Abstract
Aim
To investigate the impact of triglyceride on hypertriglyceridemic acute pancreatitis (HTG-AP) and different lipid-lowering methods on triglyceride-lowering efficiency and HTG-AP.
Methods
The patients with HTG-AP from January 2012 to December 2023 in Civil Aviation General Hospital were analyzed, retrospectively. Patients were divided and compared according to whether their triglycerides were below 5.56 mmol/L at 48 and 72 h of admission. The patients were divided into control group, insulin group, and low molecular weight heparin (LMWH)+bezafibrate group based on the different methods of lipid-lowering. Propensity score matching (PSM) was employed to balance the baseline characteristics.
Results
There was no correlation between the severity of HTG-AP and the triglyceride at admission. The incidence of severity, local complications, and persistent organ failure (POF) were significantly decreased in patients with 48-h and 72-h triglyceride attainment. Following PSM, the incidence of infectious pancreatic necrosis (IPN) (3.3% vs. 13.3%) was significantly reduced in insulin group compared with control group (p < .05). Compared with control group, LMWH + bezafibrate group had higher lipid reduction efficiency, and the incidence of IPN (0.9% vs. 10.1%) and POF (8.3% vs. 19.3%) was significantly decreased (p < .05). There was no significant difference in the efficiency of lipid-lowering, complications, and POF between LMWH + bezafibrate group and insulin group (p > .05).
Conclusion
The severity of HTG-AP is not associated with the triglyceride levels at admission. However, rapid reduction of triglyceride levels can lower the incidence of local complications and respiratory failure. Compared with conservative treatment, insulin and LMWH + bezafibrate can both reduce the incidence of IPN in patients with HTG-AP.
Introduction
Acute pancreatitis (AP) is an inflammatory disease with abnormal activation of pancreatic enzymes and damage to pancreatic tissues caused by a variety of etiologic factors [Citation1]. AP is a prevalent critical illness worldwide, common causes include choledocholithiasis, hypertriglyceridemia, and alcohol. In recent years, the incidence of hypertriglyceridemic acute pancreatitis (HTG-AP) has been increasing [Citation2–4]. Triglycerides (TG) play an important role in the development of HTG-AP, but they do not have a toxic effect on the pancreas. The pathogenesis of HTG-AP is mainly related to the damage of pancreatic cells by free fatty acid (FFA), a metabolite of TG [Citation5]. The relationship between TG level and the severity of HTG-AP is still controversial. Pothoulakis et al. [Citation6] reported that TG levels were independently associated with AP severity. However, a meta-analysis by Kiss et al. [Citation7] showed that there were no significant differences in AP severity, mortality, and organ failure between TG levels > 11.3 mol/L and TG levels at 1.7 mmol/L–11.3 mmol/L. There were two defects in previous studies, one was that the AP of biliary origin and alcohol could influence the results, and the other was whether there was still an impact after TG > 11.3 mol/L.
Although the relationship between the level of TG at onset and the severity of HTG-AP is inconclusive, it has been recognized by many scholars that rapid reduction of TG levels is required in the early stages [Citation8]. Guidelines and expert consensus in China recommend rapid reduction of serum TG to 500 mg/dl (5.56 mmol/L) [Citation9]. Currently, commonly used drugs to lower serum TG levels such as oral lipid-lowering drugs, insulin, low molecular weight heparin (LMWH), and heparin. Fasting and fluid replacement can also reduce serum TG, whether various methods to lower TG have better clinical outcomes remains controversial. A study by Dhindsa et al. [Citation10] showed that insulin did not accelerate the decline in TG levels in patients with HTG-AP compared with fasting and fluid replacement.
This study aimed to explore the following controversial clinical issues by analyzing and summarizing the HTG-AP data of the Civil Aviation General Hospital from January 2012 to December 2023: the influence of TG level on the severity of HTG-AP, the effect of different lipid-lowering methods on the efficiency of lowering TG, the severity and complications of HTG-AP.
Materials and methods
Subjects
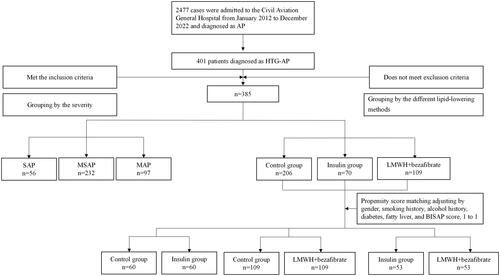
The data of patients diagnosed with AP in the Civil Aviation General Hospital from January 2012 to December 2023 were collected. The study included patients who met the inclusion criteria and did not meet the exclusion criteria. Inclusive criteria: (1) met the diagnostic criteria of HTG-AP, (2) admission within 48 h after onset, with onset measured as the start of abdominal pain, (3) over 18 years old. Exclusion criteria: (1) acute attack of chronic pancreatitis, (2) pregnancy, (3) undergone active treatment in other hospitals, (4) patients with severe infection or organ failure caused by a malignant tumor or other reasons, (5) patients who left hospital voluntarily for various reasons during disease, (6) patients with incomplete clinical information. shows the research sample selecting and grouping process.
Figure 1. The process of data inclusion and grouping. AP: acute pancreatitis; HTG-AP: hypertriglyceridemic acute pancreatitis; SAP: severe acute pancreatitis; MSAP: moderately severe acute pancreatitis; MAP: mild acute pancreatitis; LMWH: low molecular weight heparin; BISAP: bedside index for severity in acute pancreatitis.
The study was approved by the Medical Ethics Committee of the Civil Aviation General Hospital (2024-L-K-01). Informed consent was not required because it was a retrospective study, and the data were analyzed anonymously, according to (approaches to ethical review of biomedical research involving human beings) of China. The ethics committee specifically waived the need for consent. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) as reflected in a priori approval by the institution’s human research committee.
Diagnostic criteria
AP diagnostic criteria
Refer to the Revised Atlanta Definitions [Citation11]: (1) abdominal pain consistent with the clinical presentation of AP; (2) serum amylase and/or lipase were 3 times or higher than the upper limit of normal; (3) the imaging findings were by the typical imaging changes of AP. Two or more of the above three criteria were met.
HTG-AP diagnostic criteria
Based on AP diagnosis, serum TG levels should be ≥ 11.3 mmol/L or 5.65–11.3 mmol/L but serum chylous, while other causes of AP are excluded, such as biliary tract disease, alcohol, iatrogenic, and infection [Citation9].
AP severity rating
Refer to the Revised Atlanta Definitions [Citation11]: severe acute pancreatitis (SAP) is accompanied by persistent organ failure (POF). Moderately severe acute pancreatitis (MSAP) is accompanied by transient organ failure or systemic or local complications. Mild acute pancreatitis (MAP) is not accompanied by organ failure and local or systemic complications.
Definition of organ failure
The assessment is based on the Marshall score. Organ failure is defined as a respiratory, circulatory, and renal score of ≥ 2, with recovery to transient organ failure within 48 h, and POF beyond 48 h.
Research methods
The clinical data, including age, gender, smoking, drinking, diabetes, fatty liver, blood routine, biochemistry, abdominal CT, ultrasound, MRI, and vital signs, were collected from the medical record system. During subsequent treatment, we collected clinical data on local complications such as acute necrotic accumulation, acute encapsulated necrosis, acute peripancreatic fluid accumulation, pancreatic pseudocyst, and infectious pancreatic necrosis (IPN), as well as organ failure including respiratory, renal, and circulatory issues.
For MAP patients, we administered fasting, oxygen, proton pump inhibitors to inhibit gastric acid secretion, growth inhibitors to inhibit pancreatic exocrine secretion, gabexate to inhibit trypsin and other enzyme activities, fluid supplementation, correction of acid-base imbalance and electrolyte disorders, and antispasmodic and analgesic medications when necessary, and autonomous fasting as early as possible when it was tolerated. For patients admitted with local or systemic complications or organ failure, in addition to the treatments received by MAP patients, we also performed early fluid resuscitation, organ function maintenance, early enteral nutrition, rational use of antibiotics, and management of local and systemic complications. Due to the limitation of medical resources, ICU would only treat patients with combined multiple organ failure at the time of admission or patients with persistent organ failure with no improvement after 48 h of admission.
The control group did not receive any medication to lower the TG levels. The insulin group received continuous insulin infusion after admission and the blood glucose levels were monitored every hour. The insulin infusion rate was adjusted based on the blood glucose level, and intravenous glucose supplementation was provided as needed. The patients in the LMWH + bezafibrate group received LMWH 100 U/kg subcutaneously once a day, in combination with bezafibrate 0.4 g orally three times a day after admission. Patients with gastrointestinal intolerance were administered gastrointestinal nasal feeding drugs. Serum TG levels were measured at 24, 48, and 72 h of admission. Patients were categorized into MAP, MSAP, and SAP groups according to the severity of the disease. Propensity score matching (PSM) was used to compare the control group, insulin group, and LMWH + bezafibrate group. The independent variables included gender, smoking history, drinking history, diabetes, fatty liver, and BISAP score at admission. The dependent variable was the method of lipid-lowering. The nearest matching method was used for 1:1 matching, with a matching tolerance of 0.01.
Statistical analysis
SPSS 26.0 statistical software was used for statistical analysis. Normally distributed measures were expressed as mean ± standard deviation and analyzed using independent-sample t-tests or analysis of variance. Non-normally distributed measures were presented as median/interquartile range (IQR) and compared using the Kruskal–Wallis H test or Mann–Whitney U test. The categorical variables were described as percentages, and comparisons between groups were performed by chi-square test or Fisher exact probability method. The area under the receiver operating characteristic (ROC) curve was used to analyze the predictive efficacy of TG at admission, 24, 48, and 72 h of admission for SAP. Multivariate logistic regression was used to estimate the PSM score. A significant difference was observed with p < .05.
Results
The incidence of HTG-AP
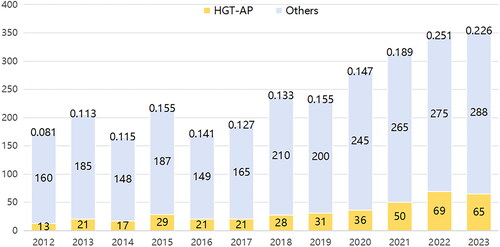
A total of 2477 patients with AP were admitted to the Civil Aeronautics General Hospital over the past 12 years, 401 patients met the inclusion criteria, while 16 patients met the exclusion criteria. Finally, 385 patients were incorporated into this study. The process of data inclusion and grouping is shown in . In our hospital, the number of patients with HTG-AP and AP increased annually, with the prevalence of HTG-AP rising from 8.1% to over 22.6% in 12 years ().
General clinical data
The patients diagnosed with HTG-AP were predominantly young and middle-aged males, with a mean age of 39 ± 9 years and a male/female ratio of 299/86. Among the HTG-AP patients, 40.5% had diabetes and 82.9% had fatty liver, including 97 with MAP, 232 with MSAP, and 56 with SAP. The differences in TG levels at admission, gender, age, smoking history, alcohol history, and concomitant diabetes were not statistically significant (p > .05). While the differences in concomitant fatty liver, lipid-lowering methods, BISAP score at admission, TG at 24, 48, 72 h of admission, the rate of achieving TG at 48 h of admission, and the rate of reaching TG at 72 h of admission were statistically significant (p < .05). shows the general clinical data of MAP, MSAP, and SAP.
Table 1. The general clinical data of MAP,MSAP and SAP.
Prognostic impact of 48-h TG attainment
Two hundred and ten patients had their TG levels fall below 5.56 mmol/L within 48 h of admission, while 175 patients did not. The differences in disease severity, local complications, acute peripancreatic fluid accumulation, pancreatic pseudocysts, POF, and incidence of respiratory failure were statistically significant (p < .05). The differences in fatty liver, diabetes, combined SIRS, and BISAP score at admission were statistically significant (p < .05) ().
Table 2. Characteristics and clinical outcomes of 48-h and 72-h TG attainment.
Prognostic impact of 72-h TG attainment
Two hundred and sixty-nine patients had their TG levels fall below 5.56 mmol/L within 72 h of admission, while 175 patients did not. The differences in the incidence of local complications, acute peripancreatic fluid accumulation, pancreatic pseudocysts, POF, and respiratory failure were statistically significant (p < .05). The differences in fatty liver, diabetes, combined SIRS, and BISAP score at admission were statistically significant (p < .05) ().
ROC curve of TG predicting SAP
ROC analysis of patients’ TG levels at admission, 24, 48, and 72 h of admission revealed that TG levels at admission were not significantly associated with the severity of HTG-AP (p > .05). However, the TG level at 48 h of admission was the most effective predictor of SAP, with an optimal cutoff value of 6.74 mmol/L, a sensitivity of 64.3%, and a specificity of 74.2% ().
Table 3. The area under the ROC curve of TG predicting SAP.
Effect of different lipid-lowering methods on the prognosis of HTG-AP
The study included 206 patients in the control group, 70 patients in the insulin group, and 109 patients in the LMWH + bezafibrate group (). The differences in initial TG levels and TG attainment rates at 48 and 72 h of admission were not statistically significant between the groups (p > .05). The severity and prognostic impact of different lipid-lowering schedules on HTG-AP showed statistically significant differences, with the lowest incidence of IPN occurring in patients receiving LMWH in combination with bezafibrate (p < .05). However, this was a retrospective study and there were differences in baseline characteristics between the groups. These statistically significant differences included alcohol history, diabetes, and SIRS at admission (p < .05). Therefore, propensity score matching analysis was used for further analysis.
Table 4. Characteristics and clinical outcomes of different lipid-lowering methods.
Effect of different lipid-lowering methods on the prognosis of HTG-AP by propensity score matching analysis
PSM was performed in a 1:1 ratio using gender, smoking history, drinking history, diabetes, fatty liver, and BISAP score at admission as independent variables and different lipid-lowering modalities as dependent variables. The matching tolerance was set at 0.01. The results showed that the differences between the insulin group and the control group for the initial TG level, and the rate of TG attainment at 48 h and 72 h were not statistically significant (p > .05). However, the incidence of IPN was reduced in the insulin group (p < .05). There were no statistically significant differences in the TG levels at 24, 48, and 72 h of admission, the rate of reaching the TG standard at 48 h and 72 h of admission between the LMWH + bezafibrate group and the control group (p > .05). However, the TG levels at admission in the control group were even lower (p < .05), which indicated that LMWH combined with bezafibrate was more efficient than the control group in lowering lipids. The incidence of IPN and POF in the LMWH + bezafibrate group was significantly lower than that in the control group (p < .05). Compared with the insulin group, the LMWH + bezafibrate group had similar baseline characteristics, and the differences in lipid-lowering efficiency, the incidence of local complications, the incidence of POF, and the severity of AP were not statistically significant (p > .05) ().
Table 5. Characteristics and clinical outcomes of different lipid-lowering methods by propensity score matching.
Discussion
The number of AP and HTG-AP cases admitted to our hospital has been increasing year by year, especially in the past two years. Over 12 years, HTG-AP accounted for over 22% of AP, a 2.7-fold increase, which was consistent with the data reported by Lin et al. [Citation12]. Compared with other etiologies of AP, HTG-AP is associated with faster disease progression and higher incidence of complications and organ failure[Citation13,Citation14].
TG is the primary cause of HTG-AP. However, the pathogenesis of HTG-AP is mainly related to the TG metabolite FFA. The FFA can accumulate in the pancreas, leading to pancreatic microcirculation disorders and calcium overload, which cause pancreatitis [Citation15,Citation16]. Additionally, it can stimulate the release of inflammatory mediators, potentially leading to pancreatic cell injury and multi-organ failure [Citation17]. It is currently controversial whether the level of TG at onset influences the prognosis of HTG-AP because TG itself does not damage the pancreas. A study [Citation6] showed that patients with elevated TG levels had a significantly higher incidence of SAP, ICU admissions, and mortality regardless of the etiology of AP. However, the study did not differentiate between pancreatitis caused by biliary origin, alcohol, or other factors. In our study, the severity of HTG-AP was not related to the level of TG at admission to hospital. Although the relationship between initial TG levels and the severity of HTG-AP is controversial, sustained high levels of TG are metabolized to produce more FFA, which can exacerbate AP. This study showed that rapid reduction of TG after admission may reduce the incidence of local complications and organ failure, particularly when TG levels are less than 5.56 mmol/L within 48 h after admission.
Fatty liver and diabetes were statistically different between the two groups based on whether or not TG levels were met at 48 or 72 h (p < .05). Fatty liver and diabetes may affect the decrease in TG levels.TG is metabolized to glycerol and FFA primarily by lipoprotein lipase (LPL) [Citation18]. Elevated blood glucose inhibits the synthesis of LPL, leading to a short-term sharp increase in TG levels inducing HTG-AP and causing impaired TG metabolism that cannot be rapidly reduced. A clinical study [Citation19] showed that elevated TG levels were strongly associated with poor glycemic control. Fatty liver is due to excessive deposition of lipids such as TG in liver cells. A large genome-wide meta-analysis conducted by Ghodsian et al. [Citation20] showed that the gene expression of LPL in the subcutaneous adipose tissues of patients with NAFLD was significantly reduced, and its expression level was negatively correlated with the severity of NAFLD. Reduced LPL gene expression and LPL synthesis in patients with fatty liver affect TG metabolism. Therefore, for patients with HTG-AP, active glycemic control and amelioration of fatty liver can not only rapidly reduce TG, but also reduce the recurrence of HTG-AP [Citation21].
Currently, there are two major categories of methods used in clinical practice to lower serum TG: non-invasive and invasive. Non-invasive therapeutic measures include oral lipid-lowering drugs, insulin, LMWH, and heparin. Invasive measures refer to blood purification techniques, such as hemofiltration, hemoperfusion, plasma exchange, and other modalities. Compared with drug therapy, blood purification can not only reduce TG faster [Citation22], but also remove celiac particles, toxins, and inflammatory factors in the blood [Citation23], and correct water, electrolyte, and acid-base balance disorders. However, several previous studies had revealed [Citation22,Citation24,Citation25] that blood purification not only failed to significantly improve clinical outcomes such as organ failure but also increased ICU admissions and additional medical costs. In addition, many hospitals were unable to implement this medical technology because of the constraints. Therefore, noninvasive lipid-lowering measures are more favored in the clinic now.
Insulin can accelerate the degradation of celiac particles by increasing the activity of LPL, which reduces the level of TG. Additionally, it can inhibit hormone-sensitive lipase in adipocytes, reducing the release of FFA from stored TG [Citation26]. This has a more significant lipid-lowering effect and is widely used in clinical practice [Citation27,Citation28]. Our study indicated that insulin reduced TG levels by up to 83.5% within 72 h. However, there was no advantage in lipid-lowering efficiency compared with conservative treatment solely. Insulin significantly reduced the incidence of IPN (p < .05), suggesting that insulin had an additional pathway to improve the clinical outcomes of HTG-AP, in addition to lowering TG. Bruce et al. [Citation29] identified that insulin-protected pancreatic alveolar cells during AP by preserving the supply of glycolytic ATP from the calcium pump, attenuated pancreatic injury. Although there are so many advantages, frequent blood glucose monitoring is required during insulin administration to prevent hypoglycemia, which will undoubtedly greatly increase the workload of medical workers and the pain of patients.
It had been reported that heparin and LMWH not only promoted the release of LPL from endothelial cells to accelerate the degradation of TG [Citation30] but also exerted anticoagulant and antithrombotic effects to enhance pancreatic microcirculation and ameliorate the prognosis of AP. In addition, heparin and LMWH exerted anti-inflammatory effects by inhibiting IFNγ and IL-6 activities [Citation31]. Several previous studies showed that LMWH significantly decreased the incidence of pancreatic necrosis, mortality, and POF [Citation32,Citation33]. Typical oral lipid-lowering drugs include fibrates, statins, niacin, and omega-3 fatty acids, with fibrates being preferred, which increase LPL and hepatic lipase activity to lower TG [Citation34]. Our study indicated that LMWH combined with bezafibrate was more efficient in lipid lowering compared with conservative treatment. The incidence of IPN and POF was also significantly decreased with LMWH combined with bezafibrate (p < .05). Meanwhile, serious side effects related to LMWH such as gastrointestinal bleeding and intracranial hemorrhage were not observed in our study. It had been reported that prolonged use of LMWH might deplete LPL on the surface of vascular endothelial cells, leading to a resurgence in blood TG levels [Citation35]. The patient in this report was given heparin for a prolonged period. Usually, heparin and LMWH are not kept for such a long time in the treatment of AP. In our study, the maximum duration of LMWH was 14 days and no re-elevation of TG was observed. Therefore, we deemed LMWH to be safe and effective during routine HTG-AP treatment, and there is no need to worry about this side effect. Kuchay et al. [Citation26] share the same view.
The differences in lipid-lowering efficiency, local complications, POF, and severity of AP disease were not statistically significant for LMWH combined with bezafibrate compared with insulin (p > .05). However, compared with insulin, LMWH combined with bezafibrate is more convenient in the clinic and can significantly reduce the workload of medical workers and the pain of patients. Based on previous experience, we recommend the use of insulin only in patients with poor glycemic control or comorbid diabetic ketoacidosis. It is necessary to closely monitor blood glucose levels during use and combine LMWH and bezafibrate if possible. Several previous studies had shown [Citation26,Citation36,Citation37] that insulin combined with LMWH in the treatment of HTG-AP had a definite lipid-lowering effect, a good safety profile, and a low medical expenditure. For patients with no requirement for glycemic control, LMWH in combination with a fibrate may be a preferable option.
The strengths of this study are the large sample size and strict inclusion and exclusion criteria to ensure the accuracy of the data and conclusions. When comparing the prognostic impact of different lipid-lowering methods on HTG-AP, variables such as gender, smoking history, drinking history, diabetes, fatty liver, and BISAP score at admission were included in the propensity score matching to minimize the effect of confounding factors, ensuring that there was no statistical difference in baseline information between the groups. This statistical approach leads to more reliable final results. The BISAP score is a good prognosticator of AP severity [Citation38]. In a previous study [Citation12], the BISAP score at admission was not taken into account but rather the severity of illness grading when doing propensity score matching to equalize baseline characteristics. The severity of the disease is the outcome of AP, and the BISAP score at admission is more reflective of the patient’s status at admission. Therefore, we believe that the BISAP score at admission is more appropriate to represent the severity of the disease at admission.
This study also has some limitations. This study was a single-center retrospective study, and the choice of lipid-lowering modality was influenced by the personal preference of different physicians thus there was a selection bias. The time from onset to admission varies from patient to patient, and our measured TG levels at admission to hospital do not reflect the actual onset TG levels.
Conclusion
In summary, we suggest that the severity of HTG-AP is not associated with the TG levels at admission. However, rapid reduction of triglyceride levels can lower the incidence of local complications and respiratory failure. Compared with conservative treatment, insulin and LMWH + bezafibrate can both reduce the incidence of IPN in patients with HTG-AP.
Ethical approval
The study was approved by the Medical Ethics Committee of the Civil Aviation General Hospital (2024-L-K-01). Informed consent was not required because it was a retrospective study and the data were analyzed anonymously, according to (approaches to ethical review of biomedical research involving human beings) of China. The ethics committee specifically waived the need for consent.
Author contributions
Yang Liu: Acquisition, curation, analysis, and interpretation of data, Writing – original draft. Jianping Cheng: Conceptualization, supervision, review. Xiaolin Zhao: Statistical analysis.
Acknowledgements
We are very grateful to the doctor in Gastroenterology Department of Civil Aviation General Hospital for their contribution to the acquisition of data.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Gukovskaya AS, Gorelick FS, Groblewski GE, et al. Recent insights into the pathogenic mechanism of pancreatitis: role of acinar cell organelle disorders. Pancreas. 2019;48(4):459–470. doi: 10.1097/MPA.0000000000001298.
- Jin M, Bai X, Chen X, et al. A 16-year trend of etiology in acute pancreatitis: the increasing proportion of hypertriglyceridemia-associated acute pancreatitis and its adverse effect on prognosis. J Clin Lipidol. 2019;13(6):947–953.e1. doi: 10.1016/j.jacl.2019.09.005.
- Pu W, Luo G, Chen T, et al. A 5-year retrospective cohort study: epidemiology, etiology, severity, and outcomes of acute pancreatitis. Pancreas. 2020;49(9):1161–1167. doi: 10.1097/MPA.0000000000001637.
- Olesen SS, Harakow A, Krogh K, et al. Hypertriglyceridemia is often under recognized as an aetiologic risk factor for acute pancreatitis: a population-based cohort study. Pancreatology. 2021;21(2):334–341. doi: 10.1016/j.pan.2021.02.005.
- Valdivielso P, Ramírez-Bueno A, Ewald N. Current knowledge of hypertriglyceridemic pancreatitis. Eur J Intern Med. 2014;25(8):689–694. doi: 10.1016/j.ejim.2014.08.008.
- Pothoulakis I, Paragomi P, Tuft M, et al. Association of serum triglyceride levels with severity in acute pancreatitis: results from an international, multicenter cohort study. Digestion. 2021;102(5):809–813. doi: 10.1159/000512682.
- Kiss L, Fűr G, Mátrai P, et al. The effect of serum triglyceride concentration on the outcome of acute pancreatitis: systematic review and meta-analysis. Sci Rep. 2018;8(1):14096. doi: 10.1038/s41598-018-32337-x.
- Christian JB, Arondekar B, Buysman EK, et al. Clinical and economic benefits observed when follow-up triglyceride levels are less than 500 mg/dL in patients with severe hypertriglyceridemia. J Clin Lipidol. 2012;6(5):450–461. doi: 10.1016/j.jacl.2012.08.007.
- Chinese pancreatic surgery association, chinese society of surgery, chinese medical association; [Guidelines for diagnosis and treatment of acute pancreatitis in China (2021)]. Zhonghua Wai ke za Zhi [Chin J Surg]. 2021;59(7):578–587. doi: 10.3760/cma.j.cn112139-20210416-00172.
- Dhindsa S, Sharma A, Al-Khazaali A, et al. Intravenous insulin versus conservative management in hypertriglyceridemia-associated acute pancreatitis. J Endocr Soc. 2019;4(1):bvz019. doi: 10.1210/jendso/bvz019.
- Banks PA, Bollen TL, Dervenis C, et al. Acute pancreatitis classification working group. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. 2013;62(1):102–111. doi: 10.1136/gutjnl-2012-302779.
- Lin XY, Zeng Y, Zhang ZC, et al. Incidence and clinical characteristics of hypertriglyceridemic acute pancreatitis: a retrospective single-center study. WJG. 2022;28(29):3946–3959. doi: 10.3748/wjg.v28.i29.3946.
- Mosztbacher D, Hanák L, Farkas N, et al. Hungarian pancreatic study group. Hypertriglyceridemia-induced acute pancreatitis: a prospective, multicenter, international cohort analysis of 716 acute pancreatitis cases. Pancreatology. 2020;20(4):608–616. doi: 10.1016/j.pan.2020.03.018.
- Li X, Ke L, Dong J, et al. Significantly different clinical features between hypertriglyceridemia and biliary acute pancreatitis: a retrospective study of 730 patients from a tertiary center. BMC Gastroenterol. 2018;18(1):89. doi: 10.1186/s12876-018-0821-z.
- Maléth J, Balázs A, Pallagi P, et al. Alcohol disrupts levels and function of the cystic fibrosis transmembrane conductance regulator to promote development of pancreatitis. Gastroenterology. 2015;148(2):427–439.e16. doi: 10.1053/j.gastro.2014.11.002.
- Hegyi P, Petersen OH. The exocrine pancreas: the acinar-ductal tango in physiology and pathophysiology. Rev Physiol Biochem Pharmacol. 2013;165:1–30. doi: 10.1007/112_2013_14.
- Navina S, Acharya C, DeLany JP, et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Sci Transl Med. 2011;3(107):107ra110. doi: 10.1126/scitranslmed.3002573.
- Cook JR, Hawkins MA, Pajvani UB. Liver insulinization as a driver of triglyceride dysmetabolism. Nat Metab. 2023;5(7):1101–1110. doi: 10.1038/s42255-023-00843-6.
- Zheng D, Dou J, Liu G, et al. Association between triglyceride level and glycemic control among insulin-treated patients with type 2 diabetes. J Clin Endocrinol Metab. 2019;104(4):1211–1220. doi: 10.1210/jc.2018-01656.
- Ghodsian N, Abner E, Emdin CA, et al. Electronic health record-based genome-wide meta-analysis provides insights on the genetic architecture of non-alcoholic fatty liver disease. Cell Rep Med. 2021;2(11):100437. doi: 10.1016/j.xcrm.2021.100437.
- Ding L, Li S, Cao L, et al. Recurrence of hypertriglyceridemia-associated acute pancreatitis: a multicenter, prospective cohort study. Eur J Intern Med. 2024;S0953-6205(24):00130–00134. doi: 10.1016/j.ejim.2024.03.022.
- He W, Cai W, Yang X, et al. Insulin or blood purification treatment for hypertriglyceridaemia-associated acute pancreatitis: a systematic review and meta-analysis. Pancreatology. 2022;22(7):846–857. doi: 10.1016/j.pan.2022.07.013.
- Padmanabhan A, Connelly-Smith L, Aqui N, et al. Guidelines on the use of therapeutic apheresis in clinical practice – evidence-based approach from the writing committee of the American society for apheresis: the eighth special issue. J Clin Apher. 2019;34(3):171–354. doi: 10.1002/jca.21705.
- Gubensek J. The role of apheresis and insulin therapy in hypertriglyceridemic acute pancreatitis-a concise review. BMC Gastroenterol. 2023;23(1):341. doi: 10.1186/s12876-023-02957-3.
- Cao L, Chen Y, Liu S, Chinese Acute Pancreatitis Clinical Trials Group (CAPCTG)., et al. Early plasmapheresis among patients with hypertriglyceridemia-associated acute pancreatitis. JAMA Netw Open. 2023;6(6):e2320802. doi: 10.1001/jamanetworkopen.2023.20802.
- Kuchay MS, Farooqui KJ, Bano T, et al. Heparin and insulin in the management of hypertriglyceridemia-associated pancreatitis: case series and literature review. Arch Endocrinol Metab. 2017;61(2):198–201. doi: 10.1590/2359-3997000000244.
- Marić N, Mačković M, Bakula M, et al. Hypertriglyceridemia-induced pancreatitis treated with continuous insulin infusion-Case series. Clin Endocrinol (Oxf). 2022;96(2):139–143. doi: 10.1111/cen.14554.
- Pulipati VP, Amblee A, Yap SET, et al. Hypertriglyceridemia-associated acute pancreatitis: response to continuous insulin infusion. PLoS ONE. 2021;16(11):e0260495. doi: 10.1371/journal.pone.0260495.
- Bruce JIE, Sánchez-Alvarez R, Sans MD, et al. Insulin protects acinar cells during pancreatitis by preserving glycolytic ATP supply to calcium pumps. Nat Commun. 2021;12(1):4386. doi: 10.1038/s41467-021-24506-w.
- Näsström B, Olivecrona G, Olivecrona T, et al. Lipoprotein lipase during continuous heparin infusion: tissue stores become partially depleted. J Lab Clin Med. 2001;138(3):206–213. doi: 10.1067/mlc.2001.117666.
- Litov L, Petkov P, Rangelov M, et al. Molecular mechanism of the anti-inflammatory action of heparin. IJMS. 2021;22(19):10730. doi: 10.3390/ijms221910730.
- Patil B, Meena LN, Sharma DC, et al. Impact of low-molecular-weight heparin in the treatment of moderately severe and severe acute pancreatitis; a randomized, single blind, phase 3 control trial. Int J Surg. 2022;101:106621. doi: 10.1016/j.ijsu.2022.106621.
- He K, Zhang Y, Song K, et al. Randomized controlled trials of low molecular weight heparin in non-mild acute pancreatitis: a systemic review and meta-analysis. Thromb Res. 2023;221:26–29. doi: 10.1016/j.thromres.2022.11.012.
- Laufs U, Parhofer KG, Ginsberg HN, et al. Clinical review on triglycerides. Eur Heart J. 2020;41(1):99–109c. doi: 10.1093/eurheartj/ehz785.
- Watts GF, Cameron J, Henderson A, et al. Lipoprotein lipase deficiency due to long-term heparinization presenting as severe hypertriglyceridaemia in pregnancy. Postgrad Med J. 1991;67(794):1062–1064. doi: 10.1136/pgmj.67.794.1062.
- Jin M, Peng JM, Zhu HD, et al. Continuous intravenous infusion of insulin and heparin vs plasma exchange in hypertriglyceridemia-induced acute pancreatitis. J Digest Dis. 2018;19(12):766–772. doi: 10.1111/1751-2980.12659.
- Anbessie ZM, Gebremeskel YB. Hypertriglyceridemic pancreatitis managed with heparin and insulin: a case report. J Med Case Reports. 2023;17(1):256. doi: 10.1186/s13256-023-03995-x.
- Mederos MA, Reber HA, Girgis MD. Acute pancreatitis: a review. JAMA. 2021;325(4):382–390. doi: 10.1001/jama.2020.20317.