Summary
Labidostomma motasi Iavorschi, 1992 was discovered and described from a single female in the newly discovered hypoxic sulfidic, ancient and isolated Movile Cave (Romania). Male and female specimens collected recently brought new data for the description of this species. The morphology shows affinities and close relation with the species allied to L. integrum Berlese, 1911 which is distributed all around the Mediterranean Basin and Asia. As representatives of the family are also known from Baltic Amber (more than 40 My), this blind species in Movile Cave first attests of a probable adaptation of an epigean species to the cave environment: a model of two congeneric and sympatric species, one living in a cave and the second (L. integrum) living in soils in a region where the labidostommatid fauna is well referenced. This species may have migrated in caves, a winning option which allowed it to survive during past climatic variation since Miocene. Genetic data obtained from three specimens of this species were compared with the data published in Genbank showing a clear distinction between the subgenera Labidostomma Kramer, 1879 and Nicoletiella Canestrini, 1882. Overall, this species completes the long list of endemic invertebrates found in the sulfidic groundwater ecosystem of Movile Cave. Knowledge of the history of this cave system allowed us to estimate the isolation of this species in the Movile labyrinth during the ancient geological episodes following the Messinian Crisis.
Résumé
Labidostomma motasi redécouvert : un témoin de la crise messinienne? (Acari : Labidostommatidae). Labidostomma motasi Iavorschi, 1992 a été décrit peu après la découverte de grotte isolée ancienne hypoxique et riche en hydrogène sulfurique de Movile (Roumanie) sur une seule femelle en 1992. La récolte de spécimens mâles et femelles permet de compléter la description des deux sexes et de conclure aux affinités avec le groupe d’espèces proches de L. integrum Berlese, 1911, espèce présente dans le bassin méditerranéen et l’Asie. Comme les représentants de la famille sont connus aussi de l’ambre de la Baltique (plus de 40 Ma) cette espèce aveugle de Movile atteste d’une probable et ancienne adaptation d’un groupe épigé à l’environnement cavernicole. Ainsi cette espèce pourrait être le vicariant d’une espèce sympatrique des biotopes forestiers de la région. Ces données justifient l’hypothèse de l’adaptation à la vie cavernicole, plausible et ayant permis la survie durant les variations climatiques depuis le Miocène. Une première caractérisation génétique de l’espèce et la confrontation avec les données déposées dans Genbank montrent nettement la distinction entre les sous-genres Nicoletiella Canestrini, 1882 et Labidostomma Kramer, 1879. Surtout, cette espèce complète la longue liste des Invertébrés endémiques du complexe de cette grotte roumaine. La connaissance de l’histoire de cette grotte permet d’estimer l’isolement de l’espèce dans le labyrinthe de Movile des anciens épisodes géologiques ayant succédé à la crise messinienne.
Keywords:
With their primitive morphology, the “Labidostommatidae”, distributed worldwide, present an easily identified silhouette, the typical pattern being conserved by their heavy sclerotization (Walter et al. Citation2009). This group is placed near the root of the phylogenetic tree of the Actinedid mites and forms, according to different authors, a suborder (Labidostommatina Oudemans, Citation1906) or a supercohort (Walter et al. Citation2009). Their pattern corresponds to the common representation of the archetype of primitive terrestrial Acari which was conserved in time more than several hundred thousand years; Labidostomma Kramer, 1879 having been recorded in Europe since the Cenozoic (Dunlop & Bertrand Citation2011; Klimov et al. Citation2018). Mainly soil mites, few small sized species belonging to this family are eu-edaphic: with shortened appendages, this way of life being accompanied by shortened sensitive setae, and regression of the ocular lenses, from vestigial to complete absence as illustrated by the genus Akrostomma Robaux, 1977 (Robaux 1977; Bertrand & Coineau Citation1979; Bertrand Citation1983). However, coloration and cuticular characteristics were not fundamentally changed. Only one surface living species of the family is also blind, i.e. the French Labidostomma glymma Grandjean, 1942, a rarely collected one, and more information is needed on its biology. To date, Labidostomma motasi Iavorschi, Citation1992 remains the only species encountered solely in caves (Iavorschi Citation1992). Two characters are linked to the cave environment: the absence of ocular organs (absence of the three lenses, the lateral pair behind (gr) setae and the single frontal eye), and the elongated legs (long appendages) equipped with dense sensory organs (usual setae, solenidia and eupathidiae) (Alberti Citation1998). We can suppose a priori that this species shows adaptations to troglobiotic life since it takes a long time to acquire these features (Peck Citation1998). In addition, the epigeic acarofauna has been studied thoroughly in this region of Romania and we feel confident to affirm with a quasi-certitude the absence of this species in the common soil fauna though other species of the family have been prospected and recorded (Feider & Vasiliu Citation1970).
Some data on the basal cave Prostigmatic mites
Some paradigms on the life in caves by basal Prostigmatic mites must be restated here: according to Řezáč et al. (Citation2023), an elongation of appendages is an adaptation to life in relatively large subterranean spaces, typically caves (troglomorphism in Zacharda Citation1979). A contrario, a minute or diminished body size represents an adaptation to life in relatively small, narrow subterranean spaces, typically of soils (edaphomorphism sensu Zacharda Citation1979). Among the other basal actinedid mites living in caves, the Rhagidiidae, predators too, are the best known. Zacharda et al. (Citation2011) have underlined the tendency to invade subterranean habitats from as early as the Pliocene, and have defined their troglomorphism. Alternately, some of the subterranean colonization occurred more recently (during or since the last glaciation). The genus Troglocheles Zacharda, 1980 revealed a pool of “endemic cave vicariants” but no epigean (common?) ancestors are known. Many of the more basal actinedids commonly collected in caves are trogloxenic (often ubiquitous, such as the genus Orchestrale Tragärdh, 1909 now corrected in Caenonychus Oudemans, 1902) (Bolton & Bauchan Citation2022). To compare the soft Rhagidiidae and the sclerotized Labidostommatidae is meaningful because (i) the species of both families retained a primitive morphology, (ii) their habitus is “standardized”, (iii) their diet is not specialized (feeding on other invertebrates), (iv) the species are collected both under and above the soil surface, and so (v) edaphic populations ‘captured’ by this subterranean habitat can survive there. Examples of Labidostomma cornutum Canestrini & Fanzago, 1877 found by Willmann (Citation1932) in Croatia, also found in French caves (Ardèche, Hérault by M.B., unpublished data), L. denticulata (Schrank, 1776) in Poland by Pax, Citation1937 and L. (Nicoletiella) jacquemarti Coineau, Citation1964 from the French “Grotte de Pouade” (Eastern Pyrenees). More recently, L. cornutum was reported from sulfidic habitats in the Fracassi caves (Italy) (Sarbu et al. Citation2022). Błoszyk et al. (Citation2003) stated the synonymies of principal different European species, including several collected in caves.
Scavenging, opportunistic predation and fungivory are typical feeding modes of these extant early-derivative Acari which succeeded in colonizing most of the available ecosystems, and endured successive changes throughout time.
The interest in the distribution of these plesiomorphic mites
The distribution of actual species results both from the consequences of more or less favorable periods in the past and from the more recent processes of expanding population from the isolated refuges (Walter & Proctor Citation1998). Currently, the European distribution of the species and of mite communities resulted from the reconquest since last glaciations. Therefore, the colonization processes may have favored extinction of clades as well as speciation among the remaining taxa (Bertrand Citation1989; Sidorchuk & Bertrand Citation2013). In the case of Halacaridae, the colonization of inland underground waters by a small number of mostly cosmopolitan species is believed to have started from marine coastal waters; similarly, true cave species may descend from surface ancestors, and be considered vicarious of the surface ones (Bartsch Citation1996). In such a context, discovery of a true cavernicolous labidostommid is of interest because (i) it is a primitive soil mite which has kept primitive characters; (ii) it is not linked to a “cave inhabiting” host such as bats (such as the Spinturnicidae or others), nor to a “secondary” food source such as guano or fungi; and (iii) they have preserved their primitive morphology which was fixed by an early acquired strong sclerotization (“Labidostommatina was recovered as a basal Prostigmata”: Pepato & Klimov Citation2015, 13). This last character allowed the conservation of a global homogeneous pattern of morphology through time. So, the identification of troglomorphisms in a cave species, where the closest species is living nearby at the surface, may attest that the capability of speciation was retained. This gives the opportunity to address some central questions such as how some species have evolved, and in response to which parameters (Huang Citation2020).
Material and methods
Abbreviations
Apo, cheliceral organ. apo sej. sejugal apodeme. apo1, apo2, apo3, apo4, sternal apodemes 1 to 4. bo.a, bo.p, anterior and posterior trichobothries. c.p.c., podocephalic canal. c.p.c., podocephalic canal. cha, chb, cheliceral setae. d1-3, v(x), dorsal and ventral tibial setae. da, db, dc, dd, de, dorsal setae of opisthosoma. cha, chb. cheliceral setae. ga, ge, gm, gr, prodorsal setae.k": special subterminal dorsal tibial seta. la, lb, lc, ld, le, lateral setae of opisthosoma. LL: lateral lips. LS, labrum. ma, mb infracapitular setae. p1, p2, p3, anal setae. pl, setae of the lateral lips. T, terminal eupathidia of the tarsus. TR, trachea. tα antiaxial tooth of the fixed digit. tπ, paraxial tooth of fixed digit. v1, v2, genital papillae. ω, unique tarsal palp solenidion. ϵ, famulus. ζπ, ζα, ζv, paraxial, antiaxial and ventral eupathidial setae. φ, tibial solenidion. ω1-ω2, dorsal tarsal solenidia.
The species
Labidostomma motasi was described from one single female collected in Movile Cave (Romania) in 1991. In 2021–2022, new specimens (eight males and one female) were collected by V. Kiss and by S.M. Sarbu, and identified by M. Manu. Three of these were preserved for genetic analysis, whereas the others were kept for confirmation of identification. The studied specimens belong to the family Labidostommatidae Oudemans, Citation1906 (syn. Nicoletiellidae Canestrini, 1891), a group of basal well-identified Prostigmata, with few genera described, and of which the range of current genera suggests that they differentiated prior to the Miocene (Bertrand et al. Citation2015). As a paradigm, these mites are more prone to endemism than other groups (Błoszyk & Napierała Citation2020). However, to date the immature stages remain unknown.
Identification of European labidostommatids
A short key here below allows identifying the three subgenera in the genus Labidostomma (adapted from Pfliegler & Bertrand Citation2011).
1. Famulus on the tarsi PI with a central fruit rounded or varied in shape, surrounded by five or six branches2 Famulus regressive, often hidden among the dorsal setae of the tarsi I, spinelike. Apical eye (if it exists) in subterminal position, body fusiform and elongated, no anterolateral dorsal projections (cornuae)subgenus Labidostomma Kramer, 1879 (the species of the integrum species group exhibit a dorsolateral line of pores surrounding the dorsal shield interrupted at the level of ocular lateral zones)
2. One gland-like organ on each side of dorsal shield, rather large and generally multiporous; famulus with spine-like branches. Frontal eye (if exists) in subterminal position. Legs: tibia and genua may be ornamented with alveoli similar to those of the dorsum or reticulate. Anterolateral projections (“cornuae”) more or less developed. Body elongated in shape, chelicerae with proximal seta inserted at the top of a long tube, inferior tooth of fixed digit prolonged in a recurrent bladesubgenus Cornutella Feider & Vasiliu, 1969
– Fruit of the famulus rounded, surrounded by simple short branches; one pair of multiporous gland-like organs present. If additional pustules present, then uniporous (additional pustules behind the lateral ones or even in lateroposterior position). If frontal eye exists, it is in terminal position, genua of legs I shorter than tibia, chelicerae with proximal seta inserted on a short tubercle.................... ...............................subgenus Nicoletiella Canestrini, 1882
Preparation of specimens
Among the specimens, two males and one female were preserved in 70% ethanol for morphological examination. Cleared in lactic acid on cavity slides, one male and one female were conserved intact, the second male being used for dissection allowing examinations under different views. The in toto animals or the different pieces resulted from with minute pins under binocular (e.g. legs, chelicerae… and separation of dorsal and ventral shields, infracapitulum, and chelicerae) were examined in temporary preparations on cavity slides with lactic acid under microscope. The different pictures and measures were taken with Motic Camera and processed with Motic pictures software MoticImage Plus 30 (provided by Moticeurope, Barcelona, Spain). Drawings were realized using Inkscape 0,92.5 software (Free Software Foundation, Inc. 51 Boston, USA). Several specimens were identified by M. Manu from pictures taken by V. Kiss (with measure calibration) in Romania.
Notation and abbreviations correspond to standard notation and nomenclature (Grandjean Citation1942a, Citation1942b).
Genetic analysis
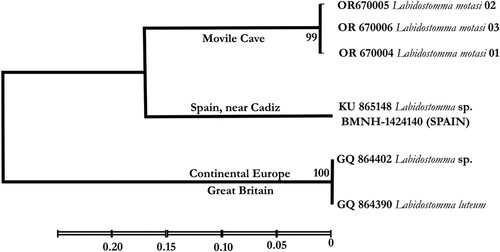
DNA was extracted from whole specimens, using the Qiagen QIAmp® DNA Mini Kit (Qiagen, Hilden, Germany) and following the manufacturer’s instructions. The COI fragment was amplified with the universal primer pair LCO1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) and HCO2198 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) (Folmer et al. Citation1994). PCR amplicons were excised from the agarose gel and purified using the FavorPrep™ Gel/PCR Purification Kit (Favorgen-Europe, Vienna, Austria). Purified amplicons were sequenced unidirectionally at a sequencing facility (Macrogen, Amsterdam, the Netherlands). The raw chromatograms were visualized in Chromas v2.6.6 (Technelysium Pty Ltd., Brisbane, Australia) and low-quality ends were trimmed. The resulting sequences had a length of 643 nucleotides (nts) and were deposited in GenBank under the following accession numbers: OR670004 Labidostomma motasi Lm01 OR670005 Labidostomma motasi Lm02 OR670006 Labidostomma motasi Lm03.
These three sequences were assembled into an alignment together with GenBank sequences KU865148, GQ864402 and GQ864390. All the sequences in the alignment were trimmed to a length of 565 nucleotides, i.e. the length of the shortest GenBank sequence. The sequences were aligned using ClustalW with default parameters. The best maximum likelihood substitution model was estimated to be the Tamura 3-parameter model with a discrete Gamma distribution of rates among sites. The phylogeny reconstruction was done by Maximum Likelihood, using 1000 bootstrap replications. The assembly and trimming of sequences, and subsequent phylogenetic analyses were performed in MEGA7 (Kumar et al. Citation2016).
Results
Identification
Only a few Labidostommatid genera live in Europe (see Beron Citation2022): the eu-edaphic Akrostomma Robaux, Citation1976 (small size, short appendages, blind or with reduced eye-lens), the large, strong and surface living mite Eunicolina Berlese, Citation1911, and the most diverse genus Labidostomma Kramer, 1879. The genus Labidostomma is known by three main categories:
the largest species, living in litter, occasionally found in caves (trogloxene): i.e. subgenus Cornutella (type species L. (C.) cornutum Canestrini & Fanzago, 1878, syn. L. spelaeophila Willmann, 1940);
the subgenus Nicoletiella (type species L. (N.) denticulatum (Schrank, 1776)) with robust species living in soil litter and the upper layers of soil; and
the subgenus Labidostomma, in warmer climatic conditions, gathering the pool of species allied to L. (L.) integrum Berlese, Citation1911 (syn. L. caucasicum Reck, 1940).
Note that some species were collected both outside and inside caves or near cave entrances (i.e. L. (N.) jacquemarti Coineau Citation1964, Grotte de Pouade, France (66)). Although the labidostommids are not rare, populations are often reduced and only few individuals are captured by traditional soil fauna sampling.
The Romanian Labidostommatidae
Iavorschi (Citation1992) described the first specimen of the troglophilic species L. motasi, which was the fifth Romanian species – Eunicolina nova Sellnick, 1931, Labidostomma (N.) denticulata (Schrank, 1776), L. (N.) romanica Feider & Vasiliu, 1968 and the common Mediterranean L. (L.) integrum Berlese, Citation1911 – (Bertrand et al. Citation2012). Each species of this former subgenus shares three characters of great importance in the family: (i) the morphology of the chelicerae, (ii) the “simple” shape of the famulus, a specialized dorsal seta on the tarsi of the first pair of legs, and (iii) the presence of the linear file of pores on the lateral fields of the dorsal shield, these pores being gathered by a sclerotized gutter (Grandjean Citation1942b). By definition, the different genera of the family share a range of characters (plesiomorphies or convergence) but these three characters identify clearly each subgenus, including the fossil species of the genus (Bertrand et al. Citation2012, Citation2015).
Additional data to the description of L. motasi
Habitat, locus typicus. Labidostomma motasi lives in the deep recesses of Movile Cave, discovered in 1986 in the eastern border of Obanu Mare, a collapsed doline at a depth of 18–20 m from the hill’s rocky surface. These mites were collected in a dry cave passage located above the Lake Room in Movile Cave. The lower level of the cave provides access to a groundwater aquifer, rich in hydrogen sulfide, methane and ammonium. Microorganisms oxidize these chemical compounds by producing food in situ by chemoautotrophy. To date, 51 invertebrate species were identified within this cave, with 37 endemic species, making Movile Cave and the local hydrothermal aquifer a hotspot of subterranean biodiversity (Brad et al. Citation2021; Drăgușin et al. Citation2021). Although the upper dry level of this cave is about 200 m long, as most of the terrestrial cave species, L. motasi was solely collected in the close proximity of the sulfidic lake – namely in the “Lake Room”, i.e. where bacteria are present, and consequently where an abundance of food is produced in situ.
General features and outline. Blind species, with oval-shaped idiosoma. Variable size from 450 to 850 µm long from the front (so excluding chelicerae and palps) to the posterior end, and 220–250 µm wide. Thick cuticle colored, brown to light yellow. The shape of the dorsal shield is elongated oval, briefly ended. The front is smooth without cornuae. Appendages are long compared to the body: first and last pairs of legs (PI and PIV) are the longest, longer than the body in complete extension (i.e. PI = 1110 µm for a dorsal shield 660 µm long, PIV = 965 µm long). The legs of the second pair are the shortest. Our specimens were measured rather smaller (420–670 µm) than in the original description due to differences in the preparation of the specimens.
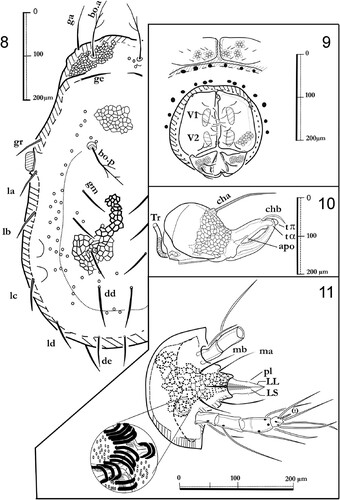
Dorsum: male () and female (). The dorsal shield corresponds to the original description (Iavorschi Citation1992). The shield surface is covered with alveolar pattern, with thicker walls in the central zone than in the lateral ones. On the cleared specimens, in dorsal view, several pores are visible, with few forward to the setae ge, more numerous (more than 20) backward the posterior pair of trichobothrias all around the central zone of the dorsal shield. None neotrichie. The prosoma: in dorsal view, the usual number of setae with the dorsal pairs ga, ge, gr and gm and the two pairs of trichobotries bo.a and bo.p on the prosoma (Grandjean's nomenclature). Except for the trichobothries (with six to eight branches), lateral and dorsal setae are simple. Remarkable development of the frontal pair of setae (90–120 µm) longer than the trichobotrial seta bo.a (80–90 µm) whilst bo.p are about 100 µm long (). The interval between the two setae gm is shorter than the inter-bothridial distance. The pre-ocular setae (gr) are short, and close to the gland-like-organs which are large (60 µm diameter). In lateral view (), this orientation allows observation of the lyrifiorm organ near the gland-like organ. A lateral line of pores is visible, interrupted near the pustule with an anterior branch continued near the posterior bothridia (bo.p) while the posterior branch of the gutter continues all along the dorsal shield from one side to the other.
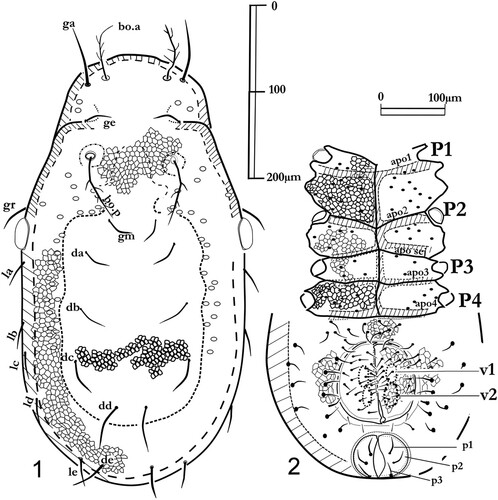
Figures 1, 2. Labidostomma motasi, male. 1, Dorsal shield. 2, Ventral view. On the epimera solely the base of setae are noted, pores are not figured.
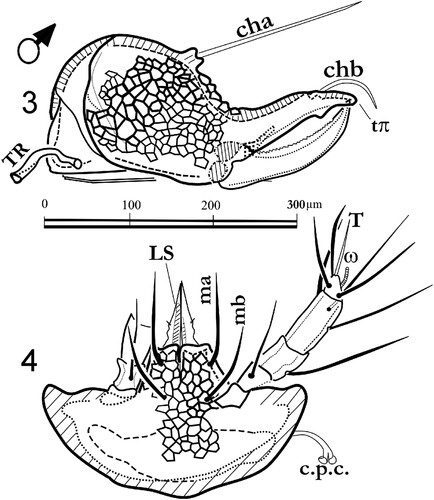
Figures 3, 4. Labidostomma motasi, male. 3, Left chelicera, internal side. 4, Infracapitulum ventral view.
Figures 5–7. Labidostomma motasi, female. 5, Paraxial view of the tarsus I. 6, Leg I, laterodorsal view of tibia and genu. 7, Ocular zone, view of the lateral line of pores.
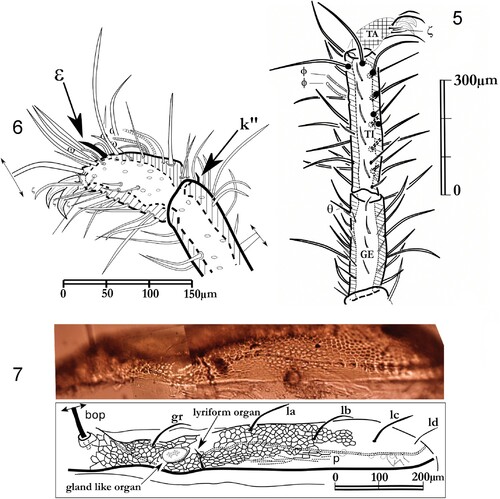
Posterior part of the idiosoma: usual pairs of setae with the dorsal files (da, db, dc, dd, de) and the lateral ones (from la to le). All the setae are smooth, about 70 µm long but the pair (dd) is the longest (about 100 µm).
One female () has been described previously: our specimens corresponded to the original description of the holotype (Iavorschi Citation1992). Some differences remained in the measurements. Notwithstanding the natural individual variations, the general pattern is very similar to that of the males.
Ventral shields. . The ventral surface is highly sclerotized with the four pairs of epimeral plates, the ventral sclerite, the genital and anal sclerites gathered in an ano-genital complex in the female. As in the female the male displays large epimeral plates (syn. coxal plates) unequal in size, the first being the largest and the second being the narrowest along the sagittal axis (in the 660 µm long individual, epimeral plate I with maximal length of 120 µm long, less than 40 µm along the sagittal axis for the second, 100 µm for the third and 70 µm for the epimeral plate IV).
The genital sclerites bear more than 20 setae each, solely 14 for the female. Both male and female exhibit two genital suckers. Anal sclerites provided each with three anal setae.
Infracapitulum and chelicerae. Male: , . Female: , . The infracapitulum is rather large in ventral view (200–300 µm wide) with alveolar ornamentation (, ) and the setae ma and mb 60–95 µm long. The mouth part is provided of a pair of lateral lips each bearing a minute seta (pl) and a superior lip longer than half of the lateral ones. The anterior part of the podocephalic canal (c.p.c.) is inserted dorsally (). The palp is four-segmented with trochanter, femur, genuo-tibia and tarsus as for the genus.
Chaetotaxy of the palps is (1-1-3-4 + ω). All the setae are rather long, notably the femoral seta (>100 µm), but are shorter in males. Dorsally, the tarsal solenidion is well observable.
The chelicerae () are strong, more than 300 µm long, with two long setae cha and chb. The proximal seta is bored by a short tubercle. A movable digit is flanked basally by the “lateral apodeme” (apo) visible on an antiaxial view (). The movable digit is regularly dented. The fixed digit is ended by two teeth (tπ and tα) and a post-terminal recurrent blade. In paraxial and antiaxial views (, ) the chelicerae exhibit a strong ornamentation with alveolar pattern. Tracheal conducts (TR) are inserted as usual on peritremes between chelicerae ().
Genital and anal areas: The male anal and genital apertures are independent. Three anal setae on each anal flap in both sexes. The ano-genital complex in the female is roughly rounded and genital and anal flaps jointed. The shelters are slightly ornamented with alveolar patterns. The genital aperture of the male is sub-circular, each flap having numerous setae (). Both male and female genital area are surrounded by pores all around and underlining the fourth epimeral plates (, ).
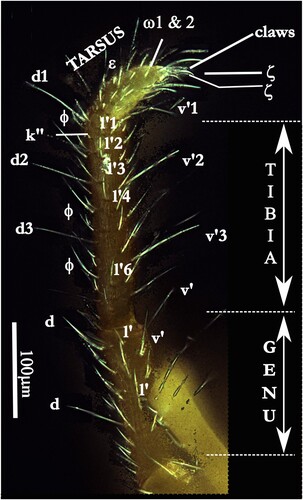
Legs. Legs (, , ) are provided with simple setae. Tarsi I show a double claw surrounded with terminal eupathidia (ζ) (), PII, PIII and PIV with three claws. Among the simple setae, the famulus is dorsal on the tarsi I, forward the two lying solenidia (ω1, ω2) common in the family. Tibia and genu of the first pair of legs are the longest (, ). As in all the species of this genus, the legs are of different lengths (see above). Dorsally, the tibiae are provided with four or five long setae on PI, at least two on PII, PIII and PIV, with smaller ones on para-dorsal files, lateral and para-ventral files. On the ventral surface of the articles, several paired setae are observed but being shorter than the dorsal ones (four on PII, three on PIII. four on PIV). In para-dorsal position, tibial solenidia are present. No alveolae on the cuticle but more or less marked small wrinkles ().
Figures 12, 13. Labidostomma motasi. 12, General silhouette of the fourth leg (antiaxial view). 13, Compared lengths of the articles of the four pairs of legs.
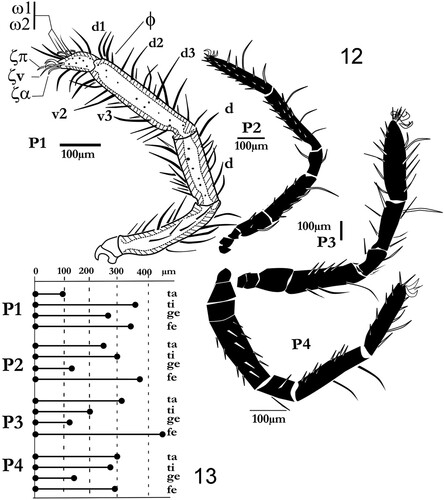
Figure 14. Labidostomma motasi, male. Lateral view of the tarsus, tibia and genu of the first of right leg showing the development of d, dorsal and v, ventral setae. Lateral line of tibial setae is noted (l’1 to l’6).
The epimeral plates are large and well developed, sclerotized and ornamented with polygonal design ().
New data on the genetic identity of L. motasi
The preliminary genetic analysis was conducted on three specimens of this species by comparison with data published in Genbank (Sayers et al. Citation2020). Three sequences from European specimens were available in GenBank for this comparison: two of them corresponded to the species L. (N.) luteum: the first by Dabert et al. (Citation2010), the second by Dr Thomas (pers. comm.) who collected mites from Europe. The third was collected from southern Spain (near Cadiz; M.L. Moraza, pers. comm.) but determined solely to genus level (Arribas et al. Citation2016). The results of the analysis showed () that L. motasi differs clearly from L. luteum and the unknown Spanish species (two species of the genus, distributed on both sides of the Mediterranean Sea are often collected in this region: L. (L.) integrum and L. (C.) cornuta). Hebert et al. (Citation2002) considered that for invertebrates (Lepidopteran families), the intraspecific variability ranges between 0.17% and 0.36% of nucleotide sequence divergence, intra-generic and intra-family variabilities range between 5.8% to 9.1% and 10% to 12.5% respectively. Data available in the literature on mite species show similar or comparable values (Stålstedt et al. Citation2013). On the basis of these results, the main acknowledgment of the analysis is the clear discrimination of the subgenera Nicoletiella represented by L. luteum and Labidostomma (L. integrum and L. motasi).
Discussion
Labidostomma (L.) motasi, a Romanian species found in a sulfidic cave, was initially described from a single female collected in Movile Cave. By different characters (e.g. development of the first pair of legs, large lateral pustule, chelicera morphology, dorsal lip shorter than the lateral lips, famulus simple), it must be considered close to L. integrum and L. intermedia Bertrand, Bagheri, Akbari, Yazdanian, Irani-Nejad, Mohajer & Saboori, Citation2012. These common features were completed by the relative proportions of the article of the legs: as a rule, in the subgenus, tibia and genu of the PI are elongated (in the family tarsus I is rather short if compared to tarsi II–IV), the total length of the distal complex [Tibia + Genu] is larger than the half of the total length of the leg (for L. motasi, the ratio [(Tibia + Genu)/(total length of the leg)] is more than 0.5). The line of pores behind the fourth epimeral plate was also observed in the Iranian species L. (L.) intermedia. Notwithstanding the lack of data, we know that L. (L.) integrum is present in the northern and southern part of the Mediterranean Sea, from the West (Spain, Algeria, Italy; France, …) to Kazakhstan, whereas, to date, L. intermedia, a closely related species, was found solely in Iran (Bertrand et al. Citation2012). Labidostomma intermedia may have been identified as L. integrum by J. Bloszyk on Iranian specimens whereas this author reported on the Turkish presence of L. integrum (Błoszyk et al. Citation2003). These distributions are organized around the long-disappeared shores of the Tethys Sea, very similar to the distribution of the three European species of the genus Eunicolina in Europe around the Mediterranean Basin. As the European fauna has been studied over the past two centuries, we can assess the scarcity or absence of this species in higher latitudes (50°N) or under wet or continental climatic conditions. Labidostomma integrum is known from Romania (Feider & Vasiliu Citation1970; Beron Citation2022). As noted among the other species of the family (Pfliegler & Bertrand Citation2011) (i.e. genus Eunicolina), speciation may have been favored by an isolation not solely spatial but in the subterranean environment. As said in the introduction, a parallel can be made with the Rhagidiid Troglocheles: however, in this case we know which is the closely related species living at the surface: L. integrum!
Adaptation to life in caves has solely changed few features: the differentiation of the length of legs article which was yet noted in several species, the absence of eyes was noted in species living in deep soils (Bertrand & Coineau Citation1979) (even on the rare Mediterranean species L. glymma Grandjean, 1942, until now collected only at the surface). Even if the morphology of the family did not allow a large plasticity, we stated the presence of limited troglomorphisms (Christiansen Citation1962). This is coherent with our knowledge of the family: retaining primitive characters, the labidostommatids maintained throughout time a homogeneous pattern.
About the multiplication of pores
To date, few data are available on the role of gland-like organs and pores, the structures of these organs being similar and of the same origin (Grandjean Citation1942a; Alberti Citation2013). There are several possible roles: inter or intra-specific communication, secretion to protect against predators by creation of a defensive atmosphere, or solely contribution to the elaboration of the epicuticle (Alberti Citation2013). All these hypotheses are congruent with life in caves (where animals are dispersed), and in an adverse environment. In the family, several examples of neotaxy, notably the pustules (considered as homologous of pores according to Alberti Citation2013) are found in the genus Eunicolina or some representative of the genus Labidostomma (i.e. L. jacquemarti Coineau, Citation1964; L. vialeae Bertrand, Citation1982). Therefore, the discovery of males and females in these caves allows both sexual or parthenogenetic reproduction, even if no eggs were seen in the females, and if no immature specimens have been collected to date. Even though the general morphology of the family was preserved, L. motasi can be qualified as a true troglobiont even if the plasticity of the family did not allow severe modifications.
Life as endemic in Movile Cave
Is L. motasi “eutroglophilous”? We stated the hypotheses that a population of one surface species may have migrated to steadily living and reproducing underground and that this species has kept resemblance to the ancestral population (see the definition in Sket Citation2008). This is very close to the definition of vicarious species, a biogeographic term commonly used “horizontally” for different surface species or subspecies or for the subterranean ones distributed in different cavities close geographically (Katz et al. Citation2018).
Ancient isolation can explain emergence of such species: according to a recent publication by Drăgușin et al. (Citation2021), the scarp-related dry valleys in southern Dobrogea resulted from a complex process which can be dated from the Pliocene, and for the deepest and oldest caves during the Messinian Crisis (Miocene) characterized by the lowest level of seas (Mediterranean and Black Seas), i.e. starting 5.97 My ago and lasting for 500,000 years. What were the consequences on the epigean and troglobitic fauna? Climate changes since Pleistocene have placed constraints on the distribution of surface species, but without marked speciation: i.e. the European species may have expanded their distribution from the southern refugia (Błoszyk et al. Citation2017). It is obvious that acquisition of troglomorphisms generally requires a long time: in North America the troglobiontic spiders were collected solely in the southern regions which were not affected by last glaciations and none in the region which was covered by the Laurentide Ice Sheet until 14,000 years ago (Peck Citation1998; Paquin et al. Citation2021). In Movile Cave, where the favorable period extended from the Messinian period, the Labidostommids were (probably) already present (apparition of ancient order of Acariformes is estimated during Paleozoic and most of the family level taxa are thought fixed in the Triassic and Jurassic) (Arribas et al. Citation2019). However, the presence of other labidostommatids in sulfidic caves (Sarbu et al. Citation2022) did not allow attribution of speciation to a chemical adaptation.
In conclusion, how must we consider L. motasi? As a representative of a terrestrial and unspecialized group whose ancestors were probably captured in subterranean cavities in a period with low sea level, and who survived the climatic changes in the sulfidic environment whereas closely related species survived in the surface? The model of general trends in the evolution of Acari being essentially regressive, the main adaptation was restricted to the loss of ocular structures, a character common to deep soil species. Labidostomma motasi represents an old vicarious species of an upper surface species. In the introduction we recalled the studies on the Rhagidiidae and the Troglocheles group: the main difference here is that we can identify L. motasi as a close relative of the surface living species (L. integrum), which was favored by nutritive resources within the sulfidic cave, while the neighboring species L. integrum remained epigeic through all the climatic changes.
Acknowledgements
The authors would like to thank V. Kiss for collecting and for providing detailed photographs of the specimens of L. motasi. We thank too the members of the ‘Group for Underwater and Speleological Exploration’ (GESS group) for their help and assistance with the fieldwork and for providing access to the GESS Lab in Mangalia (Romania) for sample processing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Alberti G. 1998. Fine stricture of receptor organs in Oribatid mites. In: Arthropod Biology; contributions to morphology, ecology and systematics. Ebermann E. (ed). Biosystematics and Ecology Series. 14:27–77. https://www.zobodat.at/pdf/BioEco_14_0027-0077.pdf
- Alberti G. 2013. Fine structure of pustules of Labidostoma luteum Kramer (Acari Actinotrichida, Labidostomatidae) with further remarks on the complex cuticle of this mite. Acarologia. 53(2):129–143. doi:10.1051/acarologia/20132083
- Arribas P, Andujar C, Hopkins K, Shepherd M, Vogler A. 2016. Metabarcoding and mitochondrial metagenomics of endogean arthropods to unveil the mesofauna of the soil. Methods in Ecology and Evolution. 7:1071–1081. doi:10.1111/2041-210X.12557
- Arribas P, Andujar C, Moraza M.-L, Linard B, Emerson B.C, Vogler AC. 2019. Mitochondrial metagenomics reveals the ancient origin and phylodiversity of soil mites and provides a phylogeny of the Acari. Molecular Biology and Evolution, Oxford University Press,
- Bartsch I. 1996. Halacarids (Halacaroidea, Acari) in freshwater. Multiple invasions from the Paleozoic onwards? Journal of Natural History. 30(1):67–99. doi:10.1080/00222939600770051
- Berlese A. 1911. Acarorum species novae quindecim. Redia. 7:429–435.
- Beron P. 2022. Acarorum Catalogus X. Advanced Books. Pensoft & National Museum of Natural History, Sofia: 424 pp. doi:10.3897/ab.e68612
- Bertrand M. 1983. Nouveaux Labidostommidae d’Espagne (Acari: Actinedida). Acarologia. 24(2):159–167.
- Bertrand M. 1989. La durée de développement Un facteur limitant l’extension de l’aire de répartition: le cas de trois espèces européennes de Labidostommidae. In: Ontogenèse et concept de stase chez les arthropodes. Société des Acarologues de langue française, (Banyuls 1986), Lions et André eds. AGAR Publishers (Wavre, Belgium): 123–128.
- Bertrand M, Bagheri M, Akbari A, Yazdanina M, Irani-Nejad H, Mahajer S., Saboori A. 2012. A new Iranian species of the subgenus Labidostomma (Prostigmata: Labidostomatidae) with new biogeographic data on the integrum species group. Acarologia. 52(3):233–245. doi:10.1051/acarologia/20122042
- Bertrand M, Coineau Y. 1979. Une nouvelle forme biologique eu-édaphique d’acarien Akrostomma coralloides n.sp. Labidostomidae aveugle. Vie et Milieu. 28-29(1), série C: 101-110.
- Bertrand M. 1982. Nouvelles espèces de Labidostommidae de Corse. Intérêt biogéographique. Acarologia. 23(1):27–38.
- Bertrand M, Sidorchuk E, Hoffeins C. 2015. Before the summer turns the winter: the third labidostommid genus from Baltic amber has subtropical kin. Acarologia. 55(3):321–336. doi:10.1051/acarologia/20152170.
- Błoszyk J, Błaszak C, Ehrensberger R. 2003. Die milben in des Zoologischen Staatssammlung München. Teil 3. Familie Labidostommidae (Acari, Actinedida). Spixiana 26(2):171–174.
- Błoszyk J, Napierała A. 2020. Endemism of Uropodina Mites: Spurious or Real? Diversity.12:283. doi:10.3390/d12070283
- Błoszyk J, Książkiewicz-Parulska Z, Adamski Z, Napierała A. 2017. Influence of Pleistocene glaciation on the distribution of three species of Labidostomma in Europe (Acari: Labidostommatidae). Systematic and Applied Acarology. 22(6):841–857. doi:10.11158/saa.22.6.9
- Bolton SJ, Bauchan GR. 2022. Caenonychus a senior synonym of Speleorchestes (Acariformes: Nanorchestidae). Systematic and Applied Acarology. 27(2):241–249. doi:10.11158/saa.27.2.6
- Brad T, Iepure S, Sarbu SM. 2021. The chemoautotrophically based Movile Cave groundwater ecosystem, a hotspot of subterranean biodiversity. Diversity. 13:128. doi:10.3390/d13030128
- Christiansen KA. 1962. Proposition pour la classification des animaux cavernicoles. Spelunca. 2:75–78.
- Coineau Y. 1964. Un nouveau Labidostoma à pustule multiple: Labidostoma jacquemarti n.sp. (Labidostomidae, Acari: Prostigmata). Revue d’Ecologie et Biologie des sols. 1:543–552.
- Dabert M, Witalinski W, Kazmierski A, Olszanowski Z, Dabert J. 2010. Molecular phylogeny of acariform mites (Acari, Arachnida): strong conflict between phylogenetic signal and long-branch attraction artifacts. Molecular Phylogenetics and Evolution. 56(1):222–241. doi:10.1016/j.ympev.2009.12.020
- Drăgușin V, Tîrlă L, Covaliov S, Cruceru N, Mirea IC, Şandric I. 2021. The unique topography from Obanul Mare (Mangalia, SE Romania): remnant of a maze cave. Géomorphologie: relief, processus, environnement [on line]. Vol 27(3)2021. Edited 14th september 2021, visited 27/09/2023.URL: http://journals.openedition.org/geomorphologie/15794; doi:10.4000/geomorphologie.15794
- Dunlop JA, Bertrand M. 2011. Fossil Labidostomatid mites (Prostigmata: Labidostommatidae) from Baltic amber. Acarologia. 51(2):191–198. doi:10.1051/acarologia/20112006
- Feider Z, Vasiliu, N. 1970. Espèces de Nicoletiellidae (Acariformes) de Roumanie. In: Livre duCentenaire Emile G. Racovitza (1868-1968), Ed.Académie de la République socialiste de Roumanie. 371–391.
- Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology Oct. 3(5):294–299. PMID: 7881515.
- Grandjean F. 1942a. Observations sur les Labidostommidae (1ere série). Bulletin du Muséum national d’Histoire naturelle Paris. 14(2):118–125.
- Grandjean F. 1942b. Observations sur les Labidostommidae (2e série). Bulletin du Muséum national d’Histoire naturelle Paris. 14(3):185–192.
- Huang JP. 2020. Is population subdivision different from speciation? From phylogeography to species delimitation. Ecology and Evolution. 10:6890–6896. doi:10.1002/ece3.6524
- Hebert PDN, Cwinska A, Ball SL. 2002. Biological identifications through DNA barcodes. Proceedings of the Royal Society, London. B (2003). 270:313–321. doi:10.1098/rspb.2002.2218
- Iavorschi V. 1992. Labidostoma motasi n.sp. (Nicoletiellidae) a new species of mite of Romania. Travaux de l’Institut de Spéléologie "Emile Racovitza", Bucharest. 31:47–51.
- Käser D. 2010. A new habitat of subsurface waters: the hyporheic biotope (translation of Orghidan's 1959 paper). Fundamental and Applied Limnology. 176:291–302. doi:10.1127/1863-9135/2010/0176-0291.
- Katz AD, Taylor SJ, Davis MA. 2018. At the confluence of vicariance and dispersal: Phylogeography of cavernicolous springtails (Collembola: Arrhopalitidae, Tomoceridae) codistributed across a geologically complex karst landscape in Illinois and Missouri. Ecology and Evolution 2018:10306–10325. doi:10.1002/ece3.4507
- Klimov P, O’Connor B, Chetverikov P, Bolton S, Pepato A, Mortazavi M, Tolstikov A, Bauchan G, Ochoa R. 2018. Comprehensive phylogeny of acariform mites (Acariformes) provides insights on the origin of the four-legged mites (Eriophyoidea), a long branch. Molecular Phylogenetics and Evolution. 119:105–117. ISSN 1055-7903, doi:10.1016/j.ympev.2017.10.017. (https://www.sciencedirect.com/science/article/pii/S1055790317303159)
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Molecular Biology and Evolution. 33(7):1870–1874. doi:10.1093/molbev/msw054
- Oudemans AC. 1906. Nieuwe classificatie der Acari. Entomologische Berichten. 2:43–46.
- Paquin P, Aubé M, Brodeur J, Desaulniers CO, Simard C. 2021. Troglobie, troglophile ou trogloxène? Meta ovalis (Gertsch 1933) (Aranae: Tetragnathidae) au Québec. Hutchinsonia 1:21–26.
- Pax F. 1937. Höhlenfauna des Glatzer Schneeberges. Wandlungen des Tierlebens in der Wolmdorfer Tropfsteinhöhle. Beiträge zur Biologie des Glatzer Schneeberges. 3:289–293.
- Peck SB. 1998. A summary of diversity and distribution of the obligate cave-inhabiting faunas of the United-States and Canada. Bulletin of the National Speleological Society. 60:18–26.
- Pepato AR, Klimov PB. 2015. Origin and higher-level diversification of acariform mites – evidence from nuclear ribosomal genes, extensive taxon sampling, and secondary structure alignment. BMC Evolutionnary Biology. 15:178. doi:10.1186/s12862-015-0458-2
- Pfliegler W, Bertrand M. 2011. A new species of Labidostomma for the fauna of Hungary (Acari: Trombidiformes: Labidostommatidae) with an overview of the family. Opuscula Zoologica Instituti Zoosystematici Universitatis Budapestinensis. 42(2):177–183.
- Řezáč M, Růžička V, Dolanský J, Dolejš P. 2023. Vertical distribution of spiders (Araneae) in Central European shallow subterranean habitats. Subterranean Biology. 45:1–16. doi:10.3897/subtbiol.45.95850
- Robaux P. 1976. Some Actinedida (Prostigmata) of the soil of North America. VI. Two new species of Labidostommidae (Acari). Acarologia. 18(3):442–461. doi10.1186/s12862-015-0458-2
- Sarbu SM, Brad T, Chauveau CA, Flot JF, Galassi DMP, Galdenzi S, Gentile G, Iepure S, Jones DS, Martin P, Montanari A, Stoch F. 2022. Sulfidic Habitats in the Frasassi Caves, Italy: A Hotspot of Subterranean Biodiversity. In: The 2nd International Electronic Conference on Diversity (IECD 2022)—New Insights into the Biodiversity of Plants, Animals and Microbes session Animals Diversity (15-31 March 2022). 1-6. doi:10.3390/IECD2022-12384
- Sayers EW, Cavanaugh M, Clark K, Pruitt KD, Schoch CL, Sherry ST, Karsch-Mizrachi I. 2020. GenBank. Nucleic Acids Research. 49 :D92–D96, doi:10.1093/nar/gkaa1023.
- Sidorchuk E, Bertrand M. 2013. New fossil Labidostommatids (Acari: Labidostommatidae) from Eocene Amber and presence of an apustulate species in Europe. Acarologia. 53 (1):25–39. doi:10.1051/acarologia/20132079
- Sket B. 2008. Can we agree on an ecological classification of subterranean animals? Journal of Natural History. 42(21-22):1549–1563. doi:10.1080/00222930801995762
- Stålstedt J, Bergsten J, Ronquist F. 2013. “Forms” of water mites (Acari: Hydrachnidia): intraspecific variation or valid species? Ecology and Evolution. 3(10):3415–3435. doi:10.1002/ece3.704
- Walter DE, Proctor HC. 1998. Feeding behaviour and phylogeny: observations on early derivative Acari. Experimental and Aplied Acarology. 22:39–50.
- Walter DE, Lindquist FE, Smith JM, Cook DR, Krantz G W. 2009. Order Trombidiformes. In: A Manual of Acarology, 3rd edition. Krantz & Walter eds. Lubbock: Texas Tech University Press, Bolton (USA): 233–420.
- Willmann C. 1932. Acari of Südostalpinen Hölen und Karstforsch. Mitteilungen über Höhlen und Karstforschung. 4:158–181.
- Zacharda M. 1979. The evaluation of the morphological characters in Rhagidiidae. In: Rodriguez JG (Ed.) Recent Advances in Acarology (Vol. I). Academic Press, New York. 509–514. doi:10.1016/B978-0-12-592202-9.50073-2
- Zacharda M, Isaiab M, Piva E. 2011. New troglobitic species of the genus Troglocheles (Acari: Prostigmata: Rhagidiidae) from caves in northern Italy and Austria, with a key to adult species of the genus. Journal of Natural History. 45(11-12):641–666. doi:10.1080/00222933.2010.535914