?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Guidelines generally recommend a combination of immunological assays and chest X-ray imaging (CXR) when screening for latent tuberculosis infection (LTBI) prior to biologic treatment in inflammatory bowel disease (IBD).
Objective
To investigate whether CXR identify patients with suspected LTBI/TB who were not identified with QuantiFERON tests (QFT) when screening for LTBI/TB before starting biologic treatment in IBD patients.
Methods
Single-center, retrospective cohort study of patients with inflammatory bowel disease who had a QFT and a CXR prior to initiation of biologic treatment in a 5-year period (October 1st, 2017 to September 30th, 2022).
Results
520 patients (56% female, mean age 40.1 years) were included. The majority had none or few risk factors for TB (as reflected by the demographic characteristics) but some risk factors for having false negative QFT results (concurrent glucocorticoid treatment and inflammatory activity). QFT results were positive in 8 patients (1.5%), inconclusive in 18 (3.5%) and negative in 494 (95.0%). Only 1 patient (0.19%) had CXR findings suspicious of LTBI. This patient also had a positive QFT and was subsequently diagnosed with active TB. All patients with negative or inconclusive QFT had CXR without any findings suggesting LTBI/TB. One patient developed active TB after having initiated biologic treatment in spite of having negative QFT and a normal CXR at screening.
Conclusion
In a population with low risk of TB, the benefits of supplementing the QFT with a CXR are limited and are unlikely to outweigh the cost in both patient test-burden, radioactive exposure, and economic resources.
Introduction
Compared to standard treatment with 5-aminosalicylates (ulcerative colitis only), glucocorticoids and thiopurines, the introduction of biologics, in particular tumor necrosis factor (TNF) inhibitors, have greatly improved treatment of inflammatory bowel diseases (IBD) – ulcerative colitis and Crohn’s disease [Citation1–3]. While the use of biologic treatments has improved the options for IBD-treatment, they can have significant side effects such as the reactivation of latent infections such as latent tuberculosis infections (LTBI) [Citation4]. Side effects such as these are also seen with JAK-inhibitor treatment [Citation5].
It is estimated that up to 25% of people worldwide are affected by LTBI with high endemic areas generally located in sub-Saharan Africa and Southeast Asia and low endemic areas in North America and Europe [Citation6,Citation7]. In most infections, the immune system creates a granuloma formation to limit further growth of the bacteria resulting in LTBI, a condition with no clinical symptoms but with the risk of later reactivation leading to active tuberculosis (TB) [Citation8]. Due to the ability of biologic treatments to reactivate TB infections/aggravate active TB, it is common practice to screen for LBTI/TB before starting biologic treatment in patients with IBD [Citation9].
LTBI is generally diagnosed based on a threefold combination of clinical data, immunological assays, and imaging. Patients are clinically screened by identifying possible risk factors for exposure to TB [Citation10]. The immunological assay tests can consist of either the tuberculin skin test (TST) or an interferon-gamma release assay (IGRA). Some IBD patients receive high doses of glucocorticoids which can weaken the T-cell response which both the TST and the IGRA tests rely on [Citation11]. This provides a potential problem when screening IBD patients. A flare up in a patients IBD disease can itself also weaken the diagnostic value of the immunological assays [Citation12].
The immunological assay tests are often supplemented with imaging, most often in the form of a chest X-ray (CXR). The CXR looks for signs of previous TB infections such as fibrotic scarring and calcified nodules. These findings are, however, unspecific and can be the result of other several differential diagnoses. Unlike the immunological assays the CXR can somewhat distinguish between active and latent TB although not as well as computed tomography [Citation13]. The role of imaging in diagnosing LTBI is contested and vary in different guidelines with some recommending it is always used in supplementing the immunological assays while others recommend it only be used under certain conditions such as in immunocompromised patients or if the immunological assay produces a positive result [Citation9,Citation14,Citation15].
While some guidelines are more reluctant with the use of CXR, most still recommend the immunological assays to be followed by a CXR regardless of result [Citation9]. Since unnecessary tests have an economic cost to the healthcare system and a negative impact on patient quality of life due to radiation and test-burden, research should be conducted to better understand the role of a CXR when screening for LTBI.
Aims
The main research objective was to investigate if a CXR identifies patients suspected of having LTBI/TB who are not identified with a QFT when screening for LTBI prior to initiating biologic treatment in inflammatory bowel disease patients.
A secondary objective was to identify possible patient characteristics which could increase the necessity for a CXR in screening for LTBI/TB in IBD patients before startup in biologic treatment.
Materials and method
Design
This study is a single center, retrospective cohort study of all IBD patients at the Department of Medical Gastroenterology at Odense University Hospital (OUH) in Denmark who initiated biologic treatment within a 5 year period.
Patients
Patients were identified by extracting a list from the Department of Clinical Microbiology, Odense University Hospital containing the unique patient identification numbers (Central Person Register number (CPR number) of all patients that has had an IGRA test requisitioned by the Department of Medical Gastroenterology, OUH from October 1st, 2017 to September 30th, 2022. The IGRA used was the QuantiFERON®-TB Gold Plus, made by Qiagen (Hilden, Germany). Since the Department of Clinical Microbiology does all the QFTs on patients from the Department of Medical Gastroenterology, all patients screened in the 5-year period were identified by this method.
Patients were excluded from the study, if they had a failed QFT, did not have a CXR taken within 3 months of the QFT, or they did not have an IBD diagnosis. A QFT could fail if insufficient blood was sampled, the wrong sample container was used, or the period between taking the sample and analysis exceeded 16 h. Some individuals featured multiple times as they had multiple QFTs done within the 5-year period. The duplicates were excluded so that an individual only featured once. The decision on which duplicate to keep was made by prioritizing the duplicate with a clear QFT result (positive/negative). If the duplicates were even in this regard, the duplicate with the shortest time span between the CXR and QFT was kept. If the duplicates were still evenly prioritized after this, the duplicate with the newest QFT was kept.
Endpoint
The primary outcome of interest in this study was the test results for the QFT and the CXR. Results of the QFT were reported as ‘positive’, ‘negative’ or ‘inconclusive’. A QFT result was considered positive if the Interferon-gamma (IFN-γ) IE/ml value was significantly higher in the TB-antigen tubes compared to the nil tube. A mitogen tube was used as a control. An IFN-γ response below 0,5 IE/ml in the mitogen tube along a negative IFN-γ response for TB-antigens resulted in an inconclusive result. In case of an inconclusive QFT result, the QFT was generally repeated after a couple of days. The CXR’s were assessed by radiologists from the Department of Radiology at OUH except for two that were assessed by doctors from the Department of Medical Gastroenterology alone. For CXR evaluated by radiologists, findings were considered suggestive of LTBI/TB if this was indicated in the radiology report. Otherwise, the CXR was classified as not being suggestive of LTBI. For the two CXR assessed by non-radiologists, calcified nodules, non-calcified nodules, pleural thickening or fibrotic scarring were considered suggestive of LTBI.
The results were accessed through the patients’ medical records which could be accessed via the electronic patient record system EPJ-SYD (Elektroniske Patient Journal Syd) system using their unique CPR numbers.
Secondary outcomes of interest were patient characteristics thought to influence either the sensitivity of the QFT or the likelihood of a patient having LTBI and could thus increase the necessity for the CXR. These patient characteristics were also collected from the patients’ medical records. The paraclinical characteristics were only gathered if data from 1 month before- or 1 week after the QFT were available. There was no time cutoff for the other characteristics.
Statistical analysis
The test results were compiled and presented together in a 3 × 2 table. The total number of patients who received a negative QFT result, but a TB suspect CXR was the outcome of interest and would represent the number of patients who would be of increased risk of LTBI reactivation when starting biologic treatment without a CXR.
The H0 assumption that CXRs identified patients with LTBI who were otherwise missed by QFTs to the same extents as QFTs identified patients with LTBI who were missed by CXRs was tested with McNemar’s test. Logistic regression was meant to be used on the different categories of patient characteristics with the characteristics being the exposure groups and the outcome being a negative QFT combined with a positive CXR and the absence of outcome being any of the three other combinations of test results. This was, however, not possible as no patients had the above-mentioned outcome. Findings was therefore presented and described.
Results
Characteristics of the study population
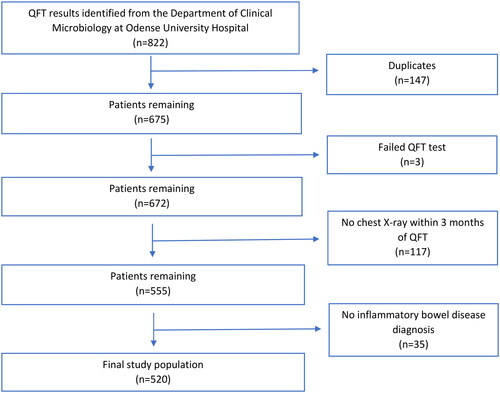
The data extract from the Department of Clinical Microbiology identified 822 test results belonging to 675 individual patients. The 147 duplicates were excluded. Out of the 675 individual patients, 3 were excluded due to failed QFT, 117 excluded due to missing CXR within 3 months of the QFT and 35 were excluded because they did not have an IBD diagnosis. This meant that 520 of the initial 675 individual patients remained after the exclusion criteria were applied (for exclusion process, see ).
Characteristics of the study population are presented in . The patients included in the study generally had few factors which increased the likelihood of having been exposed to TB. They were predominantly ethnically Danish with few of non-Danish ethnicity (8%), low levels of alcohol overconsumption (3%), drug abuse (0%), homelessness (0%), prior known TB infection (1%) and travel to high endemic areas (2%). Factors that theoretically decrease the sensitivity of the QFT were present with many in glucocorticoid treatment (44%) and having biochemical signs of inflammatory activity (high leukocytes (45%), CRP (43%), and calprotectin (48%)).
Table 1. Characteristics of the study population consisting of inflammatory bowel disease patients screened for tuberculosis before starting biologic treatment.
Some patients had a combination of characteristics which would decrease the diagnostic accuracy of the QFT, such as concurrent glucocorticoid treatment and alcohol overconsumption, non-Danish ethnicity, travel to high endemic area and known prior TB infection that should increase the risk of TB exposure. Thirty-three patients were both in glucocorticoid treatment and had 1 risk factor for TB exposure, 3 patients were in glucocorticoid treatment and had 2 risk factors, 1 was in glucocorticoid treatment and had 3 risk factors and no one was in glucocorticoid treatment and had all risk factors present. No patients without concurrent glucocorticoid treatment had more than 2 risk factors. Theoretically, these patients should be particularly at risk of getting false-negative QFT results.
QFT and CXR results
Among the 520 patients tested with both QFT and CXR, 8 patients had a positive QFT, 494 had a negative QFT whereas 18 patients had inconclusive results of their QFTs. Only one patient had CXR findings suspicious of TB and this patient also had a positive QFT. This patient was subsequently diagnosed with active TB. Thus, none of the patients with negative or inconclusive QFT results had CXR findings suspicious of TB (see ). QFTs identified patients with LTBI who were missed by CXRs statistically significantly more often than CXRs identified patients with LTBI who were otherwise missed by QFTs (p = 0.008). Even if the exclusion criteria were loosened to accept CXR’s regardless of the time from QFT as well as CT- and PET-CT scans, there still was not a single patient with a negative QFT combined with chest imaging findings indicative of LTBI/TB out of now 582 patients.As previously noted, all patients suspected of LTBI (or active TB) were referred for treatment prior to initiation of biologic treatment. Since there is no single gold standard test for LTBI it is not certain how many of these patients actually had LTBI [Citation9].
Table 2. Test results for inflammatory bowel disease patients screened for tuberculosis before starting biologic treatment.
During the inclusion period only one patient subsequently developed active TB. This patient had both a negative QFT and a negative CXR prior to initiation of biologic treatment but nevertheless developed TB during biologic therapy. The patient was a 20-year-old ethnic Danish female with ulcerative colitis and no risk factors for TB exposure or biochemical signs of inflammatory activity. She was, however, in glucocorticoid treatment and had received 200 mg of methylprednisolone intravenously prior to the QFT.
Since no patients experienced the primary outcome (negative QFT combined with suspect CXR), no specific patient characteristics associated with this outcome could be identified.
Although the included population was at low risk of TB (as judged by demographic characteristics), 2 cases of active TB was identified, one during screening prior to biologic treatment and one during biologic treatment.
Discussion
In the present study of outcome of TB screening prior to biologic treatment in IBD patients, CXR did not provide any additional diagnostic benefit compared to QFT alone. Thus, none of patients screened was identified as potentially having LTBI/TB based on radiological changes in a CXR without a positive QFT. All patients identified as being suspected of LTBI/TB, also had a positive QFT. One patient, who had both normal CXR and negative QFT subsequently developed active TB. Although post-screening exposure to TB cannot be ruled out for this patient, the findings underline the limitations of both screening tests.
While previous studies have been made examining the diagnostic accuracy of CXR’s when screening for LTBI in both low and high risk populations [Citation13,Citation16,Citation17], this study is to the authors’ knowledge the first to directly examine whether using CXRs in addition to QFTs have any value in finding cases of LTBI when screening for LTBI in a low endemic area.
A previous systematic review and meta-analysis of published studies on radiological signs of LTBI found a higher percentage of patients with LTBI-CXR lesions both with a positive and a negative QFTs with proportions of 32% and 11% respectively compared to 11% and 0% found in this study [Citation16]. The previously published review included studies from high endemic areas or studies based on screening high risk groups like immigrants from high endemic areas whereas the present study primarily included ethnic Danish patients with few risk factors for having TB (homelessness, drug abuse and travel to high endemic areas). Most likely, this explains the differences between the results of the present study and results of the before mentioned meta-analysis. However, the ambiguity of the definition of LTBI-CXR lesions and their interpretation could also in part explain the difference of the results of this study and the before-mentioned meta-analysis. Thus, while the QFT results were unambiguously negative, positive, or indeterminate, the CXR results were not, with clinical context sometimes necessary for interpretation. Several CXR’s showed changes that could be compatible with a former TB infection such as nodules, fibrotic lesions or thickening of the pleura. However, since these findings are non-specific for LTBI and compatible with aftereffects of much more common illnesses such as other bacterial or viral pneumonias, they were all interpreted by the radiologists as non-suspect for TB and biologic treatment was initialized without subsequent development of TB supporting the judgement of the radiologist. This low specificity of CXR’s have been described in several other studies showing the limitation of CXR’s in diagnosing TB [Citation13,Citation17,Citation18]. The clinical reality of these results was, that they led to the same outcome as a completely normal CXR, and they have therefore all been registered as ‘non-suspect’ in this study as none of them resulted in further tests or treatment for LTBI. The validity of the CXR evaluations made is supported by the fact, that only one patient developed TB during biologic treatment despite having a normal CXR (subsequent re-review of CXR revealed no suspicious finding whatsoever).
The primary strength of the study is the fact that it involved all IBD patients at Odense University Hospital (OUH) who were screened for LTBI during a five-year period. OUH is primary IBD center for more than half of the population of Funen (approximately 470.000 inhabitants with a TB incidence of 6.1 per 1.000.000 in 2019 [Citation19]) making the results almost fully representative for IBD patients on the island of Funen, Denmark with just a few missing due to exclusion.
With a p value of 0.008 through McNemar’s test the study had sufficient power to demonstrate that the fraction of patients with positive CXR but negative QFT was statistically smaller than the number of patients with positive QFT but negative X-CXR supporting the conclusion that in this low risk population, the extra benefit of CXR for screening for LTBI is limited.
Furthermore, all patients on Funen, who develop TB are treated at Odense University Hospital, Department of Infectious Diseases. Thus, all patients in the study population who developed active TB during the study period have been identified, making it possible to evaluate the effect of the screening program in terms of reducing risk of active TB during biologic treatment.
Furthermore, the study population had many characteristics present which could lower the sensitivity of the QFT such as obesity, inflammatory disease activity and glucocorticoid treatment [Citation20]. Thus, the study population is well suited to test if CRX provides any additional screening benefit prior to biologic treatment in IBD.
The primary limitation of the study is the retrospective nature. Thus collection of information regarding the patient characteristics and history depended on the completeness of the patients’ medical journals. In particular, history of alcohol overconsumption, drug abuse, and travel to high-endemic areas was not available for all patients probably leading to underestimation of prevalence of these risk factors.
A further limitation is the fact that the study population was at low risk of TB. Results of this study cannot be extrapolated to a high risk/TB endemic population where use of CXR for screening or even TB treatment prior to biologic treatment in spite of negative QFT may be warranted.
23% of the 675 patients initially enrolled in the study had to be excluded which could potentially affect the external validity. However, the majority of the excluded patients (115 out of 155) were excluded due to not having a CXR within 3 months of their QFTs. A large portion of these were patients hospitalized due to a flare up in their IBD. Patients rarely started biologic treatment without a CXR so many excluded only had the QFT taken in case they needed rescue treatment but never did and were therefore not a part of the target population.
There was no time-based cutoff for the data for patient characteristics, except for the paraclinical information. Information in medical journals regarding some of the characteristics such as smoking status and body mass index was sometimes years old and may no longer have been relevant at the time of the QFTs.
Even when using both the QFT and a CXR not every patient with LTBI will be identified as shown with the patient that had a negative QFT and normal CXR and still developed active TB after starting biologic treatment. Combined with the result of this study it is suggested that in a population like the one studied here, more emphasis should be put on using the chest X-ray imaging (CXR/CT) more liberally for diagnostic purposes after startup in biologic treatment rather than screening pre-startup.
CXR’s still have an important role in differentiating between active and latent TB which the QFT cannot. While in many places the CXR is always used with either the TST or QFT, this study shows that it might be better to reserve its use depending on a positive or indeterminate result of the QFT.
In conclusion, this study implies that the benefits of supplementing the QFT test with a CXR are limited and are unlikely to outweigh the cost in both patient test-burden, radioactive exposure, and economic resources when screening for LTBI in a low endemic population.
While it remains likely that imaging still plays a larger role in certain subgroups with higher TB incidence, no characteristics could be identified in this study due to the low-endemic nature of the study population.
Ethics statement
The data were collected as part of a quality assurance study approved by the Management of Odense University Hospital. Under Danish law, quality assurance studies limited to 5 years do not require approval from Ethics committee.
Disclosure statement
MAA served as an advisor to for Abbvie and Janssen. SBC has no conflicts of interest to declare.
Data availability statement
The participants of this study did not give written consent for their data to be shared publicly, so due to the sensitive nature of the research supporting data is not available.
Additional information
Funding
References
- Gomollón F, Dignass A, Annese V, et al. 3rd European evidence-based consensus on the diagnosis and management of Crohn’s disease 2016: part 1: diagnosis and medical management. J Crohns Colitis. 2017;11(1):3–25. doi:10.1093/ecco-jcc/jjw168.
- Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017;11(6):649–670. doi:10.1093/ecco-jcc/jjx008.
- Harbord M, Eliakim R, Bettenworth D, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. Part 2: current management. J Crohns Colitis. 2017;11(7):769–784. doi:10.1093/ecco-jcc/jjx009.
- Byun JM, Lee CK, Rhee SY, et al. Risks for opportunistic tuberculosis infection in a cohort of 873 patients with inflammatory bowel disease receiving a tumor necrosis factor-α inhibitor. Scand J Gastroenterol. 2015;50(3):312–320.
- Clarke B, Yates M, Adas M, et al. The safety of JAK-1 inhibitors. Rheumatology (Oxford). 2021;60(Suppl. 2):ii24–ii30. doi:10.1093/rheumatology/keaa895.
- Cohen A, Mathiasen VD, Schön T, et al. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2019;54(3):1900655. doi:10.1183/13993003.00655-2019.
- World Health O. WHO global lists of high burden countries for tuberculosis (TB). In TB/HIV and multidrug/rifampicin-resistant TB (MDR/RR-TB), 2021–2025: background document. Geneva: World Health Organization; 2021.
- Rao M, Ippolito G, Mfinanga S, et al. Latent TB Infection (LTBI) - Mycobacterium tuberculosis pathogenesis and the dynamics of the granuloma battleground. Int J Infect Dis. 2019;80s:S58–S61. doi:10.1016/j.ijid.2019.02.035.
- Hasan T, Au E, Chen S, et al. Screening and prevention for latent tuberculosis in immunosuppressed patients at risk for tuberculosis: a systematic review of clinical practice guidelines. BMJ Open. 2018;8(9):e022445. doi:10.1136/bmjopen-2018-022445.
- Fehily SR, Al-Ani AH, Abdelmalak J, et al. Review article: latent tuberculosis in patients with inflammatory bowel diseases receiving immunosuppression-risks, screening, diagnosis and management. Aliment Pharmacol Ther. 2022;56(1):6–27. doi:10.1111/apt.16952.
- Hakimian S, Popov Y, Rupawala AH, et al. The conundrum of indeterminate QuantiFERON-TB Gold results before anti-tumor necrosis factor initiation. Biologics. 2018;12:61–67. doi:10.2147/BTT.S150958.
- Lovatt J, Gascoyne-Binzi D, Hussey T, et al. Screening for TB in hospitalised patients with inflammatory bowel disease before anti-TNF therapy: is QuantiFERON(®) gold testing useful? J Clin Med. 2021;10(9):1816. doi:10.3390/jcm10091816.
- Piccazzo R, Paparo F, Garlaschi G. Diagnostic accuracy of chest radiography for the diagnosis of tuberculosis (TB) and its role in the detection of latent TB infection: a systematic review. J Rheumatol Suppl. 2014;91:32–40. doi:10.3899/jrheum.140100.
- Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl. 3):s1–s106. doi:10.1136/gutjnl-2019-318484.
- Singh JA, Furst DE, Bharat A, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken). 2012;64(5):625–639. doi:10.1002/acr.21641.
- Uzorka JW, Wallinga J, Kroft LJM, et al. Radiological signs of latent tuberculosis on chest radiography: a systematic review and meta-analysis. Open Forum Infect Dis. 2019;6(7). doi:10.1093/ofid/ofz313.
- Pepper T, Joseph P, Mwenya C, et al. Normal chest radiography in pulmonary tuberculosis: implications for obtaining respiratory specimen cultures. Int J Tuberc Lung Dis. 2008;12(4):397–403.
- Marciniuk DD, McNab BD, Martin WT, et al. Detection of pulmonary tuberculosis in patients with a normal chest radiograph. Chest. 1999;115(2):445–452. doi:10.1378/chest.115.2.445.
- Institut Ss. Tuberculosis 2019 - 2020 en.ssi.dkn.d; 2022. [updated 4. February]. Available from: https://en.ssi.dk/surveillance-and-preparedness/surveillance-in-denmark/annual-reports-on-disease-incidence/tuberculosis-2019–-2020.
- Yamasue M, Komiya K, Usagawa Y, et al. Factors associated with false negative interferon-γ release assay results in patients with tuberculosis: a systematic review with meta-analysis. Sci Rep. 2020;10(1):1607. doi:10.1038/s41598-020-58459-9.
- Organization WH. WHO global lists of high burden countries for tuberculosis (TB), TB/HIV and multidrug/rifampicin-resistant TB (MDR/RR-TB), 2021–2025: background document; 2021.