
- Jones GR, Lyons M, Plevris N, et al. IBD prevalence in lothian, Scotland, derived by capture-recapture methodology. Gut. 2019;68(11):1953–1960. doi: 10.1136/gutjnl-2019-318936.
- Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the international organization for the study of IBD (IOIBD): determining therapeutic goals for treat-to-Target strategies in IBD. Gastroenterology. 2021;160(5):1570–1583. doi: 10.1053/j.gastro.2020.12.031.
- Kennedy NA, Heap GA, Green HD, et al. Predictors of anti-TNF treatment failure in anti-TNF-naive patients with active luminal crohn’s disease: a prospective, multicentre, cohort study. Lancet Gastroenterol Hepatol. 2019;4(5):341–353. doi: 10.1016/S2468-1253(19)30012-3.
- Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting therapeutic targets in inflammatory bowel disease (STRIDE): determining therapeutic goals for treat-to-target. Am J Gastroenterol. 2015;110(9):1324–1338. doi: 10.1038/ajg.2015.233.
- Allocca M, Fiorino G, Furfaro F, et al. Maintaining the quality standards of care for inflammatory bowel disease patients during the COVID-19 pandemic. Clin Gastroenterol Hepatol. 2020;18(8):1882–1883. doi: 10.1016/j.cgh.2020.04.028.
- Bai X, Yang H, Qian J. COVID-19 outbreak and inflammatory bowel disease management: a questionnaire survey from realistic practice. J Crohns Colitis. 2020;14(10):1494–1495. doi: 10.1093/ecco-jcc/jjaa064.
- Martin Arranz E, Suarez Ferrer C, García Ramírez L, et al. Management of COVID-19 pandemic in Spanish inflammatory bowel disease units: results from a national survey. Inflamm Bowel Dis. 2020;26(8):1149–1154. doi: 10.1093/ibd/izaa142.
- Allocca M, Furfaro F, Fiorino G, et al. Point-of-care ultrasound in inflammatory bowel disease. Journal of Crohn’s and Colitis. 2020;2020:1–9.
- Taylor SA, Mallett S, Bhatnagar G, et al. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn’s disease (METRIC): a multicentre trial. Lancet Gastroenterol Hepatol. 2018;3(8):548–558. doi: 10.1016/S2468-1253(18)30161-4.
- The Hidden Cost and a Vision for Change Crohn’s and Colitis Care in the UK Acknowledgements IBD UK board members and key contributors. 2021).
- Radford SJ, Clarke C, Shinkins B, et al. Clinical utility of small bowel ultrasound assessment of Crohn’s disease in adults: a systematic scoping review. Frontline Gastroenterol. 2022;13(4):280–286. doi: 10.1136/flgastro-2021-101897.
- Radford SJ, Taylor S, Moran G. Ultrasound use to assess Crohn’s disease in the UK: a survey of british society of gastroenterology inflammatory bowel disease group members. Frontline Gastroenterol. 2022;13(6):471–476. doi: 10.1136/flgastro-2021-102065.
- Pariente B, Mary J-Y, Danese S, et al. Development of the Lémann index to assess digestive tract damage in patients with Crohn’s disease. Gastroenterology. 2015;148(1):52–63.e3. doi: 10.1053/j.gastro.2014.09.015.
- Le Berre C, Peyrin-Biroulet L, SPIRIT-IOIBD study group. Selecting end points for disease-Modification trials in inflammatory bowel disease: the SPIRIT consensus from the IOIBD. Gastroenterology. 2021;160(5):1452–1460.e21. doi: 10.1053/j.gastro.2020.10.065.
- Gilletta C, Lewin M, Bourrier A, et al. Changes in the Lémann index values during the first years of Crohn’s disease. Clin Gastroenterol Hepatol. 2015;13(9):1633–1640.e3. doi: 10.1016/j.cgh.2015.02.041.
- Bodini G, Giannini EG, De Maria C, et al. Anti-TNF therapy is able to stabilize bowel damage progression in patients with Crohn’s disease. A study performed using the Lémann index. Dig Liver Dis. 2017;49(2):175–180. doi: 10.1016/j.dld.2016.10.014.
- England N. National Tariff Payment System: a consultation notice guidance on the aligned payment and incentive approach2022/2023.
- Pariente B, Torres J, Burisch J, et al. Validation and update of the Lémann index to measure cumulative structural bowel damage in Crohn’s disease. Gastroenterology. 2021;161(3):853–864.e13. doi: 10.1053/j.gastro.2021.05.049.
- Lamb CA, Kennedy NA, Raine T, et al. British society of gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–s106. doi: 10.1136/gutjnl-2019-318484.
- House of commons health C. Managing the care of people with long–term conditions. Second Report of Session 2014–15. 2014;I:1–89. Ev1-131.
- NHS Diagnostic Waiting Times and Activity Data NHS England and NHS Improvement 2 NHS Diagnostic Waiting Times and Activity Data. August 2020 Monthly Report. 2020.
- Patient experience in adult NHS services: improving the experience of care for people using adult NHS services Clinical guideline. 2012.
- Collins B. Payments and contracting for integrated care The false promise of the self-improving health system2019.
- Castle-Clarke S, Edwards N, Buckingham H. Falling short: why the NHS is still struggling to make the most of new innovations2017.
- Johnson MJ, May CR. Promoting professional behaviour change in healthcare: what interventions work, and why? A theory-led overview of systematic reviews. BMJ Open. 2015;5(9):e008592-e. doi: 10.1136/bmjopen-2015-008592.
- Hulscher M, Schouten L, Grol R, et al. Determinants of success of quality improvement collaboratives: what does the literature show? BMJ Qual Saf. 2013;22(1):19–31. doi: 10.1136/bmjqs-2011-000651.
- Wilkens R, Dolinger M, Burisch J, et al. Point-of-care testing and home testing: pragmatic considerations for widespread incorporation of stool tests, serum tests, and intestinal ultrasound. Gastroenterology. 2022;162(5):1476–1492. doi: 10.1053/j.gastro.2021.10.052.
- Radford S, Leighton P, Coad J, et al. Stakeholder-identified barriers and enablers to ultrasound implementation in inflammatory bowel disease services in the UK: a qualitative interview study. BMJ Open. 2023;13(6):e067528. doi: 10.1136/bmjopen-2022-067528.
- Clough J, Mawdsley J, Irving P, editors. P95 inflammatory bowel disease diagnosis: choosing the right path. 2021: BMJ Publishing Group Ltd and British Society of Gastroenterology.
- Wurcel V, Cicchetti A, Garrison L, et al. The Value of Diagnostic Information in Personalised Healthcare: a Comprehensive Concept to Facilitate Bringing This Technology into Healthcare Systems. 2019.
- Marín-Jiménez I, Casellas F, Cortés X, et al. The experience of inflammatory bowel disease patients with healthcare a survey with the IEXPAC instrument. Medicine (Baltimore). 2019;98(14):e15044. doi: 10.1097/MD.0000000000015044.
- Bryant RV, Friedman AB, Wright EK, et al. Gastrointestinal ultrasound in inflammatory bowel disease: an underused resource with potential paradigm-changing application. BMJ Publishing Group; 2018. p973–985. doi: 10.1136/gutjnl-2017-315655.
- Lee DW, Koo JS, Choe JW, et al. Diagnostic delay in inflammatory bowel disease increases the risk of intestinal surgery. World J Gastroenterol. 2017;23(35):6474–6481. doi: 10.3748/wjg.v23.i35.6474.
- Schoepfer AM, Dehlavi MA, Fournier N, et al. Diagnostic delay in crohn’s disease is associated with a complicated disease course and increased operation rate. Am J Gastroenterol. 2013;108(11):1744–1753; quiz 1754. doi: 10.1038/ajg.2013.248.
- The Value of Knowing and Knowing the Value: improving the Health Technology Assessment of Complementary Diagnostics. 2016).
- Anonychuk A, Beastall G, Shorter S, et al. A framework for assessing the value of laboratory diagnostics. Healthc Manage Forum. 2012;25(3_suppl):S4–S11. doi: 10.1016/j.hcmf.2012.07.015.
- Fortney JC, Burgess JF, Bosworth HB, et al. A re-conceptualization of access for 21st century healthcare. J Gen Intern Med. 2011;26 Suppl 2(Suppl 2):639–647. doi: 10.1007/s11606-011-1806-6.
- Ghosh N, Premchand P. A UK cost of care model for inflammatory bowel disease. Frontline Gastroenterol. 2015;6(3):169–174. doi: 10.1136/flgastro-2014-100514.
- Burisch J, Zhao M, Odes S, et al. The cost of inflammatory bowel disease in high-income settings: a lancet gastroenterology & hepatology commission. Lancet Gastroenterol Hepatol. 2023;8(5):458–492. doi: 10.1016/S2468-1253(23)00003-1.
- Ahmed Ali U, Kiran RP. Surgery for crohn’s disease: upfront or last resort? Gastroenterol Rep (Oxf). 2022;10:goac063. doi: 10.1093/gastro/goac063.
- Farraj KL, Pellegrini JR, Munshi RF, et al. Chronic steroid use: an overlooked impact on patients with inflammatory bowel disease. JGH Open. 2022;6(12):910–914. doi: 10.1002/jgh3.12841.
- Melmed GY, Siegel CA. Quality improvement in inflammatory bowel disease. Gastroenterol Hepatol (N Y). 2013;9(5):286–292.
- Selinger CP, Parkes GC, Bassi A, et al. A multi-Centre audit of excess steroid use in 1176 patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;46(10):964–973. doi: 10.1111/apt.14334.
- Horsthuis K, Bipat S, Bennink RJ, et al. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology. 2008;247(1):64–79. doi: 10.1148/radiol.2471070611.
- Dong J, Wang H, Zhao J, et al. Ultrasound as a diagnostic tool in detecting active crohn’s disease: a meta-analysis of prospective studies. Eur Radiol. 2014;24(1):26–33. doi: 10.1007/s00330-013-2973-0.
- Puylaert CAJ, Tielbeek JAW, Bipat S, et al. Grading of crohn’s disease activity using CT, MRI, US and scintigraphy: a meta-analysis. Eur Radiol. 2015;25(11):3295–3313. doi: 10.1007/s00330-015-3737-9.
- Greenup AJ, Bressler B, Rosenfeld G. Medical imaging in small bowel crohn’s disease - Computer tomography enterography, magnetic resonance enterography, and ultrasound: "which one is the best for what. Inflamm Bowel Dis. 2016;22(5):1246–1261. doi: 10.1097/MIB.0000000000000727.
- Alshammari MT, Stevenson R, Abdul-Aema B, et al. Diagnostic accuracy of Non-Invasive imaging for detection of colonic inflammation in patients with inflammatory bowel disease: a systematic review and Meta-Analysis. Diagnostics. 20212021;11(10)Page:1926. 111926-. doi: 10.3390/diagnostics11101926.
- Knocke K, Wagner TW. The evolving economics of implementation. BMJ Qual Saf. 2021;31(8):555–557. doi: 10.1136/bmjqs-2021-014411.
- Wagner TH, Dopp AR, Gold HT. Estimating downstream budget impacts in implementation research. Med Decis Making. 2020;40(8):968–977. doi: 10.1177/0272989X20954387.
