Abstract
Objective
Assessing unclear biliary strictures is challenging. We analyzed the diagnostic performance of radiology, EUS, and ERCP.
Methods
All patients referred for EUS and ERCP to assess an unclear biliary stricture were prospectively included. The data from radiology, EUS, ERCP, and tissue sampling were recorded. The diagnostic modalities were analyzed separately and in combination, with a focus on PSC.
Results
Between 2013 and 2020, 78 patients were included; 31% had PSC. A cholangioscopy was not performed in this study. The final diagnosis indicated that the biliary stricture was benign in 62% of the patients and malignant in 38%. The differences among the modalities were numerical, not significant. The modalities showed an accuracy between 78 and 83% in all the patients and between 75 and 83% in the patients with PSC. The combination of radiology and EUS showed the highest sensitivity of 94% in all the patients and a sensitivity of 100% in PSC. Tissue sampling showed the highest specificity of 93% in all patients and 89% in PSC. In 22 cases with combined EUS, ERCP, and tissue sampling, the accuracy, sensitivity, and specificity were 82%, 70%, and 92%, respectively. Minor differences were observed between the intention-to-diagnose analysis and the per-protocol analysis. Adverse events were recorded in 4% of cases.
Conclusion
The combination of EUS and ERCP with tissue sampling seems to be useful and safe for excluding malignancy in unclear biliary strictures. In cases with a reduced suspicion of malignancy, radiology with an EUS may be sufficient.
Keywords:
Introduction
Malignant biliary strictures are associated with a high mortality rate and are often solitary strictures. They are mostly caused by cholangiocarcinoma (CCA) [Citation1] or pancreatic cancer [Citation2, Citation3]. Primary sclerosing cholangitis (PSC) is characterized by multiple strictures caused by the inflammation of the biliary tree. Malignancy frequently occurs in these strictures within the first year of the PSC diagnosis [Citation4–6], and the risk of malignancy is much higher than the risk in the general population [Citation7].
Diagnosing malignancy in biliary strictures is challenging. Modern radiology is efficient at detecting these strictures; however, radiology alone is not sufficient to confidently rule out malignancy in bile duct strictures. Moreover, up to 20% of cases initially suspected to be cholangiocarcinoma were found to be a benign condition after surgery [Citation3, Citation8, Citation9].
CT scans have a reported overall sensitivity of 40-77% and a specificity of 60–80% [Citation1, Citation2, Citation10]. MRCP has shown a sensitivity of 38%–90% and a specificity of 70%–85% for differentiating between malignant and benign stenosis [Citation2, Citation8, Citation10–12].
Endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP) are complementary procedures in the evaluation of biliary strictures.
ERCP can visualize the biliary tree and provide tissue samples from suspected lesions. ERCP with brush cytology has demonstrated a low overall sensitivity of 26-56% in different studies [Citation6, Citation8, Citation13, Citation14], but has a high specificity of about 99% [Citation1, Citation9, Citation12]. The newer-generation cholangioscope, SpyGlass, can provide biopsies with small forceps, and has shown an improved diagnostic accuracy in some studies compared with ERCP-based brush cytology [Citation4]. PSC strictures are difficult to characterize, and a study in PSC patients reported a sensitivity of 33% for SpyGlass biopsies [Citation15].
EUS can be used to visualize the biliary tree and pancreas, and it enables tissue sampling from suspected lesions [Citation3, Citation7, Citation9]. The overall sensitivity of EUS-FNA for the diagnosis of malignant biliary strictures is 43-85%, and the specificity is 90–97% [Citation1, Citation3, Citation9, Citation14, Citation16, Citation17]. EUS demonstrated a better diagnostic ability for malignant biliary strictures compared with CT in a study by Saifuku et al. [Citation18].
Some studies have shown the feasibility of a same-session EUS-ERCP for the evaluation of biliary strictures [Citation19]. In a multicenter study, Jo et al. found that a same-session EUS-FNA and ERCP had a better diagnostic accuracy than EUS-FNA for both pancreatic masses and biliary lesions [Citation20]. A total of 78% of the patients included in this study had pancreatic masses, and malignancy was suspected. Rösch et al. evaluated the effectiveness of EUS-FNA and ERCP-BC in the case of intermediate biliary strictures and pancreatic masses and found that the combination of these two procedures provided the highest diagnostic accuracy compared to EUS-FNA or ERCP-BC alone [Citation21]. Only a few studies have evaluated a same-session EUS and ERCP for the diagnosis of unclear biliary strictures [Citation19, Citation22], but no studies have evaluated this method in patients with PSC.
In the case of a suspicion of malignancy in PSC patients, both ERCP and EUS are recommended according to the guidelines of the British Society of Gastroenterology. However, no previous studies have prospectively assessed both modern radiology as well as EUS and ERCP with tissue sampling for the assessment of unclear bile duct strictures with a focus on PSC [Citation23].
An inaccurate malignant diagnosis of a biliary stricture may lead to unnecessary extensive surgery with a high morbidity rate and high costs. On the other hand, a missed early malignancy in a young PSC patient would exclude the possibility of a life-saving liver transplantation.
The general aim of the current study was to prospectively evaluate the diagnostic performance of modern radiology and the combination of EUS and ERCP with tissue sampling, and their ability to exclude malignancy in unclear biliary strictures.
Materials and methods
Study population
The study design and the inclusion criteria
This was a single-center, prospective study on a consecutive series of patients with a biliary stricture of an unclear origin. The patients were included at the tertiary center of Sahlgrenska University Hospital, Gothenburg, Sweden, between October 2013 and September 2020. This tertiary center is the largest liver transplantation center in Sweden and serves a local population of 2.1 million, as well as liver transplantation cases from other parts of Sweden and Iceland. All cases of unclear biliary strictures are assessed by a multidisciplinary team (MDT) comprising specialized GI radiologists, surgeons, oncologists, and endoscopists. The patients were examined by both CT and/or MRI and, often, ERCP, without a clear diagnosis regarding the biliary stricture. The MDT decides whether the available radiology and endoscopy results are insufficient to reach a therapeutic decision. The MDT also decides if a patient should be referred for EUS and ERCP or for other examinations. The MDT decided on the therapy after the EUS-ERCP.
The inclusion criteria were patients who were referred for both an EUS and ERCP and patients for which these two procedures were performed in the same session or within three months.
The author RS included all the patients referred for EUS and ERCP prospectively.
All the patients gave their signed, informed consent.
Exclusion criteria
Patients with a conclusive diagnosis were excluded.
Patients who did not provide consent for their inclusion.
The presence of any contraindications for ERCP or EUS.
The final diagnosis
The final diagnosis was based on the following information
The histopathological result from an operation (e.g., liver transplant, open liver biopsy);
In patients who did not undergo an operation, a clinical follow‐up of at least 18 months combined with the diagnostic work-up, including tissue sampling.
The absence of clinical signs of malignancy at follow-up (minimum of 18 months) and malignant tissue sampling (these biliary strictures were regarded as benign).
The tissue samples were analyzed by experienced gastropathologists.
The samples were sorted into the following categories:
(1) benign, (2) atypical, (3) suspected of malignancy, (4) malignant,
and (5) non-diagnostic (insufficient tissue sampling for a pathologic diagnosis).
The samples that were labeled malignant or suspected of malignancy were considered malignant. The categories of benign, atypical, and non-diagnostic were considered negative for malignancy.
A final diagnosis of malignancy using the results of EUS or ERCP with tissue sampling (TS) was established when at least one modality (endoscopic assessment and/or tissue sampling) was positive for malignancy.
Ethical aspects and study registration
The ethical committee approved this prospective study (Dnr 573-09: Kvalitetskontroll av endoskopiskt ultraljud). The clinical trial number was NCT02360839.
Radiology
All patients underwent CT and/or MRCP/MRI before EUS-ERCP. The radiology images were assessed by experienced GI radiologists. The results of cross-sectional imaging were regarded as negative if no signs of malignancy were observed. If the cross-sectional imaging showed direct signs of malignancy or a suspicion of malignancy, the results were regarded as positive. There were no inconclusive results in the cross-sectional imaging.
EUS assessment
All EUS were performed by an experienced endosonographer. The classification of the EUS finding was based on the endosonographer’s expert judgement of the ultrasound’s appearance during the procedure and the results of the cytopathology examination from the FNA of suspected lesions.
If the EUS showed a clear, benign etiology for the stricture, such as a diverticulum, no FNA was performed. In cases with a clear diagnosis after the EUS and no need for therapy, ERCP was not performed to avoid adverse events.
Based on the results of the EUS, the biliary stricture was classified as malignant if either of the two modalities (ultrasonography appearance or cyto/pathology) was malignant.
During the EUS, the bile duct was examined from both the stomach and the duodenum.
The findings that suggested malignancy on the EUS included the following:
Any visible lesions, for example, in the pancreas or on a lymph node or papilla, causing a compression of the bile duct;
A localized wall thickening involving the surrounding tissue and vessels.
A low-vascularized lesion causing an abrupt stricture.
A clear prestenotic dilatation.
A hard lesion on puncture.
A double-duct sign (a stricture in both the CBD and pancreatic duct with proximal dilation).
No observed benign causes for a stricture and dilatation. These could be a diverticulum, autoimmune pancreatitis, autoimmune cholangitis with a generally highly vascularized bile duct wall, or general mild wall thickening.
ERCP assessment
The signs suggesting malignancy on the ERCP included the following:
A tumor of the papilla.
An abrupt stricture with a prestenotic dilatation.
A tight stricture hardly allowing a stone extraction balloon to pass.
No benign cause of a stricture such as a diverticulum.
A double-duct sign.
During the diagnostic work-up, blood tests (liver enzymes, albumin, and CA 19-9) were taken and the patients’ previous medical history and eventual biliary diseases were recorded. We also recorded adverse events after the EUS-ERCP procedures.
The five diagnostic modalities that were compared included the following:
A radiological assessment (MRCP/MRI and/or CT) before EUS-ERCP (named as “radiology” in the results).
Radiology in combination with EUS (with tissue sampling in the cases for which sampling was performed); this is referred to as radiology–EUS.
EUS-ERCP endoscopic assessment (EUS-ERCP).
All endoscopic tissue sampling; this is referred to as TS.
EUS-ERCP with tissue sampling (EUS-ERCP + TS).
Aims
The specific primary aim of this study was to compare the diagnostic performance (sensitivity, specificity, accuracy, NPV, and PPV) between the five diagnostic modalities (radiology, radiology–EUS, EUS-ERCP, TS, and EUS-ERCP + TS) for unclear biliary strictures.
The secondary aim was to assess these modalities in patients with PSC. Another secondary aim was to assess the added value of combining tissue sampling with both an EUS and ERCP. Furthermore, we aimed to assess the adverse events of EUS-ERCP + TS.
Statistical analysis
All statistical analyses were evaluated using SPSS 27.0 software. Continuous variables were expressed as the median with ranges. Categorical variables were expressed as counts with percentages. The chi-squared test and the McNemar test were used to compare the results of different diagnostic modalities (sensitivity, specificity, positive predictive value, negative predictive value, and accuracy). A p-value of 0.05 or less was regarded as statistically significant.
Results
Patients
During 2013–2020, 80 patients with unclear biliary strictures were referred for EUS and ERCP to rule out malignancy. Nine patients had jaundice on the index EUS-ERCP. Therefore, 64(88%) ERCP were performed in non-jaundiced patients.
Two patients were excluded from the study because of the lack of a definitive biliary diagnosis. One patient died in hypercalcemia four months after the EUS-ERCP, and the other died in acute exacerbation of COPD five months after the EUS-ERCP. Obduction was not performed in these cases.
Our data analysis was based on the index EUS-ERCP combination. However, during the follow-up period of 48(18-92) months (median (range)), nine patients underwent a repeated EUS/ERCP. The data for these procedures were only used to determine the final diagnosis.
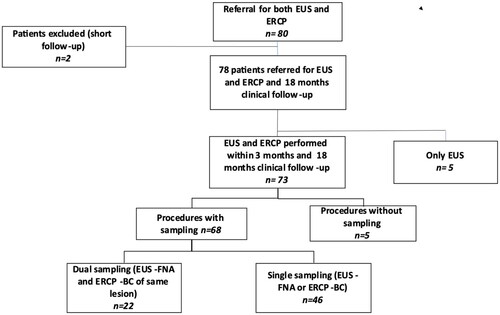
Therefore, 78 patients were included for the analysis of the diagnostic accuracy of the different methods. Three patients had an unclear mass that could be inflammatory or malignant. The inclusion and exclusion process of patients is shown in .
Figure 1. Patient selection in a flow diagram.
BC: brush cytology, EUS: endoscopic ultrasound, ERCP: endoscopic retrograde cholangiopancreatography, FNA: fine needle aspiration.
Twenty-five patients had a previous biliary disorder before the EUS-ERCP (twenty-four patients had PSC and one patient had cholelithiasis). At least one ERCP was performed in 53% of the patients before they were referred for EUS-ERCP.
The final diagnoses were benign in 48 (62%) of the cases (PSC, n = 18; other benign diagnoses, n = 30) and malignant in 30 cases (38%) (CCA, n = 16; pancreatic cancer, n = 11; other malignancy, n = 3). This study’s baseline characteristics are presented in and the final diagnoses are given in .
Table 1. The study’s baseline characteristics.
Table 2. The final diagnoses.
Blood tests
The results of the blood tests taken on the day of the EUS examination (liver function tests, CA 19-9, and albumin) are presented in relation to the final diagnosis in . The albumin and bilirubin values were found to be normal in many cases, despite malignancy.
Table 3. The Laboratory results in relation to the final diagnosis.
The diagnostic performance
Seventy-three patients underwent EUS-ERCP within 3 months; of these, 58 (79%) underwent this procedure on the same session. The median age was 58 years (range: 15-80 years), and 68% of the patients were male (m/f: 50/23). The results of the diagnostic performance of EUS-ERCP in this group were analyzed using a per-protocol analysis, as shown in . Out of the 58 patients who underwent EUS + ERCP in the same session, 56 patients were sampled.
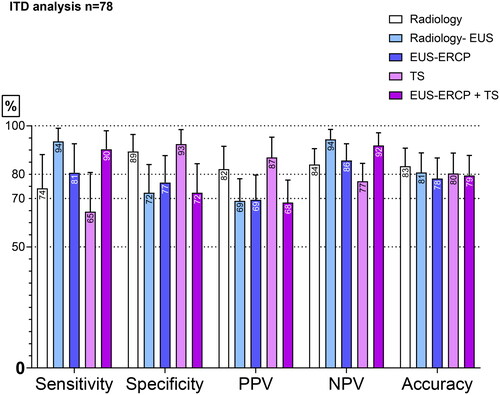
Figure 2. Sensitivity, specificity, NPV, PPV, and accuracy of the different diagnostic modalities in patients undergoing combined EUS-ERCP (n = 73). This is the per-protocol analysis.
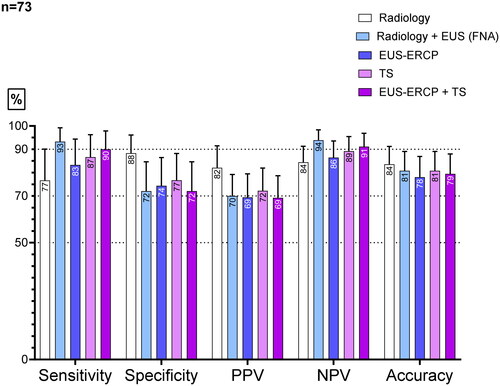
The diagnostic performance of the five compared modalities is shown in as the intention-to-diagnose analysis: n = 78. The tissue sampling showed the highest numerical specificity. Radiology–EUS and EUS-ERCP + TS had the highest numerical sensitivity and NPV. The analysis showed no significant differences between the examined groups. When comparing the intention to diagnose and the per-protocol analysis, no major differences were observed regarding the diagnostic performance. Five patients underwent only EUS. Two had a diverticulum that explained the dilatation of the bile duct and no lesions. One patient had chronic pancreatitis and no lesion. Two had adenomas at the papilla and were left for discussion by the MDT. No samples were taken in five patients. As mentioned above, two had a diverticulum on the EUS and no lesions, and one patient had chronic pancreatitis and no lesion; only EUS was performed in the three cases. Two patients had small bile duct stones that were not visible on radiology and that caused a dilatation. These last two underwent EUS-ERCP.
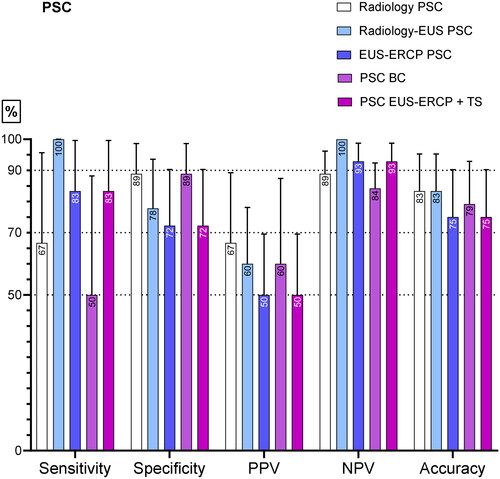
The PSC patients
In the PSC patients (n = 24), which accounted for 31% of the study population, EUS-ERCP + TS showed a high negative predictive value of 93%, indicating few missed malignancies. The combination of radiology and EUS showed a NPV and a sensitivity of 100%. ERCP brush cytology was performed in all the PSC patients, and only two had EUS-FNA at the request of the MDT because these were difficult cases to diagnose. The FNA was taken from the stricture. In 16/24 (67%) of the PSC patients, ERCP-BC was performed before their inclusion in this study, without a clear diagnosis. The diagnostic performance of all the modalities is presented in .
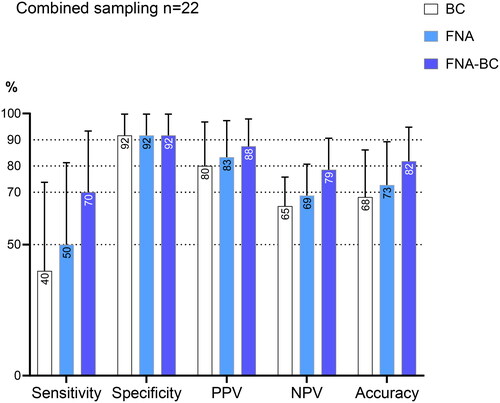
Combined tissue sampling with ERCP and EUS
In the 22 patients undergoing combined tissue sampling with ERCP and EUS from the same lesion, the diagnostic measures are presented in . These procedures were performed in the same session. The combination of EUS-ERCP with tissue sampling resulted in a numerical improvement in the accuracy, but this did not reach statistical significance.
Adverse events
For all the patients undergoing both EUS and ERCP (n = 73), the incidence of pancreatitis (n = 2) was 3% and that of cholangitis (n = 1) was 1%. These patients had an uneventful recovery with conservative management. In the patients undergoing EUS-ERCP and tissue sampling in the same session (n = 58) two patients (5%) had pancreatitis.
Discussion
This study showed that the combination of EUS-ERCP with tissue sampling has a high negative predictive value and can, therefore, exclude most malignancies in unclear biliary strictures. The combination of these two procedures is also safe. This is the first study to focus on the combination of EUS and ERCP in PSC patients. In PSC patients, this combination showed a high diagnostic performance as well.
The accurate diagnosis of an unclear biliary strictures is difficult and has a huge impact on the treatment decision. An inaccurately characterized benign stricture will put the patient into the risk of undergoing unnecessary extensive surgery. On the other hand, a PSC patient with a malignant stricture that is characterized as benign will not be offered surgery or transplantation. This will impact the survival of the patient.
There are several diagnostic modalities that can be used for the investigation of unclear biliary strictures, but none of them have proven to be robust enough as a single method for making a clinical treatment decision.
Cross-sectional imaging has a high accuracy for biliary malignancy, but in modern clinical decision-making regarding therapy, tissue acquisition is often required [Citation24].
EUS and ERCP are complementary procedures in the evaluation of biliary strictures and can be performed in the same session. Previous studies investigating the combination of EUS and ERCP have often been limited by incomplete protocols or small cohort sizes and have also focused on cases with a strong suspicion of malignancy. The value of EUS-ERCP alone or in combination with cross-sectional imaging in PSC has not been studied before.
In this study, we enrolled patients with unclear biliary strictures. In these patients, several diagnostic modalities failed to reach a definitive diagnosis. Most of the included patients 62% had a benign final diagnosis, in contrast to previous studies. The results of blood tests taken on the day of the EUS examination revealed that the albumin and bilirubin values were normal in many cases, despite malignancy. also shows a large overlap in the blood test values between the benign and malignant cases, reflecting the difficulty of diagnosing these cases.
Our hypothesis was that EUS-ERCP with tissue sampling is a useful tool for excluding malignancy in unclear biliary strictures. However, we were not sure how this tool would work in the difficult PSC patients and we were unsure about the risk of adverse events. In line with our hypothesis, the results of this study indicated that combining EUS and ERCP results in a useful tool that can be used to exclude malignancy in unclear biliary strictures in both PSC and non-PSC patients. The results also suggested that tissue sampling combined with both ERCP and EUS can improve the diagnostic performance. The combination of these two procedures is safe.
This study also demonstrated that the combination of cross-sectional imaging and EUS-FNA improves the sensitivity and NPV, minimizing the risk for complications by avoiding ERCP.
The intention-to-diagnose (n = 78) analysis showed a high NPV of 92% for EUS-ERCP with tissue sampling. The combination of cross-sectional imaging and EUS (with FNA in cases it was performed) showed a high sensitivity and NPV, both in the overall patient group (NPV of 94%, sensitivity of 94%) and in the PSC group (NPV of 100%, sensitivity of 100%).
Endoscopic ultrasounds, CT, and MRI use different techniques to produce images. Some lesions may be visible with one technique, but not another. An EUS also allows for tissue sampling. EUS-ERCP + TS is a useful tool in the diagnosis of unclear biliary strictures, showing only a few missed malignancies and resulting in a low risk for complications. This work presents novel data for this combination in the setting of PSC and non-PSC biliary strictures. Previous studies have investigated the use of EUS in the setting of choledocholithiasis [Citation25, Citation26]. One study by Jo JH et al. supports the use of a combination of EUS and ERCP for suspected malignant biliary obstruction [Citation20]. In his study malignancy was diagnosed in 90.9%. Our study with a median follow-up of 48 months showed benign strictures in 62% supporting the difficult nature of the cases included in our study.
The combination of radiology and EUS could be considered in cases with a reduced suspension of malignancy.
The accuracy of tissue sampling combined with ERCP and EUS for the same lesion (n = 22) showed a numerical improvement but did not reach significance. A larger patient group is probably needed for this improvement to reach statistical significance. The possibility of performing EUS-FNA in PSC patients is limited because of the theoretical risk for the seeding of a potential biliary malignancy.
EUS-FNA was performed in only two PSC cases. In these two patients, the punctures were discussed at the MDT and there was a high clinical need for a definitive diagnosis. It should also be mentioned that no data exist on seeding in this setting, and that avoiding a puncture is merely a precautionary measure.
This study showed that malignancy may be excluded in PSC patients, with only a few missed cases, by combining EUS and ERCP. The combination of cross-sectional imaging and EUS (mainly without FNA) showed a NPV and sensitivity of 100%, which is useful for the decision-making process.
The limitations of our study warrant mention: 1) This was a tertiary, single-center study. 2) One experienced endoscopist performed the procedures. 3) The study population with dual sampling was limited. 4) Numerical but not significant differences were shown between the modalities. The generalization of the results should be done with caution. However, the study supports the recommendation of the British Society of Gastroenterology.
In conclusion, this study on unclear biliary strictures assessed different combinations of diagnostic modalities. In cases with a reduced suspicion of malignancy, the combination of imaging and EUS-FNA seems to be a useful and safe method that can be used to exclude malignancy. EUS-ERCP with tissue sampling shows a high negative predictive value and is mandatory when a tissue diagnosis is necessary for the exclusion or confirmation of malignancy. However, further research is needed to support these results.
Author contributions
RS and EB were responsible for the study design. The clinical data were acquired by EB. EB, RS, PH, and AM analyzed the data, and the analyses were interpreted by all the authors. The manuscript was drafted by EB. All the authors made critical revisions of the manuscript’s important intellectual content and approved the final submitted draft. RS obtained the funding.
Acknowledgements
We thank Björn Lindkvist (MD, PhD) at Sahlgrenska University Hospital for the important intellectual input and discussion regarding the data.
Disclosure statement
EB, AM, and PH have no conflicts of interest or financial ties to disclose. RS received lecture fees from Cook Medical, Boston Scientific, and Olympus that were unrelated to this work.
Additional information
Funding
References
- Xie C, Aloreidi K, Patel B, et al. Indeterminate biliary strictures: a simplified approach. Expert Rev Gastroenterol Hepatol. 2017;12(2):189–199. doi: 10.1080/17474124.2018.1391090:.
- Singh A, Gelrud A, Agarwal B. Biliary strictures: diagnostic considerations and approach. Gastroenterol Rep (Oxf). 2015;3(1):22–31. doi: 10.1093/gastro/gou072.
- Huynh R, Owers C, Pinto C, et al. Endoscopic evaluation of biliary strictures: current and emerging techniques. Clin Endosc. 2021;54(6):825–832. doi: 10.5946/ce.2021.048.
- Barkin JA, Levy C, Souto EO. * Endoscopic management of primary sclerosing cholangitis. Ann Hepatol. november-december, 2017;16(6):842–850. *** doi: 10.5604/01.3001.0010.5274.
- Lutz HH, Wasmuth HE, Streetz K, et al. Endoscopic ultrasound as an early diagnostic tool for primary sclerosing cholangitis: a prospective pilot study. Endoscopy. 2012; Oct44(10):934–939. Epub 2012 Jul 2. PMID: 22752890. doi: 10.1055/s-0032-1309896.
- Bhat P, Aabakken L. Role of endoscopy in primary sclerosing cholangitis. Clin Endosc. 2021;54(2):193–201. Publication Date (Web): 2020 May 08 (Review) doi: 10.5946/ce.2020.019-IDEN.
- Fung BM, Tabibian JH. Biliary endoscopy in the management of primary sclerosing cholangitis and its complications. Liver Res. 2019;3(2):106–117. doi: 10.1016/j.livres.2019.03.004.
- Victor DW, Sherman S, Karakan T, et al. Current endoscopic approach to indeterminate biliary strictures. World J Gastroenterol. 2012;18(43):6197–6205. doi: 10.3748/wjg.v18.i43.6197.
- Wang GX, Ge XD, Zhang D, et al. MRCP combined with CT promotes the differentiation of benign and malignant distal bile duct strictures. Front Oncol. 2021;11:683869. Published 2021 Sep 14. doi: 10.3389/fonc.2021.683869.
- Lee HJ, Cho KB. Diagnosis of malignant biliary stricture: more is better. Clin Endosc. 2018;51(2):115–117. doi: 10.5946/ce.2018.035.
- Tanisaka Y, Mizuide M, Fujita A, et al. Diagnostic process using endoscopy for biliary strictures: a narrative review. J Clin Med. 2021;10(5):1048. Published 2021 Mar 3. doi: 10.3390/jcm10051048.
- Dorrell R, Pawa S, Zhou Y, et al. The diagnostic dilemma of malignant biliary strictures. Diagnostics (Basel). 2020;10(5):337. Published 2020 May 25. doi: 10.3390/diagnostics10050337.
- De Moura DT, De Moura H, Bernardo EGH, et al. Endoscopic retrograde cholangiopancreatography versus endoscopic ultrasound for tissue diagnosis of malignant biliary stricture: systematic review and meta-analysis. Endosc Ultrasound. 2018;7(1):10–19. doi: 10.4103/2303-9027.193597.
- Weilert F, Bhat YM, Binmoeller KF, et al. EUS-FNA is superior to ERCP-based tissue sampling in suspected malignant biliary obstruction: results of a prospective, single-blind, comparative study. Gastrointest Endosc. 2014; Jul80(1):97–104. Epub 2014 Feb 19. PMID: 24559784. doi: 10.1016/j.gie.2013.12.031.
- Arnelo U, von Seth E, Bergquist A. Prospective evaluation of the clinical utility of single-operator peroral cholangioscopy in patients with primary sclerosing cholangitis. Endoscopy. 2015; Aug47(8):696–702. Epub 2015 Mar 31. PMID: 25826274. doi: 10.1055/s-0034-1391845.
- Chung HG, Chang JI, Lee KH, et al. Comparison of EUS and ERCP-guided tissue sampling in suspected biliary stricture. PLoS One. 2021;16(10):e0258887. Published 2021 Oct 20. doi: 10.1371/journal.pone.0258887.
- Sadeghi A, Mohamadnejad M, Islami F, et al. Diagnostic yield of EUS-guided FNA for malignant biliary stricture: a systematic review and meta-analysis. Gastrointest Endosc. 2016; Feb83(2):290–298.e1. Epub 2015 Sep 28. PMID: 26422979. doi: 10.1016/j.gie.2015.09.024.
- Saifuku Y, Yamagata M, Koike T, et al. Endoscopic ultrasonography can diagnose distal biliary strictures without a mass on computed tomography. World J Gastroenterol. 2010;16(2):237–244. doi: 10.3748/wjg.v16.i2.237.
- Charbel SMD, Kimberly JMD, Conway JMD, et al. Same-Day combined EUS/ERCP to investigate biliary and pancreatic disorders: better together. American Journal of Gastroenterology. September 2008;103: s 70. * - volumeIssue - doi: 10.14309/00000434-200809001-00180.
- Jo JH, Cho CM, Jun JH, Research Group for Endoscopic Ultrasonography in KSGE., et al. Same-session endoscopic ultrasound-guided fine needle aspiration and endoscopic retrograde cholangiopancreatography-based tissue sampling in suspected malignant biliary obstruction: a multicenter experience. J Gastroenterol Hepatol. 2019; Apr34(4):799–805. Epub 2018 Nov 21. PMID: 30378169. doi: 10.1111/jgh.14528.
- Rösch T, Meining A, Frühmorgen S, et al. A prospective comparison of the diagnostic accuracy of ERCP, MRCP, CT, and EUS in biliary strictures. Gastrointest Endosc. 2002; Jun55(7):870–876. PMID: 12024143. doi: 10.1067/mge.2002.124206.
- Vila JJ, Fernández-Urién I, Carrascosa J. EUS and ERCP: a rationale categorization of a productive partnership. Endosc Ultrasound. 2021;10(1):25–32. doi: 10.4103/eus.eus_58_20.
- Chapman MH, Thorburn D, Hirschfield GM, et al. British Society of Gastroenterology and UK-PSC guidelines for the diagnosis and management of primary sclerosing cholangitis. Gut. 2019;68(8):1356–1378. doi: 10.1136/gutjnl-2018-317993.
- Sudan D, DeRoover A, Chinnakotla S, et al. Radiochemotherapy and transplantation allow long-term survival for nonresectable hilar cholangiocarcinoma. Am J Transplant. 2002;2(8):774–779. doi: 10.1034/j.1600-6143.2002.20812.x.
- Patel R, Ingle M, Choksi D, et al. Endoscopic ultrasonography can prevent unnecessary diagnostic endoscopic retrograde cholangiopancreatography even in patients with high likelihood of choledocholithiasis and inconclusive ultrasonography: results of a prospective study. Clin Endosc. 2017; Nov50(6):592–597. Epub 2017 Aug 9. PMID: 28793395; PMCID: PMC5719909 doi: 10.5946/ce.2017.010.
- Prachayakul V, Aswakul P, Bhunthumkomol P, et al. Diagnostic yield of endoscopic ultrasonography in patients with intermediate or high likelihood of choledocholithiasis: a retrospective study from one university-based endoscopy center. BMC Gastroenterol. 2014; Sep 2614(1):165. PMID: 25257935; PMCID: PMC4182833 doi: 10.1186/1471-230X-14-165.