Abstract
Objectives
Most patients with pancreatic cancer who have undergone surgical resection eventually develop disease recurrence. This study aimed to investigate whether there is evidence to support routine surveillance after pancreatic cancer surgery, with a secondary aim of analyzing the implementation of surveillance strategies in the Nordic countries.
Materials and Methods
A scoping review was conducted to identify clinical practice guidelines globally and research studies relating to surveillance after pancreatic cancer resection. This was followed by a survey among 20 pancreatic units from four Nordic countries to assess their current practice of follow-up for operated patients.
Results
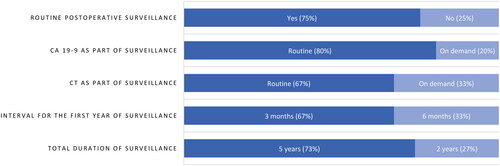
Altogether 16 clinical practice guidelines and 17 research studies were included. The guidelines provided inconsistent recommendations regarding postoperative surveillance of pancreatic cancer. The clinical research data were mainly based on retrospective cohort studies with low level of evidence and lead-time bias was not addressed. Active surveillance was recommended in Sweden and Denmark, but not in Norway beyond the post-operative/adjuvant period. Finland had no national recommendations for surveillance. The Nordic survey revealed a wide variation in reported practice among the different units. About 75% (15 of 20 units) performed routine postoperative surveillance. Routine CA 19-9 testing was used by 80% and routine CT by 67% as part of surveillance. About 73% of centers continued follow-up until 5 years postoperatively.
Conclusion
Evidence for routine long-term (i.e. 5 years) surveillance after pancreatic cancer surgery remains limited. Most pancreatic units in the Nordic countries conduct regular follow-up, but protocols vary.
Introduction
Pancreatic cancer constitutes a substantial global burden in terms of cancer mortality. The incidence is lower than many other cancer types, but pancreatic cancer consistently ranks as one of the leading causes of cancer deaths [Citation1]. This highlights the aggressive nature of the disease and the challenges associated with its early detection and treatment.
Surgical resection remains the cornerstone of curative therapy for pancreatic cancer [Citation2]. Nevertheless, recurrence of the tumor occurs in up to 80% of patients, typically within two years after the operation [Citation3]. The most common site for recurrence is the liver (26.5%), followed by locoregional sites (20.8%), peritoneal dissemination (13.5%) and lungs (11.4%) [Citation4]. There are still no clear treatment strategies for recurrent disease, in terms of which treatment to offer and how this may potentially affect survival. Indeed, there is a risk of lead-time-bias () involved in the analyses of patients treated for recurrence. A variety of treatment options are available for recurrent pancreatic cancer, ranging from surgical re-resection, to chemotherapy, radiotherapy and local ablative therapies, with a selection of patients achieving potential clinical benefit [Citation5–7] and also depending on the site of recurrence (e.g. lungs or liver) [Citation8]. These types of data have sparked interest in early detection of disease recurrence to enable salvage therapy while the tumor burden is still limited [Citation9,Citation10]. Therefore, surveillance programs are increasingly recommended, but protocols vary and the clinical benefits remain uncertain [Citation11]. For example, a recent UK study reported a wide variation in surveillance practice after pancreatic cancer surgery between but also within units [Citation12].
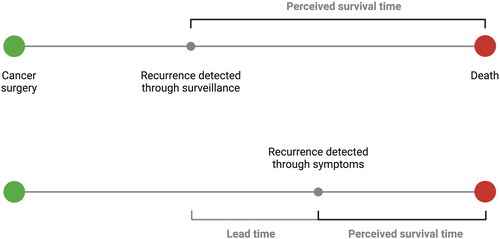
Figure 1. Potential for lead-time bias as a result of systematic surveillance. Recurrence may be detected earlier at an asymptomatic stage in the surveillance group (top part of diagram), leading to a perceived increased survival time attributed to treatment, compared to treatment only initiated after symptoms (bottom panel). Provided the oncological treatment has no or only limited effect, the time of death may remain about the same in both groups, and hence actual survival is similar even if perceived as longer in the surveillance group. Created with BioRender.com.
The aim of the present study was to examine the available literature on the role of routine surveillance after pancreatic cancer surgery and, as a secondary aim, to assess postoperative surveillance strategies in the Nordic countries using a survey among pancreatic surgeons within the Nordic Pancreatic Cancer Network (NPCN).
Methods
Scoping review
This review was reported according to the PRISMA extension for scoping reviews [Citation13]. Clinical practice guidelines in pancreatic cancer surgery were retrieved from society websites. A search of PubMed was done from 2000 to 2023 using the search string “pancreatic cancer AND (surveillance OR follow-up) AND recurrence” to identify relevant clinical research studies. Studies were considered eligible if they reported on survival, cost-effectiveness or quality of life related to postoperative surveillance after pancreatic cancer surgery. Data points extracted and charted from the studies comprised year of publication, country of origin, study design, number of patients and main findings. No additional data were required from the authors of the included papers. Results were compiled as a narrative synthesis.
Survey
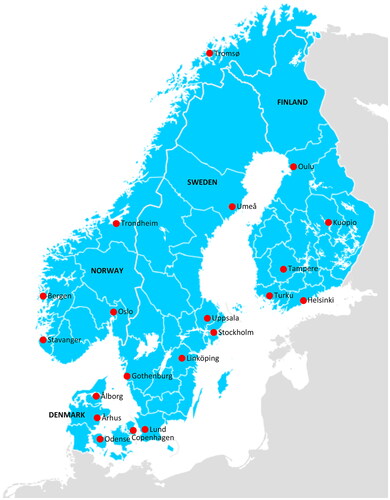
This was a survey of all 20 Nordic tertiary referral centers providing curative-intent surgery for pancreatic cancer. The Nordic countries cover a population of approximately 27.5 million inhabitants, with Sweden having a population of 10.6 million, Denmark 5.9 million, Norway 5.5 million, and Finland 5.5 million (year 2024). The healthcare systems in the Nordic countries are mainly public-based, especially for treatments requiring specialized level of care, such as pancreatic cancer surgery and oncological management. The participating centers are shown in . All surgeon members of the NPCN were sent an online questionnaire distributed via email to evaluate whether routine surveillance is performed after pancreatic cancer surgery and requesting the specific surveillance strategy for each center.
Figure 2. HPB Centers in the nordic countries. All centers (n = 20) participated in the survey. Created with www.nordmap.se.
Results and discussion
The literature search retrieved 4 418 articles. After full-text assessment, 16 clinical practice guidelines () and 17 research studies () were included. describes the PRISMA flow diagram of study inclusion.
Table 1. Guidelines for surveillance after pancreatic cancer surgery.
Table 2. Studies evaluating surveillance after pancreatic cancer surgery.
Guidelines
Current guidelines vary widely in their recommendations regarding surveillance after pancreatic cancer surgery, from no routine follow-up to regular clinical examination, tumor markers and computed tomography (CT)/magnetic resonance imaging (MRI) every 3-6 to months for up to 5 years.
Nordic countries
Swedish guidelines recommend postoperative surveillance with clinical examination every 3 months for 1 year, then individualized follow-up for up to 5 years; CA 19-9, HbA1C and CT after 6 and 12 months on an individual basis [Citation14]. Danish guidelines recommend follow-up with clinical examination and CA 19-9 every 3 months for 1 year, then every 6 months for up to 2 years; CT after 6 and 12 months on an individual basis [Citation15]. Norwegian guidelines recommend clinical examination, CA19-9 and CT before initiation and after completion of adjuvant chemotherapy (i.e. 2-3 months and 7-8 months after surgery), then clinical follow up in the general practitioner (GP) office [Citation16]. Finland currently has no national guidelines for pancreatic cancer surveillance and each university hospital has its own postoperative strategy.
The rest of Europe
The European Society for Medical Oncology (ESMO) recommends surveillance after resection of pancreatic cancer, but no information is provided regarding method, interval and duration of surveillance. The International Association of Pancreatology/European Pancreatic Club (IAP/EPC) has no recommendation for surveillance. Surveillance is recommended by French [Citation20] and Italian [Citation17] guidelines, with clinical examination, CA 19-9, and CT to monitor recurrence with varying surveillance intervals and duration, while Dutch [Citation18], German [Citation21], Spanish [Citation23] and UK guidelines [Citation24] do not recommend routine surveillance.
North America
The National Comprehensive Cancer Network (NCCN) recommends routine clinical examination, CA 19-9 and CT every 3-6 months for 2 years, then every 6-12 months for 2 years, then every 6-12 months as clinically indicated. The American Society of Clinical Oncology (ASCO) also recommends surveillance, but provides no clear role for imaging tests.
Asia
The Chinese guidelines recommend clinical examination, CA 19-9 and CT/MRI to monitor for recurrence up to 5 years after surgery. The frequency and method of surveillance may be adjusted according to the regional differences and the accessibility of healthcare resources. The Japanese guidelines recommend CA 19-9 and CT every 3-6 months for 2 years, then every 6-12 months up to 5 years post-surgery.
Oceania
The Australian guidelines have no recommendation on surveillance.
Diagnostic performance
CA 19-9 is the most widely used serum marker for pancreatic cancer. However, approximately 5-10% of the population have a Lewis-negative phenotype and cannot produce the CA 19-9 tumor antigen. Postoperative CA 19-9 has a sensitivity of 68-89% and a specificity of 77-89% for the detection of pancreatic cancer recurrence () [Citation46–50]. Interestingly, it has been shown that elevation in CA 19-9 levels can precede radiological evidence of recurrence by 3-6 months [Citation38, Citation40]. CEA has a lower diagnostic yield with a sensitivity of 50% and a specificity of 65% [Citation46, Citation48]. CT is the imaging method most frequently used for postoperative surveillance. However, the sensitivity is moderate at 70% with a specificity of 80% [Citation51]. PET-CT is less frequently employed, but the sensitivity is higher at 88% with a specificity of 89% [Citation51]. FDG-PET reliably detects locoregional tumor recurrences, while CT or MRI is more sensitive for the detection of hepatic metastases [Citation54]. MRI with diffusion-weighted imaging (DWI) has shown higher sensitivity than MRI alone for detecting postoperative tumor recurrence [Citation52,Citation53].
Table 3. Diagnostic performance of postoperative serum tumor markers and imaging modalities for the detection of local and/or distant disease recurrence after pancreatic cancer resection.
Survival
The effects of routine surveillance on survival after resection of pancreatic cancer remains largely unclear due to conflicting results ().
A retrospective cohort study among 327 patients reported that regularly scheduled clinical and radiographic surveillance program may aid in the detection of asymptomatic recurrence, especially within the first 2 years [Citation31]. Asymptomatic patients more often received treatment and demonstrated improved post-diagnosis overall survival (29.6 vs 18.0 months; p = 0.003), as well as post-recurrence overall survival (13.0 months vs 5.1 months; p < 0.001).
Another retrospective cohort study among 229 patients found that routine imaging surveillance detected recurrent disease earlier and more patients received chemotherapy for recurrent disease and had longer postoperative overall survival (30.4 months vs 17.1 months; p = 0.002) [Citation36].
One retrospective cohort study among 525 patients [Citation38] demonstrated that an elevated CA 19-9 level was associated with significantly reduced overall survival when measured at six monthly intervals for the first 24 months after resection [Citation38]. A retrospective cohort study among 80 patients [Citation40] reported that patients who started salvage treatment only based on rising CA19-9 levels had a significantly longer postoperative overall survival than patients with treatment changes based on radiological examinations (28.1 vs 20.7 months; p = 0.049) [Citation40].
A Dutch multicenter observational study among 836 patients [Citation43] reported that routine follow-up imaging, as compared with symptomatic follow-up, was associated with improved survival (25 vs 15 months; p < 0.001). Using propensity-score matching, treatment of recurrence was found to be independently associated with longer overall survival for both asymptomatic and symptomatic recurrences. The median postoperative overall survival was 20 months among patients with asymptomatic tumor recurrence and 15 months among symptomatic patients (p < 0.001).
Similarly, a recent retrospective cohort study of 368 patients found that tumor recurrence detected within a scheduled follow-up compared to relapse at an unplanned visit was associated with a significantly improved survival [Citation44]. Compared to patients with recurrence detected by clinical deterioration, patients with recurrence detected by routine imaging or laboratory tests had longer postoperative overall survival (24.8 vs 15.1 months; p = 0.007).
A retrospective cohort study of 85 patients evaluated the consequences of a symptomatic surveillance strategy without routine imaging [Citation37]. Although imaging testing was not part of the symptomatic follow-up strategy, most patients underwent additional imaging procedures to detect recurrence. Most of the recurrences were diagnosed at a late stage and after the manifestation of clinical symptoms, with only a minority of patients receiving additional treatment for their relapse. Importantly, post-recurrence overall survival was significantly longer for patients receiving salvage therapy compared to the group receiving best supportive care (7 vs 3 months; p = 0.016).
In contrast, a retrospective cohort study among 2217 patients found no significant survival benefit among patients who received annual CT scans on a routine basis [Citation30]. Similarly, another retrospective cohort study among 147 patients found no survival benefit from surveillance despite earlier detection of tumor recurrence [Citation39].
In 2021, a systematic review [Citation42] was conducted regarding postoperative surveillance based on 10 studies [Citation12, Citation31–35, Citation37,Citation38, Citation40, Citation43], with 5 studies suitable for meta-analysis [Citation31, Citation33, Citation35, Citation37, Citation43] and 3 studies included in the survival analysis [Citation31, Citation33, Citation43]. Routine surveillance more frequently detected asymptomatic recurrence, leading to higher treatment rates and longer survival. However, the longer survival may be a result of lead-time bias, i.e. detecting a recurrence earlier, and hence starting a treatment, may project a longer survival time, even if the patient’s actual survival time is not extended by the treatment ().
Cost-effectiveness of surveillance
A retrospective cohort study [Citation32] evaluated the cost-effectiveness of five surveillance strategies using a Markov model, including no scheduled surveillance and clinical evaluation and CA 19-9 without/with routine radiological examination at either 3 or 6 month intervals. Limited surveillance was found to be the most cost-effective strategy, consisting of clinical evaluation and serum CA 19-9 testing every 6 months and imaging in case of symptoms, clinical findings or increased CA 19-9 levels.
Another retrospective cohort study [Citation30] estimated the average costs for CT-scans associated with surveillance. On average, in 1991 the costs per patient were approximately $879 compared to $1797 in 2005, in 2011 monetary terms. The increase in costs reflected the increase in the amount of CT-imaging during surveillance. The study found no significant survival benefit among patients who received more CT scans.
Quality of life during surveillance
Patients are generally positively inclined towards routine surveillance after surgery [Citation34]. They feel safe participating in follow-up appointments and report few difficulties with the overall follow-up process [Citation34]. It has been shown that patients often overemphasize the importance of CA 19-9 and essential symptoms may go unaddressed [Citation12]. Structured postoperative surveillance frequently leads to initiation or modifications of symptom-directed, oncological or diabetic treatments for the benefit of quality of life [Citation35]. This is supported by a recent Danish study which also highlights the importance of symptom management after pancreatic cancer surgery [Citation45].
Nordic Survey results
All 20 consultant surgeons from the 20 pancreatic units responded to the survey. The reported practice between the pancreatic units demonstrated wide variation (). About 75% had routine postoperative surveillance after pancreatic cancer surgery. Routine CA 19-9 testing was used by 80% and routine CT by 67% as part of surveillance. Most common interval for the first year of follow-up was 3 months (67%). About 73% of centers continued follow-up until 5 years postoperatively.
Conclusion
Current guidelines provide conflicting recommendations regarding postoperative surveillance of pancreatic cancer. Some guidelines favor surveillance based on evidence suggesting that routine surveillance can detect asymptomatic disease, allowing earlier start of recurrence treatment and longer survival. The optimal surveillance method and duration remains to be determined. Further development of existing or novel liquid biopsy methods may lead to more accurate tumor detection and guidance of treatment. However, the subject of lead-time bias remains a major concern and whether postoperative cancer surveillance can be translated into improved survival rates remains to be evaluated in prospective trials.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. doi:10.3322/caac.21820.
- Kirkegård J, Gaber C, Heide-Jørgensen U, et al. Effect of surgery versus chemotherapy in pancreatic cancer patients: a target trial emulation. J Natl Cancer Inst. 2024;116(7):1072–1079. doi:10.1093/jnci/djae024.
- Groot VP, Rezaee N, Wu W, et al. Patterns, timing, and predictors of recurrence following pancreatectomy for pancreatic ductal adenocarcinoma. Ann Surg. 2018;267(5):936–945. doi:10.1097/SLA.0000000000002234.
- Tanaka M, Mihaljevic AL, Probst P, et al. Meta-analysis of recurrence pattern after resection for pancreatic cancer. Br J Surg. 2019;106(12):1590–1601. doi:10.1002/bjs.11295.
- Groot VP, van Santvoort HC, Rombouts SJ, et al. Systematic review on the treatment of isolated local recurrence of pancreatic cancer after surgery; re-resection, chemoradiotherapy and SBRT. HPB (Oxford). 2017;19(2):83–92. doi:10.1016/j.hpb.2016.11.001.
- Serafini S, Sperti C, Friziero A, et al. Systematic review and meta-analysis of surgical treatment for isolated local recurrence of pancreatic cancer. Cancers (Basel). 2021;13(6):1277. doi:10.3390/cancers13061277.
- Okusaka T. Treatment for postoperative recurrence of pancreatic cancer: a narrative review. Chin Clin Oncol. 2022;11(3):19–19. doi:10.21037/cco-21-87.
- Guerra F, Barucca V, Coletta D. Metastases or primary recurrence to the lung is related to improved survival of pancreatic cancer as compared to other sites of dissemination. Results of a systematic review with meta-analysis. Eur J Surg Oncol. 2020;46(10 Pt A):1789–1794. doi:10.1016/j.ejso.2020.06.013.
- Larsson P, Søreide K. Surgery for oligometastatic pancreatic cancer: next frontier? Br J Surg. 2024;111(1):znad419. doi:10.1093/bjs/znad419.
- Omiya K, Maekawa A, Oba A, et al. A proposal of ABCD metastasectomy criteria for synchronous/metachronous metastatic pancreatic cancer in the era of multidisciplinary treatment. Br J Surg. 2024;111:znad417.
- Andersson R, Ansari D, Haglund C. Surveillance after resection of pancreatic ductal adenocarcinoma: how to do it and what are the benefits? Scand J Surg. 2024;113(2):184–185. doi:10.1177/14574969231156353.
- Elberg Dengsø K, Tjørnhøj-Thomsen T, Oksbjerg Dalton S, et al. It’s all about the CA-19-9. A longitudinal qualitative study of patients’ experiences and perspectives on follow-up after curative surgery for cancer in the pancreas, duodenum or bile-duct. Acta Oncol. 2019;58(5):642–649. doi:10.1080/0284186X.2018.1562212.
- Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi:10.7326/M18-0850.
- Regionala cancercentrum i samverkan. Nationellt vårdprogram för pankreascancer. Version 3.1; 2021.
- Sundhedsstyrelsen. Pakkeforløb for kræft i bugspytkirtel, galdegange og lever; 2021.
- Helsedirektoratet. Pancreaskreft (bukspyttkjertelkreft) – handlingsprogram, last update; August 2023. https://www.helsedirektoratet.no/retningslinjer/pancreaskreft-bukspyttkjertelkreft-handlingsprogram/oppfolging-og-etterkontroll-etter-kurativ-behandling/sammendrag-av-anbefalinger#sammendrag-av-anbefalinger.
- Silvestris N, Brunetti O, Bittoni A, et al. Clinical practice guidelines for diagnosis, treatment and follow-up of exocrine pancreatic ductal adenocarcinoma: evidence evaluation and recommendations by the Italian Association of Medical Oncology (AIOM). Cancers (Basel). 2020;12(6):1681. doi:10.3390/cancers12061681.
- Federatie Medisch Specialisten. Follow-up na resectie pancreascarcinoom. 2024. https://richtlijnendatabase.nl/richtlijn/pancreascarcinoom/chirurgische_behandeling_pancreascarcinoom/follow-up_na_resectie.html.
- Conroy T, Pfeiffer P, Vilgrain V, et al. Pancreatic cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34(11):987–1002. doi:10.1016/j.annonc.2023.08.009.
- Neuzillet C, Gaujoux S, Williet N, et al. Pancreatic cancer: French clinical practice guidelines for diagnosis, treatment and follow-up (SNFGE, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, ACHBT, AFC). Dig Liver Dis. 2018;50(12):1257–1271. doi:10.1016/j.dld.2018.08.008.
- Seufferlein T, Mayerle J, Böck S, et al. S3-Leitlinie zum exokrinen Pankreaskarzinom – Langversion 2.0 – Dezember 2021 – AWMF-Registernummer: 032/010OL. Z Gastroenterol. 2022;60(11):e812–e909. doi:10.1055/a-1856-7346.
- Takaori K, Bassi C, Biankin A, et al. International Association of Pancreatology (IAP)/European Pancreatic Club (EPC) consensus review of guidelines for the treatment of pancreatic cancer. Pancreatology. 2016;16(1):14–27. doi:10.1016/j.pan.2015.10.013.
- Gómez-España MªA, Montes AF, Garcia-Carbonero R, et al. SEOM clinical guidelines for pancreatic and biliary tract cancer (2020). Clin Transl Oncol. 2021;23(5):988–1000. doi:10.1007/s12094-021-02573-1.
- O’Reilly D, Fou L, Hasler E, et al. Diagnosis and management of pancreatic cancer in adults: a summary of guidelines from the UK National Institute for Health and Care Excellence. Pancreatology. 2018;18(8):962–970. doi:10.1016/j.pan.2018.09.012.
- Khorana AA, McKernin SE, Berlin J, et al. Potentially curable pancreatic adenocarcinoma: ASCO clinical practice guideline update. J Clin Oncol. 2019;37(23):2082–2088. doi:10.1200/JCO.19.00946.
- Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19(4):439–457. doi:10.6004/jnccn.2021.0017.
- Cui J, Jiao F, Li Q, et al. Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of pancreatic cancer. J National Cancer Center. 2022;2(4):205–215. doi:10.1016/j.jncc.2022.08.006.
- Okusaka T, Nakamura M, Yoshida M, et al. Clinical practice guidelines for pancreatic cancer 2022 from the Japan Pancreas Society: a synopsis. Int J Clin Oncol. 2023;28(4):493–511. doi:10.1007/s10147-023-02317-x.
- Australasian Gastro-Intestinal Trials Group (AGITG). Definition of surgical standards for pancreatic cancer: a consensus statement by the Australasian Gastro-Intestinal Trials Group; 2015.
- Witkowski ER, Smith JK, Ragulin-Coyne E, et al. Is it worth looking? Abdominal imaging after pancreatic cancer resection: a national study. J Gastrointest Surg. 2012;16(1):121–128. doi:10.1007/s11605-011-1699-z.
- Tzeng CW, Fleming JB, Lee JE, et al. Yield of clinical and radiographic surveillance in patients with resected pancreatic adenocarcinoma following multimodal therapy. HPB (Oxford). 2012;14(6):365–372. doi:10.1111/j.1477-2574.2012.00445.x.
- Tzeng CW, Abbott DE, Cantor SB, et al. Frequency and intensity of postoperative surveillance after curative treatment of pancreatic cancer: a cost-effectiveness analysis. Ann Surg Oncol. 2013;20(7):2197–2203. doi:10.1245/s10434-013-2889-6.
- Nordby T, Hugenschmidt H, Fagerland MW, et al. Follow-up after curative surgery for pancreatic ductal adenocarcinoma: asymptomatic recurrence is associated with improved survival. Eur J Surg Oncol. 2013;39(6):559–566. doi:10.1016/j.ejso.2013.02.020.
- Deobald RG, Cheng ES, Ko YJ, et al. A qualitative study of patient and clinician attitudes regarding surveillance after a resection of pancreatic and peri-ampullary cancer. HPB (Oxford). 2015;17(5):409–415. doi:10.1111/hpb.12378.
- Tjaden C, Michalski CW, Strobel O, et al. Clinical impact of structured follow-up after pancreatic surgery. Pancreas. 2016;45(6):895–899. doi:10.1097/MPA.0000000000000564.
- Elmi A, Murphy J, Hedgire S, et al. Post-whipple imaging in patients with pancreatic ductal adenocarcinoma: association with overall survival: a multivariate analysis. Abdom Radiol (NY). 2017;42(8):2101–2107. doi:10.1007/s00261-017-1099-2.
- Groot VP, Daamen LA, Hagendoorn J, et al. Use of imaging during symptomatic follow-up after resection of pancreatic ductal adenocarcinoma. J Surg Res. 2018;221:152–160. doi:10.1016/j.jss.2017.08.023.
- Rieser CJ, Zenati M, Hamad A, et al. CA19-9 on postoperative surveillance in pancreatic ductal adenocarcinoma: predicting recurrence and changing prognosis over time. Ann Surg Oncol. 2018;25(12):3483–3491. doi:10.1245/s10434-018-6521-7.
- Samawi HH, Yin Y, Lim HJ, et al. Primary care versus oncology-based surveillance following adjuvant chemotherapy in resected pancreatic cancer. J Gastrointest Cancer. 2018;49(4):429–436. doi:10.1007/s12029-017-9988-8.
- Li J, Li Z, Kan H, et al. CA19-9 elevation as an indication to start salvage treatment in surveillance after pancreatic cancer resection. Pancreatology. 2019;19(2):302–306. doi:10.1016/j.pan.2019.01.023.
- Wu H, Guo JC, Yang SH, et al. Postoperative imaging and tumor marker surveillance in resected pancreatic cancer. J Clin Med. 2019;8(8):1115. doi:10.3390/jcm8081115.
- Halle-Smith JM, Hall L, Daamen LA, et al. Clinical benefit of surveillance after resection of pancreatic ductal adenocarcinoma: a systematic review and meta-analysis. Eur J Surg Oncol. 2021;47(9):2248–2255. doi:10.1016/j.ejso.2021.04.031.
- Daamen LA, Groot VP, Besselink MG, et al. Detection, treatment, and survival of pancreatic cancer recurrence in the netherlands: a nationwide analysis. Ann Surg. 2022;275(4):769–775. doi:10.1097/SLA.0000000000004093.
- Zhang D, Kruger S, Schirle K, et al. Clinical impact of structured post-operative surveillance in resected pancreatic adenocarcinoma: results from a retrospective cohort study. Oncol Res Treat. 2023;46(3):106–115. doi:10.1159/000528722.
- Elberg Dengsø K, Thomsen T, Christensen BM, et al. Physical and psychological symptom burden in patients and caregivers during follow-up care after curative surgery for cancers in the pancreas, bile ducts or duodenum. Acta Oncol. 2023;62(7):782–793. doi:10.1080/0284186X.2023.2185541.
- Daamen LA, Groot VP, Heerkens HD, et al. Systematic review on the role of serum tumor markers in the detection of recurrent pancreatic cancer. HPB (Oxford). 2018;20(4):297–304. doi:10.1016/j.hpb.2017.11.009.
- Jung W, Jang J-Y, Kang MJ, et al. The clinical usefulness of 18F-fluorodeoxyglucose positron emission tomography–computed tomography (PET–CT) in follow-up of curatively resected pancreatic cancer patients. HPB (Oxford). 2016;18(1):57–64. doi:10.1016/j.hpb.2015.06.001.
- Mataki Y, Takao S, Maemura K, et al. Carcinoembryonic antigen messenger RNA expression using nested reverse transcription-PCR in the peripheral blood during follow-up period of patients who underwent curative surgery for biliary-pancreatic cancer: longitudinal analyses. Clin Cancer Res. 2004;10(11):3807–3814. doi:10.1158/1078-0432.CCR-03-0130.
- Safi F, Schlosser W, Kolb G, et al. Diagnostic value of CA 19-9 in patients with pancreatic cancer and nonspecific gastrointestinal symptoms. J Gastrointest Surg. 1997;1(2):106–112. doi:10.1016/s1091-255x(97)80097-2.
- Sperti C, Pasquali C, Bissoli S, et al. Tumor relapse after pancreatic cancer resection is detected earlier by 18-FDG PET than by CT. J Gastrointest Surg. 2010;14(1):131–140. doi:10.1007/s11605-009-1010-8.
- Daamen LA, Groot VP, Goense L, et al. The diagnostic performance of CT versus FDG PET-CT for the detection of recurrent pancreatic cancer: a systematic review and meta-analysis. Eur J Radiol. 2018;106:128–136. doi:10.1016/j.ejrad.2018.07.010.
- Saponjski D, Djuric-Stefanovic A, Jovanovic MM, et al. Utility of MRI in detection of PET-CT proven local recurrence of pancreatic adenocarcinoma after surgery. Med Oncol. 2024;41(2):47. doi:10.1007/s12032-023-02271-8.
- Shin N, Kang TW, Min JH, et al. Utility of diffusion-weighted MRI for detection of locally recurrent pancreatic cancer after surgical resection. AJR Am J Roentgenol. 2022;219(5):762–773. doi:10.2214/AJR.22.27739.
- Ruf J, Lopez Hänninen E, Oettle H, et al. Detection of recurrent pancreatic cancer: comparison of FDG-PET with CT/MRI. Pancreatology. 2005;5(2-3):266–272. doi:10.1159/000085281.