Abstract
Background
The incidence of celiac disease (CD) has increased rapidly in the late 20th and early 21st centuries, but there are recent reports of rates levelling off in countries with a high prevalence. The aim of this study was to investigate current trends in CD in southern Sweden.
Patients and methods
Children and adults diagnosed with CD by biopsy or serology in the region of Skåne, southern Sweden, from 2010–2022 were included. The home address was identified through registers to analyze temporal and geographical trends.
Results
A total of 3218 CD-patients were identified (52.2% children), the vast majority detected in clinical care but a few children by screening studies. The age-standardized incidence rate was 18.6 cases/105. The incidence decreased at a rate of −0.75 cases/105 (95% CI −1.14 to −0.35, p 0.002). The incidence among girls under 18 years almost halved throughout the study period, decreasing by −2.94 cases/105 (95% CI −4.59 to −1.29, p 0.002), while there only were small changes among men. The most common age of onset was 3–9 years. CD incidence varied by place of living and was more common in small towns than urban or rural areas.
Conclusions
The incidence of CD in southern Sweden is decreasing, primarily in children and women who traditionally have had the highest risk of CD. CD was diagnosed most frequently in children 3–9 years old. There were regional variations in incidence. CD was most common in small towns, pointing to the importance of environmental factors in CD etiology.
Introduction
Celiac disease (CD) is a chronic autoimmune disorder characterized by small intestinal villous atrophy which is triggered by the consumption of gluten in genetically susceptible individuals [Citation1]. About 1% of the population worldwide has been estimated to suffer from the disease, although it is more common in Scandinavia [Citation2]. The incidence has increased substantially throughout the world during the late twentieth century and early twenty first century [Citation3], which is not only due to improved diagnostics [Citation4, Citation5]. CD has traditionally been considered a pediatric disease since the most common age of onset is children younger than five years. However, many patients are adults at diagnosis and a second peak of onset has been observed in people 50–69 years old [Citation6, Citation7].
In the mid-1980s the CD incidence rose to very high levels among the youngest children in Sweden. The ‘Swedish CD epidemic’ lasted for a decade before the incidence suddenly dropped [Citation8]. A later screening study in teenagers born during this period showed a CD prevalence as high as 3% in this group [Citation9]. The sudden changes in incidence indicate the importance of environmental factors in the CD pathogenesis. A later study has shown that a high gluten consumption in infants increase the risk of CD [Citation10]. With time, the incidence of CD in Sweden began to rise again in all age groups, though it seems to have levelled off in the late 2000s [Citation6]. Interestingly, the same pattern can be seen in Finland. The increase in incidence has levelled off among children [Citation11], and there even seems to be a slight decline among adults [Citation12].
The CD incidence varies greatly between different nations and over time [Citation2, Citation3, Citation8]. Less is known about possible variations in smaller geographical areas. There are a few indications that there are differences also on regional levels. Two Swedish studies in children diagnosed 1998 to 2003 revealed differences in incidence both on a regional level and between different parts of cities [Citation13, Citation14]. Another study found examples of CD case clustering in southern England [Citation15]. The CD prevalence has been found to be much higher in Finland than in the adjacent Russian Karelia region, pointing to the importance of environmental factors [Citation16].
The aim of the present study is to give an update on CD incidence, both among children and adults, from 2010 to 2022 in southern Sweden and to investigate if there are any temporal or geographical variations in the region.
Patients and methods
A retrospective register-based study of both children and adults was designed to investigate temporal and regional trends in CD epidemiology in southern Sweden. All CD cases from 2010 to 2022 among Swedish citizens in Region Skåne, a county in southernmost Sweden, were identified. There were 1.24 million residents in the region in 2010 and 1.41 million in 2022 [Citation17]. All residents in Sweden have their own unique personal identity number (PIN), given at birth or after immigration to the country. The number corresponds to date of birth and gender and can be used to identify individuals within the healthcare registries. Healthcare in Sweden is tax-funded and hence assumed to provide equal access to all citizens.
Biopsy-verified CD cases were retrieved from the registries at the pathology departments in Region Skåne. To identify CD cases, Systematized Nomenclature of Medicine (SNOMED) clinical terms D6218 (celiac disease), M58005 (partial villous atrophy) and M58006 (subtotal/total villous atrophy) were used. M58007 (total villous atrophy) is not used in Region Skåne. The SNOMED topography codes in the database search were T64 (duodenum) and T65 (jejunum/ileum). Due to variations in time interval between the arrival and examination of the specimen, the biopsy’s arrival date to the pathology department (usually the day after the gastroscopy) was used as the date of CD diagnosis. To exclude control biopsies during follow-up the date of the first biopsy indicating CD was used as the date of diagnosis. Patients with a biopsy indicating CD before the start of the study period were excluded. The use of villous atrophy in biopsy reports from Swedish pathology departments to identify CD cases has been validated previously, with a specificity of 95% for a clinical CD diagnosis [Citation18].
In accordance with the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) criteria from 2012 and the 2020 Swedish national guidelines for CD diagnosis in adults, a CD diagnosis does not require a biopsy in all cases [Citation19, Citation20]. A serology-based diagnosis is possible if the patient presents with at least two separate IgA or IgG tissue transglutaminase antibody (tTG-ab) tests more than 10 times the upper limit of normal. In cases with both a biopsy and serology indicating CD, the first positive test was used as the date of onset. tTG-ab titers were analyzed at the Department of Clinical immunology at Skåne University Hospital according to established procedures. Levels of tTG-ab >10 kilo-arbitrary units per liter (kU/L) were considered positive. At a few hospitals a very high tTG-ab test was validated with analysis of endomysial IgA antibodies (EMA-ab) in young children. The combination of a tTG-ab and an EMA-ab at two separate occasions, both more than 10 times the upper limit of normal, was also considered sufficient for a CD diagnosis in this study.
In the region there are some studies where genetically susceptible children are screened for CD, including The Environmental Determinants of Diabetes in the Young (TEDDY) study. Children in such studies who have received a serology-based diagnosis will not be covered by the method described above since their blood tests have not been analyzed by public labs. Therefore, data was also collected from the National Swedish Childhood Celiac Disease Register. All cases of CD in children below the age of 15 years ought to be reported to the register and the diagnosis will be based on the ESPGHAN criteria. The use of this register has been validated previously and used extensively in studies [Citation8, Citation13, Citation14, Citation21, Citation22]. However, since the register also includes children with possible but not verified CD, the patient charts of these children were scrutinized manually to verify that they met the inclusion criteria. The use of this register also made it possible to include children diagnosed by the gluten provocation criteria.
With the help of every individual’s PIN, the exact home address at the time of diagnosis could be identified through registers at Region Skåne. Statistics Sweden provides information on what type of neighborhood someone lives in. All municipalities are categorized into a total of 789 demographical statistical areas (DeSO) comprising of a bit more than 1000 inhabitants in each. The areas are classified as A (rural), B (mixed–villages and small towns) and C (urban). Twelve percent of the population lives in rural areas, 14% in mixed areas and 74% in urban areas [Citation17].
Incidence calculations were performed with population data from Statistics Sweden [Citation17]. Yearly population data is based on the population in the region or municipality as of November 1. Crude incidence rates were calculated and then age-standardized to the 2022 Swedish Standard Population with the help of the direct method as described by Boyle and Parkin [Citation23]. A Poisson distribution was assumed when calculating confidence intervals for crude incidence rates. The lifetime risk of CD was estimated from the yearly incidence rates by calculating cumulative risk up to 85 years of age. Trends over time were analyzed using linear regression as recommended by Boyle and Parkin [Citation23]. Groupwise comparisons for baseline characteristics were done with Chi2 for binary data. Statistical analyses were performed with Stata Statistical Software 18.0 for Windows (StataCorp, College Station, Texas). A p-value <0.05 was considered significant in all analyses.
The study was approved by the Swedish Ethical Review Authority (protocol number 2020-06631). Since the present study was strictly register-based informed consent was not required. Waiver of informed consent was approved by the Swedish Ethical Review Authority. All methods used in the study were carried out in accordance with relevant guidelines and regulations.
Results
A total of 3218 patients were diagnosed with CD during the study period 2010–2022. The majority, 1681 patients (52.2%), were children. Almost all cases were found during the initial search of pathology and serology registers. Since some patients could have been found through screening studies or misclassified in registers, information from the National Swedish Childhood Celiac Disease Register was obtained. An additional 58 children (3.5% of all childhood cases) could be identified through this register, and these are included in the total number of CD cases reported in the present study. It was not possible to analyze clinically and screening detected cases separately.
The median age was 16.7 years at the time of diagnosis but ranged from 0.8 to 93.7 years. In total, 2036 patients (63.3%) were women. During the first half of the study (2010–2015) 65.0% of patients were women in comparison to the second half (2016–2022) where 61.5% were women, a statistically significant drop in the proportion of women with newly diagnosed CD (p 0.042). Following the implementation of the ESPGHAN diagnostic criteria in 2012 around 42% of childhood CD diagnoses were serology based and the rest pathology based. In adults on the other hand, only 6% of patients were diagnosed through serology after the implementation of the new national guidelines in 2020 ().
Table 1. Baseline characteristics of the celiac disease cases from 2010 to 2022.
The crude incidence rate for the entire study period was 18.7 cases of CD per 100 000 person-years. The age-standardized incidence rate (ASR) was 18.6 cases per 100 000 person-years (95% CI 17.9–19.2). The ASR in women was 23.7 cases per 100 000 person-years (95% CI 22.7–24.8). It was lower in men, who had an ASR of 13.7 cases per 100 000 person-years (95% CI 12.9–14.5). This resulted in an age-standardized incidence rate ratio (IRR) of 1.73 (95% CI 1.62–1.86).
The cumulative risk, or lifetime risk, for CD in this study was 1.59% (95% CI 1.53–1.64%). In women the cumulative risk was 2.01% (95% CI 1.92–2.10%), while it was as low as 1.18% in men (95% CI 1.11–1.25%).
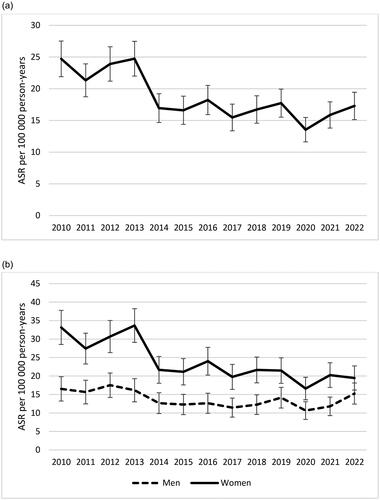
There were significant changes in the CD incidence over time. In 2010 the total ASR in people of all ages was 24.7 cases per 100 000 person-years (95% CI 21.9–27.5) and in 2022 the ASR was as low as 17.3 (95% CI 15.1–19.4). The CD incidence was high until 2013 and then began to decrease. The annual decrease over the study period was −0.75 cases per 100 000 person-years (95% CI −1.14 to −0.35, p 0.002; ). The observed trend could primarily be explained by a decrease in CD incidence among women, in whom an annual decrease of −1.17 cases per 100 000 person-years (95% CI −1.70 to −0.63, p 0.001) was found. There was also a significant, but rather small, decrease among men of −0.34 cases per 100 000 person-years (95% CI −0.64 to −0.04, p 0.031, ). Of note, the age-standardized IRR in 2010 was 2.01 (95% CI 1.58–2.54) but only 1.28 in 2022 (95% CI 0.99–1.64).
Figure 1. Age-standardized incidence rates (ASR) of celiac disease in the region of Skåne, Sweden from 2010 to 2022. The total ASR (a) and the ASR for men and women separately (b) are presented. Bars indicate 95% confidence intervals. N = 3218.
Despite the decrease in CD cases in the middle of the 2010s, the total number of analyzed tTG-ab tests increased in the same period. It then varied during the late 2010s and early 2020s but was not lower than in the early 2010s (). When the incidence of CD decreased in the mid-2010s, so did the proportion of positive tTG-ab tests.
Table 2. Positive and total number of tissue transglutaminase tests from 2010 to 2022.
Next, trends over time were analyzed after stratification into groups of children and adults. The total incidence in children during the study period was 46.9 cases per 100 000 person-years (95% CI 44.7–49.2). CD was more common among girls than boys under the age of 18 years. In girls the incidence was 61.1 cases per 100 000 person-years (95% CI 57.5–64,9), and in boys 33.5 (95% CI 30.9–36.3). The incidence among girls almost halved throughout the study period, decreasing by −2.94 cases per 100 000 person-years (95% CI −4.59 to −1.29, p 0.002). However, it remained rather constant over time in boys, decreasing insignificantly by −0.38 cases per 100 000 person-years (95% CI −1.24–0.48, p 0.35, ). To further illustrate the difference between the sexes, CD was much more common in girls in 2010 with an IRR of 2.19 (95% CI 1.56–3.13) than in 2022 when the IRR was only 1.21 (95% CI 0.85–1.73).
Figure 2. Yearly incidence rates of celiac disease in the region of Skåne, Sweden stratified by children (a) and adults (b). Bars indicate 95% confidence intervals. N = 1681 children, 1537 adults.
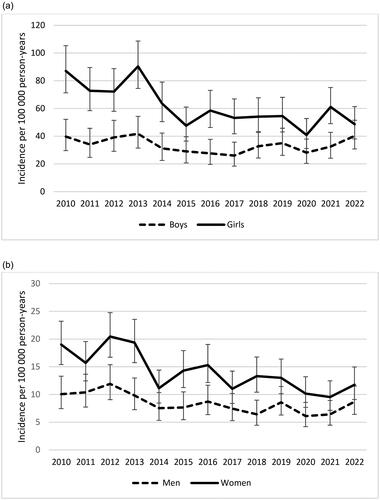
CD was less common in adults. The total incidence in adult women was 14.1 cases per 100 000 person-years (95% CI 13.2–15.0) and in men 8.4 cases per 100 000 person-years (95% CI 7.7–9.1). The incidence decreased over time in women at a rate of −0.73 cases per 100 000 person-years (95% CI −1.12 to −0.35, p 0.001). A similar, but less pronounced, trend could be observed in men where the incidence decreased with −0.32 cases per 100 000 person-years (95% CI −0.53 to −0.10, p 0.007, ). As in children, the gender difference also got less pronounced over time in adults. In 2010 the IRR was 1.89 (95% CI 1.33–2.72) and in 2022 it was 1.35 (95% CI 0.92–2.00).
The highest incidence of CD could be observed in the youngest age groups. After that, the incidence remained low in younger adults and the middle-aged. There was a second but rather small peak among, primarily male, 60–79-year-olds. CD was more common in women than men aged under 50 years. After that there was little to no gender difference (). In children CD was most frequently diagnosed in those aged 3–4 and 5–9 years. Interestingly, the incidence was lower in the youngest children aged 1–2 years, both in girls and boys ().
Figure 3. Age-specific incidence of celiac disease in the region of Skåne, Sweden from 2010 to 2022. Bars indicate 95% confidence intervals. N = 3218.
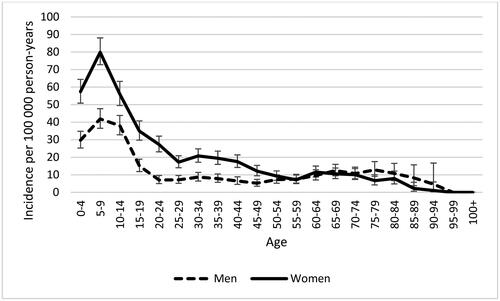
Table 3. Age-specific celiac disease incidence in people diagnosed from 2010 to 2022.
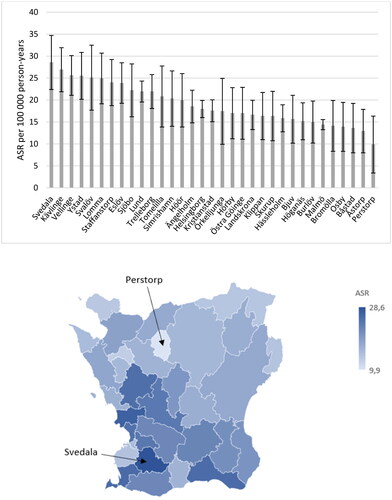
The ASRs for each of the 33 municipalities in the region were determined. A registered home address at the time of diagnosis was available for 3189 out of 3218 CD patients. There were clear regional differences. CD was most common in the municipality of Svedala, located in the southwest, where the ASR was 28.6 cases per 100 000 person-years (95% CI 22.4–34.7). The municipality with the lowest incidence was Perstorp, located in the northwest, with an ASR of 9.9 cases per 100 000 person-years (95% CI 3.4–16.3). In whole, CD was more common in the southwest of the region, while it was less prevalent in the north ().
Figure 4. Age-standardized incidence rates (ASR) of celiac disease from 2010 to 2022 in each municipality in the region of Skåne, Sweden. Bars indicate 95% confidence intervals. N = 3189.
To further analyze the regional differences, ASRs were calculated for different demographic areas, that is whether the CD patients lived in rural, mixed or urban areas at the time of diagnosis. CD was most common in the mixed neighborhoods, that is in small towns, in which the ASR was 22.2 cases per 100 000 person-years (95% CI 20.4–24.1). CD was significantly more common in the mixed neighborhoods than in rural or urban areas, while no difference in the incidence was found between rural and urban areas ().
Table 4. Age-standardized incidence rates of celiac disease in different demographic areas.
Discussion
The main finding of the study is the decreasing incidence of CD in southern Sweden from 2010 to 2022. The largest risk-reduction was observed in those traditionally considered to be at the highest risk of CD, namely women and young children. The results are in stark contrast to the reports of a continuously increasing incidence of CD during the late twentieth century and the first decade of the twenty first century [Citation3].
The CD incidence has increased greatly worldwide the last decades at a pace of approximately 7.5% per year [Citation3]. That trend has been observed in both the United States [Citation5, Citation24] and different countries in Europe [Citation25, Citation26]. Pediatric CD has become more and more prevalent during the late 20th and early 21st centuries in both southern Europe, the United Kingdom and Scandinavia [Citation27]. In a previous study of CD in Sweden from 1990 to 2015, the incidence gradually increased until around 2005, whereafter it remained at a constant but high rate. During the very last years of the study there was a tendency to decreasing rates, but the study included only biopsy-verified cases and did not account for the new diagnostic criteria established by the ESPGHAN in 2012 [Citation6]. A similar trend has been observed in Finnish children, in which CD incidence rose between 2001 and 2007 and then levelled off for the next six years [Citation11]. In Finnish adults the incidence actually decreased between 2005 to 2014, although it was a relatively small change of four cases per 100 000 person-years over the ten year study period [Citation12]. The present study of southern Sweden provides an update on CD incidence in more recent times and not only for the first decade of the twenty first century. The incidence of CD was high until 2013 and then began to drop. To the best of our knowledge, this is the first study that reports a significant decrease in CD incidence in both children and adults. The countries where the CD incidence has levelled off or decreased all have in common that CD is a prevalent disease there. It is possible that a risk maximum for CD has been reached in these places.
The decrease of incidence we found was most prominent in women who traditionally has had the highest risk of CD. Over the study period, the IRR between women and men decreased, although CD still is more common in women. A similar trend in gender was observed in the early 2010s in the Swedish national study by Bergman et al. [Citation6].
The CD incidence in Sweden has been reported to be among the highest in the world for a long time, a position shared mainly with other Scandinavian countries such as Finland [Citation3, Citation27]. This observation is further strengthened by the results from the TEDDY study where children with risk HLA haplotypes were followed for a median of 11.5 years in six sites in USA, Finland, Germany, and Sweden. Sweden had the highest CD incidence at 10 years reaching 3% [Citation28]. Although CD now seems to become less common in Sweden, the incidence reported in this study is still markedly higher than in the rest of the Western world. In the meta-analysis by King et al. [Citation3], the worldwide incidence of CD in the twenty first century was reported to be 17.4 and 7.8 cases per 100 000 person-years for women and men, respectively. In our study the ASR was 23.7 cases per 100 000 person-years in women and 13.7 in men. The difference is even clearer in children where King et al. reported an incidence of 21.3 cases per 100 000 person-years, while in this study it was as high as 46.9 cases per 100 000 person-years. In a Swedish setting, the incidence reported here in southern Sweden is close to what has been reported nationally earlier. In 1990–2015 the mean ASR in Sweden was 19.0 cases per 100 000 person-years [Citation6]. In this study the ASR was 18.6 cases per 100 000 person-years, indicating that the study setting is representative in a national perspective. However, the proportion of children (52.2%) in this study was somewhat larger than previously reported [Citation29], which could be explained by the concurrent screening studies among children in the region.
The most common age of onset for CD has for long been the first years of life [Citation25, Citation30, Citation31]. In a Swedish study the incidence was twice as high in the age group <2 years of age compared to those 2–4 years [Citation6]. There have been reports that the median age at diagnosis is increasing, but that could be a consequence of higher awareness of the fact that the disease can be found in all ages [Citation11]. In the present study, CD was more common in the age groups 3–4 and 5–9 years than in 1–2-year-olds. The incidence in the youngest children was markedly lower than previously reported [Citation6]. In the TEDDY study that followed children born 2004–10 the median age of diagnosis was 4.75 years, SD 3.4 years, a finding in accordance with the results in the present study [Citation28]. There are a few other studies reporting similar changes. A regional Swedish study found a decreasing incidence in children <2 years of age from the 1980s to the beginning of the 2000s, although some of that can be explained by the ‘Swedish CD epidemic’ in the 1980s and 1990s [Citation31]. A recent Spanish study in the region of Catalonia screened children for CD in the first and second decade of the twenty first century. While CD was more common in 1–2-year-olds than 3–4-year-olds in the first decade, the difference was eliminated in the second decade, something that was primarily explained by a reduced incidence in the youngest children [Citation30]. Their findings are in accordance with our observations.
Rapid changes in incidence must be explained by environmental factors. Arau et al. [Citation30] hypothesized that rotavirus vaccination could protect against CD and explain the changing epidemiology in young children. However, in southern Sweden rotavirus vaccination was not implemented in the vaccination program until 2019 and cannot explain the reduced risk of CD in children observed in our study. Other triggers that have been considered in the etiology of CD are dietary factors. A high consumption of gluten in early life has been found to increase CD risk [Citation10]. It is uncertain if this is true also for adults. When looking into food habits in the general Swedish population there are some noteworthy trends relating to CD. The total consumption of flour per person has been rather constant from the 1980s until today. However, the consumption of premade or processed gluten-containing foods such as bread, confectionery and pasta has increased from 50 kg per person and year in the 1980s to around 80 kg in the first decade of the twenty first century. During the second decade of the twenty first century the consumption was reduced to a bit below 75 kg per person and year [Citation32]. This trend coincides with the observed CD incidence in Sweden, which increased rapidly until the early 2010s and then started to decrease. It raises the question of whether these kinds of processed gluten-containing foods may be triggering CD. A higher total amount of gluten in these foods, the processing of gluten itself, or possibly other additives used when making these foods might trigger CD [Citation33], although these theories are hypothetical and beyond the scope of this study.
The incidence of CD in the region differed both over time and between different municipalities. CD was frequent in the southwest of the region. Examples of regional differences have been reported before in Sweden with variations between larger regions in the country [Citation14] but also a difference in the frequency of CD in children with type 1 diabetes mellitus between the region of Skåne and neighboring areas in Denmark [Citation34]. Case-clustering has also been observed on a much more local level in southern England [Citation15]. In the related disease microscopic colitis, variations in time and place has also been found in the region of Skåne [Citation35]. These differences point to the importance of environmental factors in the etiology of gastrointestinal inflammatory disorders. In this study, CD was more common in small towns and villages, compared to urban or more rural areas. It is possible that this is one factor contributing to the observed regional difference, although the data could not be adjusted for factors such as socio-economic status. Data on the importance of urban or rural settings in CD are scarce. However, the case-clustering reported in southern England was mainly focused to small towns [Citation15]. Another study found that the population density per se did not affect the risk of CD in children, but living in areas with a high level of industrial and commercial activity reduced the risk [Citation13]. Such areas would correspond to urban areas in our study and the results are hence in line with ours.
The study is strengthened by the use of several sources to retrieve data to include all CD cases in the region. The use of pathology reports to identify CD cases has been validated before [Citation18]. After the implementation of the ESPGHAN criteria in 2012, epidemiological studies can no longer be strictly pathology based. Here, both biopsy- and serology-verified cases were included. The additional search of the National Swedish Childhood Celiac Disease Register provided an extra possibility to identify all new cases. The 58 extra cases (3.5% of the total) found were mainly children diagnosed through screening studies such as TEDDY, second opinion analyses of biopsies and some cases of misclassification regarding the SNOMED codes. Although there is no similar register for CD in adults, the low number of extra cases found in the childhood register indicates that the methods used in this study to identify CD cases are appropriate. Additionally, the Swedish healthcare system is organized so that almost all analyses of biopsies and tTG-ab are made at the public hospitals in the region, which gives the possibility to study CD without any relevant risk of missing large numbers of cases. The study is further strengthened by the possibility to find the exact address of living at the time of diagnosis, making it possible to analyze geographical trends.
There are some limitations that need to be addressed. The study analyzes a specific region in Sweden, and it is not clear if the observed decreasing trend is due to local risk factors or a more general change in epidemiology in countries where CD is prevalent. However, similar trends in Finland and partly Spain indicate the latter [Citation11, Citation12, Citation30]. Although the results are age-standardized, and there are no relevant gender differences compared to the rest of Europe, there are factors that could not be accounted for on an individual level. These include for example socio-economic factors, smoking and diet. While the risk of CD seems to be higher in small towns and villages, it was not possible to adjust for such confounding factors. Furthermore, the incidence numbers reported are based on cases found through the healthcare system and there is no data on people with undiagnosed CD. Such information is not possible to gather in an epidemiological study. However, as the incidence of CD started decreasing the mid-2010s, the total number of analyzed tTG-ab tests increased. Hence, it is unlikely that the observed trends would be to reduced healthcare accessibility or some kind of loss of awareness among physicians. In addition, the observed decrease in children primarily started in 2014, which is a few years after the implementation of serology-based diagnostics, indicating that changes in CD guidelines cannot explain the trends. Another problem is the fact that some of the 1681 children with CD were found through screening studies. The TEDDY study screened 2120 children with genetical risk born 2004–10 in the region, out of which 240 had been diagnosed with CD as of 2020 [Citation36]. The exact time of diagnosis and their PIN numbers are not available, so it is not possible to estimate the total impact of the TEDDY study on our results. Considering the median age of onset in the TEDDY study, many cases must have been diagnosed before the start of this study. Consequently, the vast majority of cases in this study are clinically detected and the impact of the TEDDY study will thus be rather low. It is also impossible to know whether some of the screening detected cases would have been found in the healthcare system anyway if they were not followed in the TEDDY study. Indeed, the number of CD cases in our study will be slightly higher than in a region with no screening, but not with more than a bit over 10 cases per year. Most importantly, the trends observed over time ought not to be impacted to any larger extent.
Future studies should investigate whether the observed trends were due to a temporary change in risk factors or if the incidence keeps decreasing over time. To prevent disease onset, it is crucial to understand which risk factors are involved in CD pathogenesis. It would be of value to investigate if the changing dietary habits affect the risk of CD, and which factors that could be involved in the higher risk of CD in small towns and villages.
In conclusion, CD incidence decreased in southern Sweden from 2010 to 2022. The decrease was most prominent in groups traditionally considered at high risk of CD, namely women and young children. CD was diagnosed most frequently in children 3–9 years old instead of in the age span below 3 years. There were regional variations in incidence, and CD was most common in small towns and villages, both factors pointing to the importance of environmental factors in CD etiology.
Acknowledgements
The authors wish to thank Ingegerd Sundqvist at the Department of Pathology and Lotta Wilke at the Department of Immunology, both Skåne University Hospital, Malmö and Lund, Sweden, for help with retrieving data from the registers.
Disclosure statement
The authors report there are no competing interests to declare.
Additional information
Funding
References
- Lebwohl B, Sanders DS, Green PHR. Coeliac disease. Lancet. 2018;391(10115):70–81. doi: 10.1016/S0140-6736(17)31796-8.
- Singh P, Arora A, Strand TA, et al. Global prevalence of celiac disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16(6):823–836.e2. doi: 10.1016/j.cgh.2017.06.037.
- King JA, Jeong J, Underwood FE, et al. Incidence of celiac disease is increasing over time: a systematic review and meta-analysis. Am J Gastroenterol. 2020;115(4):507–525. doi: 10.14309/ajg.0000000000000523.
- Lohi S, Mustalahti K, Kaukinen K, et al. Increasing prevalence of coeliac disease over time. Aliment Pharmacol Ther. 2007;26(9):1217–1225. doi: 10.1111/j.1365-2036.2007.03502.x.
- Rubio-Tapia A, Kyle RA, Kaplan EL, et al. Increased prevalence and mortality in undiagnosed celiac disease. Gastroenterology. 2009;137(1):88–93. doi: 10.1053/j.gastro.2009.03.059.
- Bergman D, King J, Lebwohl B, et al. Two waves of coeliac disease incidence in Sweden: a nationwide population-based cohort study from 1990 to 2015. Gut. 2022;71(6):1088–1094. doi: 10.1136/gutjnl-2021-324209.
- West J, Fleming KM, Tata LJ, et al. Incidence and prevalence of celiac disease and dermatitis herpetiformis in the UK over two decades: population-based study. Am J Gastroenterol. 2014;109(5):757–768. doi: 10.1038/ajg.2014.55.
- Ivarsson A, Persson LA, Nyström L, et al. Epidemic of coeliac disease in Swedish children. Acta Paediatr. 2000;89(2):165–171. doi: 10.1111/j.1651-2227.2000.tb01210.x.
- Myléus A, Ivarsson A, Webb C, et al. Celiac disease revealed in 3% of Swedish 12-year-olds born during an epidemic. J Pediatr Gastroenterol Nutr. 2009;49(2):170–176. doi: 10.1097/MPG.0b013e31818c52cc.
- Andrén Aronsson C, Lee H-S, Koletzko S, et al. Effects of gluten intake on risk of celiac disease: a case-control study on a Swedish birth cohort. Clin Gastroenterol Hepatol. 2016;14(3):403–409.e3. doi: 10.1016/j.cgh.2015.09.030.
- Kivelä L, Kaukinen K, Lähdeaho M-L, et al. Presentation of celiac disease in Finnish children is no longer changing: a 50-year perspective. J Pediatr. 2015;167(5):1109–1115.e1. doi: 10.1016/j.jpeds.2015.07.057.
- Virta LJ, Saarinen MM, Kolho KL. Declining trend in the incidence of biopsy-verified coeliac disease in the adult population of Finland, 2005-2014. Aliment Pharmacol Ther. 2017;46(11–12):1085–1093. doi: 10.1111/apt.14335.
- Namatovu F, Strömgren M, Ivarsson A, et al. Neighborhood conditions and celiac disease risk among children in Sweden. Scand J Public Health. 2014;42(7):572–580. doi: 10.1177/1403494814550173.
- Olsson C, Stenlund H, Hörnell A, et al. Regional variation in celiac disease risk within Sweden revealed by the nationwide prospective incidence register. Acta Paediatr. 2009;98(2):337–342. doi: 10.1111/j.1651-2227.2008.01086.x.
- Fowell AJ, Thomas PW, Surgenor SL, et al. The epidemiology of coeliac disease in East Dorset 1993-2002: an assessment of the ‘coeliac iceberg’, and preliminary evidence of case clustering. QJM. 2006;99(7):453–460. doi: 10.1093/qjmed/hcl061.
- Kondrashova A, Mustalahti K, Kaukinen K, et al. Lower economic status and inferior hygienic environment may protect against celiac disease. Ann Med. 2008;40(3):223–231. doi: 10.1080/07853890701678689.
- Statistics Sweden (SCB). Statistical database [Internet]; 2024 [cited 2024 April 6]. Available from: http://www.statistikdatabasen.scb.se/pxweb/en/ssd/.
- Ludvigsson JF, Brandt L, Montgomery SM, et al. Validation study of villous atrophy and small intestinal inflammation in Swedish biopsy registers. BMC Gastroenterol. 2009;9(1):19. doi: 10.1186/1471-230X-9-19.
- Husby S, Koletzko S, Korponay-Szabó IR, et al. European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54(1):136–160. doi: 10.1097/MPG.0b013e31821a23d0.
- Sandström O, Agardh D, Ekstav L, et al. Nationellt vårdprogram för celiaki. Svensk förening för Pediatrisk Gastroenterologi, Hepatologi och Nutrition, Svenska barnläkarföreningen, Svensk gastroenterologisk förening, Svensk förening för allmänmedicin; 2020. Available from: https://gastro.barnlakarforeningen.se/wp-content/uploads/sites/10/2020/01/SPGHN_Celiaki_v%C3%A5rdprogram_20200114.pdf.
- Olsson C, Hernell O, Hörnell A, et al. Difference in celiac disease risk between Swedish birth cohorts suggests an opportunity for primary prevention. Pediatrics. 2008;122(3):528–534. doi: 10.1542/peds.2007-2989.
- Namatovu F, Lindkvist M, Olsson C, et al. Season and region of birth as risk factors for coeliac disease a key to the aetiology? Arch Dis Child. 2016;101(12):1114–1118. doi: 10.1136/archdischild-2015-310122.
- Boyle P, Parkin DM. Cancer registration: principles and methods. Statistical methods for registries. IARC Sci Publ. 1991;95:126–158.
- Lee RU, Stahlman SL, Magee JS. Celiac disease on the rise in the US military population: a 22 year retrospective epidemiologic study. Dig Dis Sci. 2023;68(7):3115–3118. doi: 10.1007/s10620-023-07964-8.
- Conrad N, Misra S, Verbakel JY, et al. Incidence, prevalence, and co-occurrence of autoimmune disorders over time and by age, sex, and socioeconomic status: a population-based cohort study of 22 million individuals in the UK. Lancet. 2023;401(10391):1878–1890. doi: 10.1016/S0140-6736(23)00457-9.
- Taavela J, Kurppa K, Jääskeläinen T, et al. Trends in the prevalence rates and predictive factors of coeliac disease: a long-term nationwide follow-up study. Aliment Pharmacol Ther. 2024;59(3):372–379. doi: 10.1111/apt.17803.
- Roberts SE, Morrison-Rees S, Thapar N, et al. Systematic review and meta-analysis: the incidence and prevalence of paediatric coeliac disease across Europe. Aliment Pharmacol Ther. 2021;54(2):109–128. doi: 10.1111/apt.16337.
- Stahl M, Li Q, Lynch K, et al. Incidence of pediatric celiac disease varies by region. Am J Gastroenterol. 2023;118(3):539–545. doi: 10.14309/ajg.0000000000002056.
- Lebwohl B, Green PHR, Söderling J, et al. Association between celiac disease and mortality risk in a Swedish population. JAMA. 2020;323(13):1277–1285. doi: 10.1001/jama.2020.1943.
- Arau B, Dietl B, Sudrià-Lopez E, et al. A population-based cross-sectional study of paediatric coeliac disease in catalonia showed a downward trend in prevalence compared to the previous decade. Nutrients. 2023;15(24):5100. doi: 10.3390/nu15245100.
- Tapsas D, Hollén E, Stenhammar L, et al. Unusually high incidence of paediatric coeliac disease in Sweden during the period 1973–2013. PLoS One. 2015;10(12):e0144346. doi: 10.1371/journal.pone.0144346.
- The Swedish Board of Agriculture. Statistical database [Internet]. Sweden; 2024 [cited 2024 May 9]. Available from: https://statistik.sjv.se/PXWeb/pxweb/sv/Jordbruksverkets%20statistikdatabas/.
- Lerner A, Matthias T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun Rev. 2015;14(6):479–489. doi: 10.1016/j.autrev.2015.01.009.
- Adlercreutz EH, Svensson J, Hansen D, et al. Prevalence of celiac disease autoimmunity in children with type 1 diabetes: regional variations across the Øresund strait between Denmark and southernmost Sweden. Pediatr Diabetes. 2015;16(7):504–509. doi: 10.1111/pedi.12200.
- Larsson JK, Clarkson S, Sjoberg K. Regional differences in the incidence of lymphocytic and collagenous colitis over time. Scand J Gastroenterol. 2023;58(12):1445–1452. doi: 10.1080/00365521.2023.2248536.
- TEDDY Study. Årsrapport 2020 [Internet]. Sweden; 2020 [cited 2024 June 23]. Available from: https://www.teddy.lu.se/sites/teddy.lu.se/files/2021-11/TEDDY%20%C3%85rsrapport%202020.pdf.
