Abstract
Background and objective
Iron deficiency affects more than 60% of colorectal cancer patients at the time of diagnosis. Iron deficiency ultimately leads to anemia, but additionally, iron deficiency might impact other domains of colorectal cancer patients’ health and well-being. The aim of this study was to evaluate the impact of iron deficiency on fatigue, quality of life, cognition, and physical ability in patients undergoing evaluation for colorectal cancer.
Methods
Multicenter, prospective, observational cross-sectional study (2021–2023). Fatigue was the primary outcome, measured using the Focused Assessment of Cancer Treatment-Anemia questionnaire (FACT-An). Quality of Life, Cognition, Aerobe capacity, mobility, and peripheral muscle strength were tested as secondary outcomes. Multivariate analysis was performed to estimate the impact of iron deficiency on all outcomes.
Results
Two hundred and one patients were analyzed, 57% being iron deficient. In multivariate regression analysis, iron deficiency was not associated with fatigue: FACT-An (r = −1.17, p = 0.57, 25% CI: −5.27 to 2.92). Results on quality of life, cognition, and mobility were non-significant and with small regression coefficients. Iron deficiency showed a nearly significant association with reduced hand-grip-strength (r = −3.47 kg, p = 0.06, 25%CI −7.03 to 0.08) and reduced 6 min walking distance (r = −40.36 m, p = 0.07, 25%CI: −84.73 to 4.00).
Conclusion
Iron deficiency in patients undergoing evaluation for colorectal cancer was not associated with fatigue, quality of life, or cognition, but might affect aerobic endurance and peripheral muscle strength to a degree that is clinically relevant.
Introduction
More than half of patients presenting with colorectal cancer (CRC) have iron deficiency (ID) and/or anemia at the time of diagnosis [Citation1]. Anemia is associated with adverse short-term surgical outcomes, increased rates of red blood cell transfusion, and impaired long-term oncological outcomes in patients operated for CRC [Citation2–5]. Although preoperative intravenous (i.v.) iron treatment has been recommended for anemic CRC patients, the clinical effect is equivocal. Preoperative i.v. iron treatment effectively raises hemoglobin levels when administered in sufficient time before surgery and has been shown to modestly reduce perioperative red blood cell transfusion rates in CRC patients [Citation6]. Preoperative i.v. iron treatment is included in several CRC-treatment guidelines despite that the beneficial impact on clinical endpoints, such as postoperative complication rates, length of hospital stay, postoperative recovery, or CRC recurrence has not been clearly identified [Citation7–11].
Considering the essential role of iron as a micronutrient responsible for oxygen transport in the blood and skeletal muscle, as well as its involvement in electron transfer reactions, gene regulation, and cell growth and differentiation, the impact on CRC patients likely extends beyond anemia alone [Citation12].
Iron deficiency with or without anemia may result in fatigue, exhaustion, ‘brain fog’, muscle weakness, and cognitive impairment [Citation12]. Studies conducted on iron-deficient and anemic rats showed treadmill performance to significantly improve three days after intravenous iron infusion, indicating that the effect was primarily due to the correction of iron deficiency [Citation13]. In humans iron deficiency affects mitochondrial function in cardiomyocytes, resulting in decreased ATP levels and contractility, which was also reversed shortly after i.v. iron substitution [Citation14]. In addition, a small non-blinded study including CRC patients showed increased cardiopulmonary exercise performance 25 days after i.v. iron administration [Citation15].
In a sub-study of the IVICA trial, comparing preoperative i.v. iron treatment with oral iron treatment in surgical CRC patients, the group receiving i.v. iron treatment exhibited higher quality of life (QoL) scores two to three months following surgery [Citation16]. Beyond this study, research on CRC has been limited in terms of evaluating endpoints other than hemoglobin, transfusion risk, complications, and survival. Therefore, hypotheses must be drawn from studies conducted on different patient cohorts. Notably, iron deficiency has been associated with reduced QoL, fatigue and impaired cognition [Citation17–21]. Iron deficiency anemia is associated with lower physical performance measured on endurance and aerobe functionality [Citation22]. I.v. iron treatment improves hand grip strength in cancer patients [Citation23] a measure that is associated with a significantly higher risk of postoperative complications and mortality in CRC patients [Citation24].
The aim of the study was to explore the hypothesis that iron deficiency is independently associated with higher levels of fatigue and lower quality of life, cognitive function, and physical ability in patients undergoing evaluation for colorectal cancer, compared to patients without iron deficiency.
Materials and methods
This is a multicenter prospective observational cross-sectional study. Reporting follows the STROBE guidelines [Citation25]. Inclusion was performed at three hospital-based endoscopy clinics within the Region of Southern Denmark from 11 February 2021 to 31 May 2023.
Patients and setting
Patients were recruited from the endoscopy clinic if a lesion, clinically suspected as colorectal malignancy was detected at colonoscopy. After information on the suspicion of colorectal cancer, patients received written study information. Patients were excluded if aged under 18 years, if they did not master the Danish language, or if cerebral impairment made informed consent unobtainable. Patients who only wished to submit the questionnaires without participating in the study visit were still included.
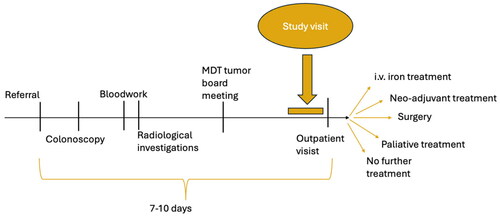
Approximately one week after the endoscopy the patients were scheduled for a clinical outpatient visit according to our clinical routine. Here the results from the histological examination of the biopsies, the laboratory analysis, the radiological examination, and the multidisciplinary team’s discussion regarding further examinations and/or treatment options are presented to and discussed with the patient. On the same day, but before this clinical outpatient visit, the included patients had a 1-h study visit facilitated by a research assistant. This timing of the research visit was chosen to avoid confounding from the disease related knowledge to be obtained at the following out-patient visit. The included patients’ participation in the study ended following the study visit and before any determination of possible treatment options was conveyed ().
Outcome variables
To evaluate the primary outcome fatigue, the Danish version of the Focused Assessment of Cancer Treatment-Anemia questionnaire (FACT-An) was used [Citation26]. As part of this 20 items questionnaire, 13 items are exclusively related to fatigue. This fatigue subscale is also known as the Functional Assessment of Chronic Illness Therapy-Fatigue Scale (FACIT-Fatigue), targeting fatigue in a broader non-cancer-specific population [Citation27]. Missing answers were handled as instructed by the FACIT.org cooperation and imputed using the average of the other answers provided. Quality of life was measured by the Danish version of the European Organization for Research and Treatment of Cancer C30 questionnaire (EORTC-30) and scored according to the official recommendations using the summary score (based on 27 items) and the Global health status/QoL (based on two items) [Citation28,Citation29]. Both the FACT-An and the EORTC-30 were completed by the patients at home one or two days before the study visit.
The remaining four outcomes were measured at the study visit. Cognition was evaluated using the Montreal cognitive assessment (MoCA) test [Citation30], administered by a research assistant. Peripheral muscle strength was evaluated using a handheld dynamometer (JAMAR) according to the instructions provided by The National Institute for Health Research and measured as a one-time test attempt [Citation31]. Mobility and balance were evaluated using the Timed Up and Go (TUG) test [Citation32] while endurance was tested with the 6 min’ walk test (6MWT) [Citation33].
Additional variables and bias handling
From the laboratory charts, we recorded Hemoglobin (Hb), p-ferritin (ug/L), transferrin saturation (TSAT), and p- albumin (g/l). Iron deficiency was defined as a p-ferritin <30 µg/l or TSAT <0.2 [Citation34,Citation35].
During the study visit, the patients were interviewed briefly to gather baseline characteristics on alcohol consumption, smoking habits, and on prior (two months) iron or blood transfusion treatments. Height and body weight were measured. The American Society of Anesthesiologist (ASA) score, histopathology, and the Union for International Cancer Control (UICC) tumor node metastasis classification was determined. The UICC T- and N-stage were determined based on the pathology report if up-front surgery was performed and based on the clinical evaluation in patients not undergoing surgery or in those receiving neo-adjuvant therapy.
To address potential expectation bias, patients were not aware of the study hypothesis or of the evaluation of iron deficiency. To reduce researcher bias, all involved research staff was blinded to the information on iron deficiency status, histopathological evaluation, UICC tumor stage, and treatment options until after the study visit had finished.
Power calculation
Power calculation was done with respect to our primary outcome measure, fatigue as measured by FACT-An. In a previous study, anemic CRC patients experienced a 9 point (SD ± 16) increase on FACT-An from baseline to day-of-surgery following i.v. iron treatment [Citation16]. Since we include both anemic and non-anemic patients, we expected our observed difference to be lower, estimating a difference of 7 points. This expected difference is still well above the minimal clinically important difference of 4.24 [Citation36,Citation37]. With a power of 0.8, a significance level of 0.05, and a distribution of iron deficient vs. non-iron deficient patients of 60 vs. 40%, the estimated sample size required was 175 patients. To allow for incomplete entries, drop-outs, etc. we arrived at a final target of 200 patients to be included.
Statistics
Statistical analyses were performed using Stata/IC 18.0 (Stata Corp, Texas, USA). All six outcome variables were compared between patients being iron deficient and patients not being iron deficient. Statistical significance was taken at p < .05. Normality was determined using the Shapiro–Wilk test. Non-parametric data was reported as medians with interquartile ranges, while parametric data was reported as means with standard deviations.
Included in multivariate linear regression analysis (complete case only) were iron deficiency as the dichotomous exposure variable, together with age, sex, albumin, ASA-classification UICC T- and M-stage, as explanatory variables. The inclusion of confounding variables was based on the association between iron deficiency and sex, albumin, and t-stage as documented in CRC patients [Citation1]. To account for some of the known associations with iron deficiency not available to us in this study we included age, ASA-classification, and tumor M-stage as confounding variables also. Anemia was not considered a confounding variable as it is the direct result of iron deficiency. Patients without cancer were recorded as T- and M-stage 0 in the multivariate analysis. Subgroup analysis was performed disregarding patients without cancer or patients who had received iron treatment or blood transfusion before the study visit was conducted. If data were missing on ferritin or TSAT-levels or if the FACT-An questionnaire was not handed in, the patient was excluded from analysis.
A subgroup analysis on iron deficient patients with a ferritin level <30 ug/l was carried out. The demographics and the average outcome scores are presented for this subgroup in the results section. Also, a more detailed examination of patients with confirmed colorectal cancer has been included as Supplementary Appendix A replicating all of the included analyses but on this sub-cohort only.
Ethics and trial registration
Data handling was approved by the Region of Southern Denmark (Journal ID: 21/6250). Ethical board approval was waivered due to the non-interventional design. The study was conducted in accordance with the Declaration of Helsinki, and patients were included only after informed consent was obtained.
Before enrolment of the first patient, the study was registered at Clinical Trials.Gov (ID NCT04749589).
Results
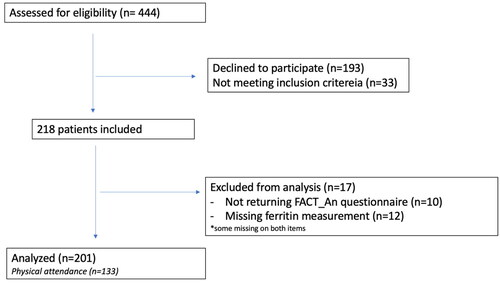
A total of 218 patients were recruited across the three centers (). After the exclusion of those who did not hand-in the FACT-An questionnaire or who were not evaluated for iron deficiency 201 patients were included in the analysis. Of these 133 patients completed the study visit in addition to answering the questionnaires.
Fifty-seven percent (n = 115/201) of the patients were iron deficient. Demographical and clinical details of patients included for analysis are shown in . The group of iron deficient patients was composed of 39 patients with ferritin <30 ug/l and of 76 with ferritin ≥30 ug/l and TSAT <0.2. With respect to the variables included in , there were statistically significant differences between these subgroups regarding only the median Hb-value (10.47 vs. 12.73 g/dl, p < .001), ferritin-value (16 vs. 120 ug/l, p < .001), and TSAT-levels (0.06 vs. 0.13, p = .002).
Table 1. Baseline patient characteristics—comparing patients with and without iron deficiency during evaluation for colorectal malignancy.
When comparing the iron deficient group with the non-iron deficient group the average scores were lower on all outcome measures except for the timed-up-and-go test, where it was higher (). Missing values were present in 14 of the 201 FACT-An questionnaire, with a median number of one item missing among those. In the subgroup of patients with iron deficiency based on ferritin <30 ug/l (n = 39) the outcome scores were similar to those of the total group of iron deficient patients with a FACT-An total score of 63 (47–71), FACT-An fatigue subscale score of 41 (30–47), EORTC-30 Global health status/QoL score of 75 (50–83) and an EORTC-30 Summary score of 88.2 (72.6–93.5). The remaining secondary outcomes scores in this group (ferritin <30 ug/l) were as follows: Six MWT median distance: 450 m (420.5–520.5, n = 20), timed-up-and-go median score: 9 (7–10, n = 22), Hand-grip mean strength: 30.5 (8.6, n = 22), MOCA mean score: 24 (2.2, n = 22).
Table 2. Average outcome scores on fatigue, QoL, cognition, hand-grip-strength, mobility, and walking endurance in patients under evaluation for colorectal cancer, comparing patients with and without iron deficiency.
These differences in outcome measures comparing the two groups were statistically significant in univariate analysis only. In the multivariate model, the precision estimates decreased considerably and none of the outcome measures were associated with iron deficiency with statistical significance (). Repeated multivariate analysis after exclusion of patients without final histopathological diagnosis of cancer, revealed similar results, also considering the association between iron deficiency and hand-grip-strength (correlation coefficient: −4 kg, p = 0.04, 25%CI −7.86 to −0.11) now reaching statistical significance (Supplementary Appendix A).
Table 3. Regression analysis on the association between iron deficiency and fatigue, QoL, cognition, hand-grip-strength, mobility, and walking endurance in patients under evaluation of colorectal cancer.
Before inclusion and baseline laboratory testing, 16 patients had received iron medication (14 in the iron deficiency group) and seven patients had received blood transfusions (all in the iron deficiency group). Performing a subgroup analysis disregarding those patients did not alter the direction, magnitude, or significance of the results.
Discussion
With this prospective study on iron deficiency, we showed a very modest, if any, impact on fatigue, QoL, cognition, and mobility. At the same time, our results suggest that aerobic endurance and peripheral muscle strength might be more substantially affected by iron deficiency, possibly to a degree that is clinically relevant.
While iron deficiency is a common comorbidity in CRC patients, the majority of existing research has focused on the implications of anemia with limited attention given to the potential consequences of iron deficiency alone. We focused exclusively on iron deficiency and took a comprehensive approach to assess the multifaceted influence of iron deficiency in CRC patients, incorporating a range of outcome variables.
Modest but statistically significant associations with iron deficiency were found in univariate analysis, suggesting that addressing iron deficiency may be essential for optimizing patient well-being and functional outcomes before surgery. These findings align with prior research on the effects of iron deficiency in various clinical contexts [Citation11–13,Citation15–19]. However, the transition to non-significance in multivariate analyses warrants a more detailed discussion. CRC is a complex disease and the overall health and functional status of CRC patients is likely shaped by a myriad of patient-specific and tumor-related factors, that may overshadow the influence of iron deficiency. The adjusted regression coefficients on FACT-An, EORTC-30, MoCA, and TUG-test suggest the impact, if any, to be minor while the impact on the physical endpoints seems closer to clinical relevance. With a difference of 40 m in the 6MWT and of a little <4 kg in the hand grip, both point estimates are close to their minimal clinically important differences [Citation38,Citation39]. Their confidence intervals are close to zero on one end, and for that reason, it seems fair to conclude that the magnitude of impact lies somewhere between no clinical relevance and a possible functional limitation associated with iron deficiency. As a stratified analysis, we included patients with iron deficiency based on ferritin <30 ug/l only. This introduced a rather big decline in the median Hb level compared to the combined group of iron deficient patients. Despite this, the average outcome scores for the primary and secondary outcomes did not substantially change. Thus, patients identified with iron deficiency based on ferritin alone were of lower hb-levels but of similar outcome scores as those with iron deficiency based on either low ferritin or low TSAT.
Previous randomized clinical trials together with observational studies from our own research team, have struggled to document the clinical benefits of preoperative i.v. iron treatment in CRC patients [Citation7–11,Citation40,Citation41]. With this study, we sought to clarify the negative impact of iron deficiency on more patient centered outcomes. Such results would have implicated that preoperative i.v. iron treatment while possibly not reducing the risk of surgical complications or colorectal recurrence, could still benefit patients in other domains of patient well-being. However, the results on the primary outcome fatigue, suggest that iron deficiency does not have a clinically relevant impact at this time in the patient journey. The possible impact of iron deficiency on muscle strength and aerobe capacity uncovered with this study could suggest that iron deficiency carries with it a risk of prolonged hospital stay or of later return to daily activities. This could be studied in longitudinal studies or in randomized trials on perioperative i.v. iron treatment, instead of a continued focus on Hb-level increase, surgical complications, and transfusion rates. Iron deficiency at the time of discharge instead of at the time of diagnosis would also be interesting to evaluate to see if those without iron deficiency before surgery develop iron deficiency during their treatment and it this affects the outcome measures suggested above.
Our study was prospective, well-powered, and conducted with rigorous blinding procedures to minimize bias. A limitation to mention is the cross-sectional design that hinders us from seeing if iron deficiency influences with greater weight further down the road of the patient’s journey. Keeler et al. showed a higher QoL score three months following surgery when comparing patients receiving i.v. iron to CRC patients receiving oral iron treatment [Citation16]. At the point in time when our study was conducted, patients suffered the mental stress of not knowing the extent of their disease or the treatment options available. It is possible that once such uncertainties have lifted, the impact of iron deficiency will be greater. It is also important to note that considering the cross-sectional design the study only measures between-group differences and not the longitudinal comparison in the same individual which is likely of higher clinical relevance. It is also important to note that power calculation was made with respect to the FACT-An questionnaire, for univariate analysis and based on paired longitudinal data. There is a risk that our study might be under-powered to document the impact of iron deficiency with statistical significance in multivariable analysis and also if the actual difference is smaller than what was expected when doing the sample size calculations. Then it should also be considered that the observed difference was rather small and not necessarily clinically relevant. It should also be acknowledged that different definitions of iron deficiency exist, both in the general population and in CRC patients specifically. As ferritin is raised due to inflammation restricting the iron definition to a ferritin threshold carries the risk of diverting truly iron deficient patients to the non-iron deficient group due to inflammation. It would have been a strength if CRP levels were measured to aid in this distinction. As this was unfortunately not included, the definition of iron deficiency included TSAT in cases where ferritin was above 30 ng/l. This follows guidance on how to diagnose iron deficiency in CRC patients in the absence of CRP but carries the risk that non-iron deficient patients have been included in the iron deficient group. To explore if this was likely to affect the results, a subgroup analysis was included.
In patients under evaluation for colorectal cancer, our results suggest that iron deficiency may not have clinically important consequences in the domains of fatigue, Quality of Life, cognition, and mobility. Yet it might negatively impact on aerobic endurance and peripheral muscle strength, and it would be interesting to test those domains in longitudinal studies.
Author contributions
MP: conceptualization, methodology, formal analysis, writing-original draft, funding acquisition. BJ: conceptualization, writing-review and editing. JJ: methodology, formal analysis, review and editing. NQ, TK, and RQ: conceptualization, methodology, writing-review and editing, supervision.
Supplemental Material
Download MS Word (108.8 KB)Acknowledgements
The authors extend their sincere thanks to the Department of Surgical Gastroenterology at Grinsted Hospital, Esbjerg Hospital, and at Sygehus Sønderjylland for participating in the study. We are particularly grateful to the endoscopy teams for their assistance with patient identification and enrollment, as well as to the research assistants whose dedication was essential in the execution of the study visits.
Disclosure statement
The authors MP, RK, and TK have all received personal fees and travel expenses from Pharmacosmos A/S outside the submitted work. The authors have no further potential conflict of interest.
Additional information
Funding
References
- Ploug M, Kroijer R, Qvist N, et al. Iron deficiency in colorectal cancer patients: a cohort study on prevalence and associations. Colorectal Dis. 2021;23(4):853–859. doi:10.1111/codi.15467.
- Leichtle SW, Mouawad NJ, Lampman R, et al. Does preoperative anemia adversely affect colon and rectal surgery outcomes? J Am Coll Surg. 2011;212(2):187–194. doi:10.1016/j.jamcollsurg.2010.09.013.
- Wilson MJ, Dekker JWT, Harlaar JJ, et al. The role of preoperative iron deficiency in colorectal cancer patients: prevalence and treatment. Int J Colorectal Dis. 2017;32(11):1617–1624. doi:10.1007/s00384-017-2898-1.
- Kim J, Konyalian V, Huynh R, et al. Identification of predictive factors for perioperative blood transfusion in colorectal resection patients. Int J Colorectal Dis. 2007;22(12):1493–1497. doi:10.1007/s00384-007-0347-2.
- Wilson MJ, van Haaren M, Harlaar JJ, et al. Long-term prognostic value of preoperative anemia in patients with colorectal cancer: a systematic review and meta-analysis. Surg Oncol. 2017;26(1):96–104. doi:10.1016/j.suronc.2017.01.005.
- Lederhuber H, Massey LH, Abeysiri S, et al. Preoperative intravenous iron and the risk of blood transfusion in colorectal cancer surgery: meta-analysis of randomized clinical trials. Br J Surg. 2023;111:1–11.
- Dickson EA, Keeler BD, Ng O, et al. Preoperative intravenous iron therapy and survival after colorectal cancer surgery: long-term results from the IVICA randomised controlled trial. Colorectal Dis. 2020;22(12):2018–2027. doi:10.1111/codi.15342.
- Ploug M, Qvist N, Kroijer R, et al. Preoperative intravenous iron treatment – a cohort study on colorectal cancer recurrence. Surg Open Sci. 2023;16:22–27. doi:10.1016/j.sopen.2023.09.003.
- Ploug M, Kroijer R, Qvist N, et al. Preoperative intravenous iron treatment in colorectal cancer: experience from clinical practice. J Surg Res. 2022;277:37–43. doi:10.1016/j.jss.2022.03.004.
- Keeler BD, Simpson JA, Ng O, et al. Randomized clinical trial of preoperative oral versus intravenous iron in anaemic patients with colorectal cancer. Br J Surg. 2017;104(3):214–221. doi:10.1002/bjs.10328.
- Richards T, Baikady RR, Clevenger B, et al. Preoperative intravenous iron to treat anaemia before major abdominal surgery (PREVENTT): a randomised, double-blind, controlled trial. Lancet. 2020;396(10259):1353–1361. doi:10.1016/S0140-6736(20)31539-7.
- Beard JL. Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr. 2001;131(2s-2):568S–579S; discussion 580S. doi:10.1093/jn/131.2.568S.
- Finch CA, Miller LR, Inamdar AR, et al. Iron deficiency in the rat. Physiological and biochemical studies of muscle dysfunction. J Clin Invest. 1976;58(2):447–453. doi:10.1172/JCI108489.
- Hoes MF, Grote Beverborg N, Kijlstra JD, et al. Iron deficiency impairs contractility of human cardiomyocytes through decreased mitochondrial function. Eur J Heart Fail. 2018;20(5):910–919. doi:10.1002/ejhf.1154.
- Plumb JOM, Otto JM, Kumar SB, et al. Cardiopulmonary exercise testing before and after intravenous iron in preoperative patients: a prospective clinical study. Perioper Med. 2023;12(1):31. doi:10.1186/s13741-023-00319-x.
- Keeler BD, Dickson EA, Simpson JA, et al. The impact of pre-operative intravenous iron on quality of life after colorectal cancer surgery: outcomes from the intravenous iron in colorectal cancer-associated anaemia (IVICA) trial. Anaesthesia. 2019;74(6):714–725. doi:10.1111/anae.14659.
- Kelkitli E, Yazıcıoğlu DA. Assessment of cognitive functions in iron deficiency anemia with Montreal Cognitive Assessment. Int J Sci Res. 2013;2(9):348–349.
- Qin T, Yan M, Fu Z, et al. Association between anemia and cognitive decline among Chinese middle-aged and elderly: evidence from the China health and retirement longitudinal study. BMC Geriatr. 2019;19(1):305.
- Clénin GE. The treatment of iron deficiency without anaemia (in otherwise healthy persons). Swiss Med Wkly. 2017;147:w14434. doi:10.4414/smw.2017.14434.
- Yokoi K, Konomi A. Iron deficiency without anaemia is a potential cause of fatigue: meta-analyses of randomised controlled trials and cross-sectional studies. Br J Nutr. 2017;117(10):1422–1431. doi:10.1017/S0007114517001349.
- Enjuanes C, Klip IT, Bruguera J, et al. Iron deficiency and health-related quality of life in chronic heart failure: results from a multicenter European study. Int J Cardiol. 2014;174(2):268–275. doi:10.1016/j.ijcard.2014.03.169.
- Haas JD, Brownlie T. Iron deficiency and reduced work capacity: a critical review of the research to determine a causal relationship. J Nutr. 2001;131(2s-2):688S-690S. doi:10.1093/jn/131.2.676S.
- Wyart E, Hsu MY, Sartori R, et al. Iron supplementation is sufficient to rescue skeletal muscle mass and function in cancer cachexia. EMBO Rep. 2022;23(4):e53746. doi:10.15252/embr.202153746.
- Sánchez-Torralvo FJ, González-Poveda I, García-Olivares M, et al. Poor physical performance is associated with postoperative complications and mortality in preoperative patients with colorectal cancer. Nutrients. 2022;14(7):1484. doi:10.3390/nu14071484.
- Vandenbroucke JP, von Elm E, Altman DG, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Int J Surg. 2014;12(12):1500–1524. doi:10.1016/j.ijsu.2014.07.014.
- Cella D. The Functional Assessment of Cancer Therapy-Anemia (FACT-An) Scale: a new tool for the assessment of outcomes in cancer anemia and fatigue. Semin Hematol. 1997;34(3 Suppl 2):13–19.
- Cella D, Yount S, Sorensen M, et al. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol. 2005;32(5):811–819.
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi:10.1093/jnci/85.5.365.
- EORTC_Quality_of_Life_Group. Scoring of the QLQ-C30 summary score. Available from: https://qol.eortc.org/app/uploads/sites/2/2018/02/scoring_of_the_qlq-c30_summary_score.pdf
- Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x.
- Procedure for Measuring hand grip strength using the Jamar dynamometer [Internet]; 2014. National Institute of Health Research. [cited 2023 Aug 10]. Available from: https://www.uhs.nhs.uk/Media/Southampton-Clinical-Research/Procedures/BRCProcedures/Procedure-for-measuring-gripstrength-using-the-JAMAR-dynamometer.pdf
- Podsiadlo D, Richardson S. The timed “up & go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39(2):142–148. doi:10.1111/j.1532-5415.1991.tb01616.x.
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi:10.1164/ajrccm.166.1.at1102.
- Quinn EM, Meland E, McGinn S, et al. Correction of iron-deficiency anaemia in colorectal surgery reduces perioperative transfusion rates: a before and after study. Int J Surg. 2017;38:1–8. doi:10.1016/j.ijsu.2016.12.029.
- Camaschella C. New insights into iron deficiency and iron deficiency anemia. Blood Rev. 2017;31(4):225–233. doi:10.1016/j.blre.2017.02.004.
- Nordin Å, Taft C, Lundgren-Nilsson Å, et al. Minimal important differences for fatigue patient reported outcome measures-a systematic review. BMC Med Res Methodol. 2016;16:62.
- Patrick DL, Gagnon DD, Zagari MJ, et al. Assessing the clinical significance of health-related quality of life (HrQOL) improvements in anaemic cancer patients receiving epoetin alfa. Eur J Cancer. 2003;39(3):335–345. doi:10.1016/s0959-8049(02)00628-7.
- Wise RA, Brown CD. Minimal clinically important differences in the six-minute walk test and the incremental shuttle walking test. COPD. 2005;2(1):125–129. doi:10.1081/copd-200050527.
- Bohannon RW. Minimal clinically important difference for grip strength: a systematic review. J Phys Ther Sci. 2019;31(1):75–78. doi:10.1589/jpts.31.75.
- Froessler B, Palm P, Weber I, et al. The important role for intravenous iron in perioperative patient blood management in major abdominal surgery: a randomized controlled trial. Ann Surg. 2016;264(1):41–46. doi:10.1097/SLA.0000000000001646.
- Ploug M, Knudsen T, Qvist N, et al. Decrease in hemoglobin following colorectal surgery – a cohort study with focus on iron deficiency. Perioper Care Oper Room Manag. 2023;34:100363. doi:10.1016/j.pcorm.2023.100363.