Abstract
Objectives
In patients evaluated for hepatocellular carcinoma (HCC), magnetic resonance imaging (MRI) is often used secondarily when multiphase contrast-enhanced computed tomography (ceCT) is inconclusive. We investigated the clinical impact of adding MRI.
Materials and methods
This single-institution retrospective study included 48 MRI scans (44 patients) conducted from May 2016 to July 2023 due to suspicion of HCC on a multiphase ceCT scan. Data included medical history, preceding and subsequent imaging, histology when available, and decisions made at multidisciplinary team meetings.
Results
In case of possible HCC recurrence, 63% of the MRI scans were diagnostic of HCC. For 80% of the negative MRI scans, the patients were diagnosed with HCC within a median of 165 days in the suspicious area of the liver. In case of possible de-novo HCC in patients with cirrhosis, 22% of the scans were diagnostic of HCC and 33% of the negative MRI scans were of patients diagnosed with HCC within a median of 109 days. None of the non-cirrhotic patients with possible de-novo HCC and negative MRI scans (64%) were later diagnosed with HCC, but 3/5 of the indeterminate scans were of patients diagnosed with HCC in a biopsy.
Conclusions
Secondary MRI to a multiphase ceCT scan suspicious of HCC is highly valuable in ruling out HCC in non-cirrhotic patients and in diagnosing HCC non-invasively in cirrhotic patients and patients with prior HCC. Patients with cirrhosis or prior HCC are still at high risk of having HCC if MRI results are inconclusive or negative.
Introduction
Hepatocellular carcinoma (HCC) is the fourth-leading cause of cancer-related death worldwide [Citation1]. In a cirrhotic liver, HCC can be diagnosed without a biopsy using multiphase contrast-enhanced computed tomography (ceCT) or magnetic resonance imaging (MRI), if imaging exhibits specific diagnostic hallmarks such as arterial contrast enhancement and venous washout [Citation2–5]. In non-cirrhotic livers, the diagnosis of HCC always requires a biopsy. International guidelines consider MRI and ceCT equal for non-invasive diagnosis of HCC in cirrhosis and as such, the initial imaging modality is individually chosen based on institutional factors and patient characteristics [Citation2,Citation3].
In a cirrhotic liver, a lesion may be suspicious of HCC, but without all the diagnostic hallmarks required for a definite diagnosis. Since such nodules can be benign lesions, premalignant lesions, HCC, and other malignancies, correct diagnostic management of the patient is crucial. The optimal diagnostic approach is a matter of debate and studies investigating whether such lesions are best examined by repeated imaging, alternative imaging, or biopsy are lacking [Citation4]. Current European (European Association for the Study of the Liver (EASL)) and American (American Association for the Study of Liver Diseases (AASLD)) guidelines recommend decision-making based on the clinical scenario [Citation2,Citation3]. It is well-known that the pre-test risk of HCC is higher in cirrhotic patients who have previously had HCC than in patients with no malignant history [Citation6,Citation7].
The diagnostic algorithm for HCC diagnosis at Aarhus University Hospital, a tertiary liver center covering approximately 2 million people, follows the EASL Clinical Practice Guidelines [Citation2]. For the diagnosis of de-novo HCC, a multiphasic ceCT scan is used as the first imaging modality. If the multiphasic ceCT scan is suspicious but indeterminate for HCC, MRI is used as the second-line imaging.
In this retrospective study, we aimed to determine whether using MRI as second-line imaging in patients with a multiphase ceCT suspicious of HCC provided a definite clinical conclusion. The data were stratified according to patients’ history of HCC or cirrhosis status.
Patients and methods
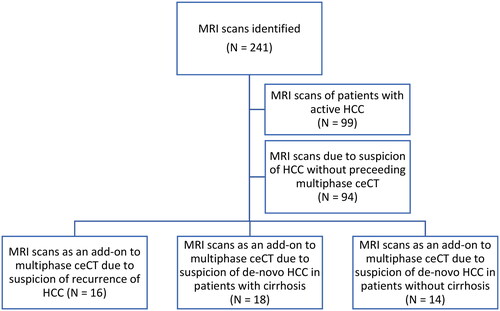
Eligible MRI scans were identified in the Picture Archiving and Communications System (PACS; Agfa, Impax, BE) and included all MRI examinations performed between May 1, 2016, and July 31, 2023, with suspicion of HCC either stated in the conclusion or the referral to the MRI. We excluded MRI scans that were part of treatment planning or evaluation of treatment response for a patient with known active HCC or did not have a multiphase ceCT scan including non-contrast, arterial, portal venous, and delayed phases suspicious of HCC within 100 days before the MRI.
A final total of 48 MRI scans of 44 patients were included in the study. The scans were grouped as MRI scans of 1) patients with possible recurrence of HCC (previously received treatment with curative intent i.e. radiofrequency ablation (RFA), resection, or transplantation), 2) patients with cirrhosis and no history of HCC, and 3) patients without cirrhosis and no history of HCC. These groups were considered at high, medium, and low risk for HCC, respectively ().
Patient information was obtained from the electronic medical record and included age, sex, preceding and subsequent imaging, decisions made at multidisciplinary team (MDT) meetings evaluating liver tumors, previous treatment for HCC, presence of cirrhosis, etiology of chronic liver disease, biochemistry at the time of MRI scan, Child-Pugh score, WHO performance status, and histology of liver biopsy, if available.
MRI was performed using either a 1.5 T MRI scanner (Philips Ingenia, Philips Healthcare, Best, The Netherlands) or a 3 T MRI scanner (Siemens Magnetom Vida, Siemens Healthcare GmbH, Erlangen, Germany; or Philips Achieva dStream, Philips Healthcare, Best, The Netherlands). The contrast used was either gadoterate meglumine (Dotarem, Guerbet, Aulnay-sous-Bois, France; or Clariscan, GE HealthCare AS, Oslo, Norway) (56%) or gadoxetate disodium (Gd-EOB-DTPA, gadoxetic acid, Primovist, Bayer HealthCare, Berlin, Germany) (44%). The liver was scanned using axial and coronal T2-weighted imaging, axial DWI (minimum b-values of 50 and 800), and axial T1-weighted gradient echo Dixon sequence contrast scan consisting of pre-contrast phase, arterial phase, followed immediately by porto-venous phase and late phase at 3 min and, for the gadoxetate disodium, a hepatobiliary phase at 15 min. The MRI scans were read by one of two experienced radiologists, each with more than 10 years of experience of MRI of the liver.
Radiological diagnosis of HCC was based on the EASL clinical practice guidelines for HCC (contrast-enhancement in the arterial phase followed by washout in the portal venous phase and/or late phase and in MRI scans with gadoxetate disodium also an assessment of the hepatobiliary phase). In patients without cirrhosis, a biopsy is required unless the imaging is benign. If the MRI did not provide a definite diagnosis, further clinical management followed international guidelines and included biopsy, continuous surveillance, or treatment [Citation2].
Ethics
The project was designed as a quality improvement project which by Danish law only needs approval by the hospital board which was obtained.
Statistics
Statistical analysis was performed using Microsoft Excel (Microsoft Office Home and Student 2019, version 2106, Redmond, WA, USA) and Stata version 17 (StataCorp LLC, Texas, USA). Comparisons between groups were performed using the Wilcoxon-Mann-Whitney test. Statistical significance was assumed for p-values < 0.05.
Results
Baseline
shows the baseline characteristics of the included 44 patients at the time of the first MRI scan. Overall, 30% had a prior diagnosis of HCC, 39% had cirrhosis but no previous diagnosis of HCC, and 32% did not have cirrhosis or a previous diagnosis of HCC. The patients without cirrhosis and no prior HCC diagnosis were significantly younger than those with cirrhosis and no prior HCC diagnosis (p = 0.03) and those with previous HCC (p = 0.02).
Table 1. Patient characteristics.
Patients with previous HCC
demonstrates the results of 16 MRI scans of 15 patients with a record of previous HCC treated with curative intent.
Figure 2. Sankey diagram of the 16 MRI scans as an add-on to a multiphase ceCT scan suspicious of HCC of patients with a previous record of HCC treated with curative intent. One patient is represented twice in the group ruling out HCC.
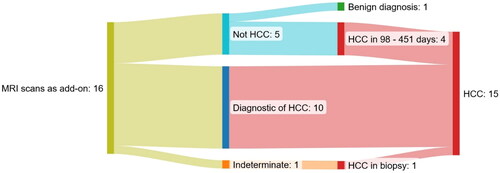
Four out of five MRI scans ruled out the suspicion of present HCC in patients who were later diagnosed with HCC within a median of 165 days; One patient had a portal vein thrombosis that was considered benign on two MRIs following two indeterminate ceCT scans; the thrombosis progressed in size and was finally deemed a tumor thrombosis 451 and 226 days after the first and second MRI scans. Two patients with nodules of 13 and 8 mm respectively suspicious of HCC on a ceCT scan were diagnosed with multifocal HCC 98 and 104 days after the MRI scans, respectively, and one of the patients had developed extrahepatic disease at that time point.
One MRI scan of a patient with a liver transplant after HCC ruled out the diagnosis. The patient has not been diagnosed with HCC 246 days after the MRI scan.
The majority of the MRI scans were diagnostic of HCC. One patient underwent a concurrent biopsy and MRI scan, and pathology revealed no malignant cells. An MRI scan five months later was diagnostic for HCC again, now with progression. A new biopsy confirmed HCC and the first biopsy was thus false negative.
Following guidelines, the patient with an indeterminate lesion (20 mm in size) was referred for a biopsy which showed HCC.
Patients with cirrhosis and no previous HCC
demonstrates the results of 18 supplementary MRI scans of 17 patients with cirrhosis and no previous record of HCC.
Figure 3. Sankey diagram of the 18 MRI scans as an add-on to a multiphase ceCT scan suspicious of HCC of patients with cirrhosis and no previous record of HCC. One patient is represented twice in the group ruling out HCC without a later diagnosis of HCC. All patients with an indeterminate MRI scan were referred to biopsy.
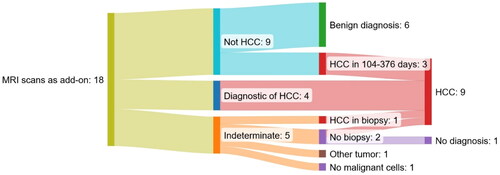
In two of the cases where MRI ruled out HCC (one with suspicious lesions of 8 and 17 mm and one with lesions of 17 and 24 mm), the lesions were found to be diagnostic of HCC 109 and 376 days from the MRI scan on a CT scan and in a biopsy, respectively. In a third case, the lesion found to be diagnostic of HCC 104 days after the MRI scan was also seen on the original MRI scan but found to be less than 10 mm. None of the remaining six patients has been diagnosed with HCC for a median of 1,274 days (interquartile range 896 − 1,617 days).
Following guidelines, biopsies were planned in the five patients where the MRI scans did not add to a definitive diagnosis. One patient had a lesion of 23 mm, but a planned biopsy was canceled due to severe comorbidities. In two patients, one with a diffuse lesion of 90 mm and one with two lesions of 25 and 35 mm, the biopsies showed HCC and angiosarcoma, respectively. In one patient with elevated AFP (163 ng/ml), the two lesions of 34 and 10 mm were not visible on ultrasound and a biopsy was thus not performed. However, the patient was diagnosed with HCC on a new MRI scan 270 days later. One patient with a lesion of 16 mm had a biopsy and RFA treatment in the same procedure. The biopsy was without malignant cells and the patient has not been diagnosed with HCC within 934 days.
Patients without cirrhosis and no previous HCC
shows the results of 14 MRI scans of 14 patients without cirrhosis and no previous record of HCC.
Figure 4. Sankey diagram of the 14 MRI scans as an add-on to a multiphase ceCT scan suspicious of HCC of patients without cirrhosis and no previous record of HCC. All patients with an indeterminate MRI scan were referred to biopsy.
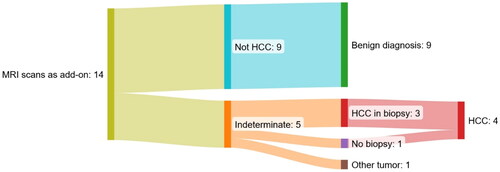
Nine (64%) of the MRI scans ruled out the HCC diagnosis and deemed the lesions definite benign (eight patients with focal nodular hyperplasia and one patient with a liver adenoma that was also verified by biopsy). None of the patients with a negative MRI has been diagnosed with HCC for a median of 780 days (interquartile range = 500 – 918 days) after the MRI.
In the five cases (36%) where MRI scans could not rule out HCC the size of the biggest lesion was 16, 25, 30, 30, and 70 mm, respectively. Evaluation at an MDT conference referred these patients to a subsequent biopsy following guidelines. The biopsies showed three cases of HCC and one case of bile duct adenoma. In one case, the tumor was not visible on ultrasound and a biopsy was thus not performed. However, the patient was diagnosed with a large infiltrative HCC on a CT scan 790 days after the MRI scan in that area of the liver.
Supplementary information
The time from a ceCT scan to a supplementary MRI was 20 days (Supplementary Table 1) and the type of contrast media used did not differ between subgroups (Supplementary Table 2). The size of the suspicious lesion was similar in patients diagnosed with HCC and those who were not (Supplementary Table 3).
Discussion
The main finding of this single-center study representing clinical daily life was that the clinical value of adding an alternative imaging technique such as MRI to patients with lesions suspicious of HCC on multiphase ceCT is highly dependent on the patient’s history. The results thus contribute to previous research demonstrating the challenges of evaluating indeterminate liver lesions suspicious of HCC [Citation4, Citation8–10], but to our knowledge, this is the first study to evaluate the diagnostic benefits of adding MRI scans in patients with ceCT scans suspicious of HCC as recommended by guidelines.
For patients at high risk of HCC, MRI was valuable for a non-invasive diagnosis of HCC. However, for patients at both high and intermediate risk of HCC, a significant proportion (80% and 33%) of the MRI scans ruling out HCC were of patients later diagnosed with HCC or another malignant disease in that area of the liver, most of them within four months. EASL Clinical Practice Guidelines recommend that a biopsy is performed from lesions that remain inconclusive. The current findings are likely to lead us to change our clinical practice and also consider a biopsy for patients with negative supplementary imaging in selected cases.
Previous studies have found an incidence of HCC of 14 – 29% in liver lesions found to be suspicious of HCC without fulfilling the diagnostic criteria in patients with cirrhosis without a prior history of HCC [Citation11–14]. The reason for a higher risk of HCC in lesions not fulfilling the diagnostic criteria for HCC in our study may be due to the clinical context. The included MRI scans were of patients who underwent secondary imaging due to the risk for HCC on a ceCT scan as evaluated by the MDT board as opposed to later follow-up.
In patients without cirrhosis and no history of HCC, the risk of HCC is low and MRI proved to be of high value in ruling out malignancy as none of the patients with MRI scans deeming the lesions benign were later diagnosed with HCC or another malignancy. MRI thus prevented unnecessary biopsies in 64% of patients. However, 80% of the patients with MRI scans that could not rule out HCC, eventually had the diagnosis. Noteworthy, the last patient had a bile duct adenoma.
The American guidelines stratify recommendations for indeterminate nodules based on the probability of the nodule representing HCC according to imaging criteria, Liver Imaging Reporting and Data System 3 or 4 (LI-RADS 3 or 4). The guidelines recommend that patients with a high likelihood of HCC (LI-RADS 4) are evaluated at an MDT conference and undergo biopsy or close-interval follow-up imaging depending on the clinical scenario, but do not further define the clinical scenario [Citation3]. For patients with LI-RADS 3 observations, the guidelines recommend repeat or alternative imaging in 3 – 6 months which is in line with a recent retrospective cohort study that found an 8.4% annual incidence of HCC in patients with LI-RADS 3 lesions [Citation15]. However, there is still little guidance on the management and follow-up for indeterminate nodules regardless of the LI-RADS classification [Citation9, Citation16]. Our data were not classified according to LI-RADS, as this is not implemented in the European guidelines [Citation2], but the MRI scans deemed indeterminate most likely correspond to LI-RADS 3 or 4. Yet, our results emphasize the importance of considering the patient’s clinical history rather than imaging criteria when assessing the risk of indeterminate nodules representing HCC. The results are in line with the EASL guidelines that state that a biopsy of an indeterminate liver nodule is indicated when supplementary imaging is also indeterminate [Citation2]. Further research in larger, multicentre studies is required to investigate the need for biopsy in patients at high or intermediate risk of HCC with negative secondary imaging.
The main concern about a tumor biopsy is the risk of a false-negative result which is up to 30% at the first assessment of fine needle biopsy of small HCCs < 20 mm [Citation17]. In adherence with this, AASLD underlines that a negative biopsy does not rule out HCC [Citation3]. In the present study, at least one out of ten biopsies was false-negative which highlights the risk of false-negative findings in both biopsy and imaging. The overall risk of tumor seeding is 2.7% [Citation18] and should not, together with the risk of bleeding, be considered a reason to refrain from a diagnostic biopsy of a liver tumor [Citation19].
Previous studies have defined a diagnostic delay in diagnosing HCC as more than 60 – 90 days from presentation until firm diagnosis [Citation20, Citation21]. Monitoring of indeterminate nodules or false-negative biopsies are common reasons for a diagnostic delay [Citation20–22]. In total, we found seven (30%) of the negative MRI scans to be of patients later diagnosed with HCC within 98 – 451 days. Hepatocarcinogenesis is considered a process by which hepatocellular nodules evolve from precancerous nodules to hepatocellular carcinoma [Citation23, Citation24]. In some cases, the lesion on the MRI thus might represent a dysplastic nodule which later evolves to hepatocellular carcinoma. Yet, it is likely that some of the MRI scans in our study did cause a delay in diagnosis as four of the patients were diagnosed with HCC less than 110 days after the MRI scan. Two patients with a small suspicious nodule were diagnosed with multifocal HCC within this timeframe, which has substantial implications for treatment possibilities.
It is a strength of our study that only two experienced radiologists read the MRI scans. The study, being a single-center study, is limited by the relatively small number of MRI scans, resulting in even smaller subgroups. However, the data include all MRI scans following an indeterminate ceCT scan over a period of 7 years at a tertiary liver center servicing approximately 2 million people. The incidence of HCC is relatively low in Denmark [Citation25], and the issue with indeterminate scans is minor but important for the individual patient. The results presented here are thus likely to influence our diagnostic approach in these patients, though the results’ generalizability to other centers should be viewed in light of the size of the study population, local preferences, and experiences. Another potential limitation of the study is that the radiologists had access to clinical and biochemical data, which may have biased the interpretation of the scans. We consider this to be of minor importance, as the diagnosis of HCC relies on specific radiological hallmarks.
In conclusion, at our institution, a tertiary liver center servicing the western part of Denmark the use of MRI as a secondary imaging modality following a ceCT scan suspicious for HCC can contribute to the final diagnosis by ruling out HCC in non-cirrhotic patients with no history of HCC. For patients at high or intermediate risk of HCC, MRI is valuable in providing a non-invasive diagnosis but if the supplementary MRI scan is either negative or indeterminate, there is a high risk of the suspicious lesion being HCC or another malignancy. This supports international guidelines that recommend either close surveillance or biopsy. However, particularly in high-risk patients, a more aggressive approach could be considered if curative treatment is an option.
Supplemental Material
Download MS Word (43.1 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Devarbhavi H, Asrani SK, Arab JP, et al. Global burden of liver disease: 2023 update. J Hepatol. 2023;79(2):516–537. doi:10.1016/j.jhep.2023.03.017.
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236.
- Singal AG, Llovet JM, Yarchoan M, et al. AASLD Practice Guidance on prevention, diagnosis, and treatment of hepatocellular carcinoma. Hepatology. 2023;78(6):1922–1965. doi:10.1097/HEP.0000000000000466.
- Roberts LR, Sirlin CB, Zaiem F, et al. Imaging for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatology. 2018;67(1):401–421. doi:10.1002/hep.29487.
- Ronot M, Purcell Y, Vilgrain V. Hepatocellular carcinoma: current imaging modalities for diagnosis and prognosis. Dig Dis Sci. 2019;64(4):934–950. doi:10.1007/s10620-019-05547-0.
- Tabrizian P, Jibara G, Shrager B, et al. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261(5):947–955. doi:10.1097/SLA.0000000000000710.
- Galia M, Agnello F, Sparacia G, et al. Evolution of indeterminate hepatocellular nodules at Gd-EOB-DPTA-enhanced MRI in cirrhotic patients. Radiol Med. 2018;123(7):489–497. doi:10.1007/s11547-018-0873-8.
- Kanneganti M, Marrero JA, Parikh ND, et al. Clinical outcomes of patients with Liver Imaging Reporting and Data System 3 or Liver Imaging Reporting and Data System 4 observations in patients with cirrhosis: a systematic review. Liver Transpl. 2022;28(12):1865–1875. doi:10.1002/lt.26562.
- Konerman MA, Verma A, Zhao B, et al. Frequency and outcomes of abnormal imaging in patients with cirrhosis enrolled in a hepatocellular carcinoma surveillance program. Liver Transpl. 2019;25(3):369–379. doi:10.1002/lt.25398.
- Mitchell DG, Bashir MR, Sirlin CB. Management implications and outcomes of LI-RADS-2, -3, -4, and -M category observations. Abdom Radiol (NY). 2018;43(1):143–148. doi:10.1007/s00261-017-1251-z.
- Beal EW, Kearney JF, Chakedis JM, et al. Interval Magnetic Resonance Imaging: an Alternative to Guidelines for Indeterminate Nodules Discovered in the Cirrhotic Liver. J Gastrointest Surg. 2017;21(9):1463–1470. doi:10.1007/s11605-017-3454-6.
- Beal EW, Albert S, McNally M, et al. An indeterminate nodule in the cirrhotic liver discovered by surveillance imaging is a prelude to malignancy. J Surg Oncol. 2014;110(8):967–969. doi:10.1002/jso.23765.
- Cococcia S, Dutta P, Moghim M, et al. The fate of indeterminate liver lesions: what proportion are precursors of hepatocellular carcinoma? BMC Gastroenterol. 2022;22(1):118. doi:10.1186/s12876-022-02135-x.
- Khalili K, Kim TK, Jang HJ, et al. Indeterminate 1-2-cm nodules found on hepatocellular carcinoma surveillance: biopsy for all, some, or none? Hepatology. 2011;54(6):2048–2054. doi:10.1002/hep.24638.
- Arvind A, Joshi S, Zaki T, et al. Risk of hepatocellular carcinoma in patients with indeterminate (LI-RADS 3) liver observations. Clin Gastroenterol Hepatol. 2023;21(4):1091–1093.e3. doi:10.1016/j.cgh.2021.11.042.
- Mitchell DG, Bruix J, Sherman M, et al. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology. 2015;61(3):1056–1065. doi:10.1002/hep.27304.
- Forner A, Vilana R, Ayuso C, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47(1):97–104. doi:10.1002/hep.21966.
- Silva MA, Hegab B, Hyde C, et al. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57(11):1592–1596. doi:10.1136/gut.2008.149062.
- Aubé C, Oberti F, Lonjon J, et al. EASL and AASLD recommendations for the diagnosis of HCC to the test of daily practice. Liver Int. 2017;37(10):1515–1525. doi:10.1111/liv.13429.
- Choi DT, Davila JA, Sansgiry S, et al. Factors associated with delay of diagnosis of hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol. 2021;19(8):1679–1687. doi:10.1016/j.cgh.2020.07.026.
- Rao A, Rich NE, Marrero JA, et al. Diagnostic and therapeutic delays in patients with hepatocellular carcinoma. J Natl Compr Canc Netw. 2021;19(9):1063–1071.
- Huo T-I, Huang Y-H, Chiang J-H, et al. Survival impact of delayed treatment in patients with hepatocellular carcinoma undergoing locoregional therapy: is there a lead-time bias? Scand J Gastroenterol. 2007;42(4):485–492. doi:10.1080/00365520600931402.
- Fung A, Shanbhogue KP, Taffel MT, et al. Hepatocarcinogenesis: radiology-Pathology Correlation. Magn Reson Imaging Clin N Am. 2021;29(3):359–374. doi:10.1016/j.mric.2021.05.007.
- Narsinh KH, Cui J, Papadatos D, et al. Hepatocarcinogenesis and LI-RADS. Abdom Radiol (NY). 2018;43(1):158–168. doi:10.1007/s00261-017-1409-8.
- Jepsen P, Andersen MW, Villadsen GE, et al. Time-trends in incidence and prognosis of hepatocellular carcinoma in Denmark: a nationwide register-based cohort study. Liver Int. 2017;37(6):871–878. doi:10.1111/liv.13340.