Abstract
Herbicide application may affect dissolved oxygen (DO), dissolved carbon dioxide (CO2), pH, and RpH in ponded water, and RpH of the water which is the water pH aerated with the atmosphere. In the present study, DO concentration did not reach supersaturated state after herbicide application, and variation in DO decreased. The herbicide application reduced the diurnal variation in dissolved CO2 and increased the overall concentration. During the day, before the herbicide application, variations in pH and RpH were large, with pH higher than RpH, allowing absorption of CO2 from the air, whereas at night, the relationship between pH and RpH was inverted. Application of the herbicide caused a reduction in pH and RpH, and RpH exceeded pH throughout the remainder of the day. After the herbicide application, average CO2 concentration in ponded water increased, and RpH was greater than pH, which caused CO2 emission to the air throughout the day. Moreover, the ponded water absorbed oxygen (O2) from the air during the day because DO in the ponded water did not reach saturated concentrations. These facts indicated that the ponded water absorbed O2 from the air and emitted CO2 to the air throughout the day after the herbicide application. Thus, herbicide application affects not only ponded water, but also the surrounding atmosphere.
Introduction
Many physical, chemical, and biological phenomena take place in the ponded water of a paddy field. The water temperature of ponded water is nearly isothermal by convection irrespective of water depth (Rose and Chapman Citation1968; Mowjood et al. Citation1997; Mowjood and Kasubuchi Citation1998a,b), and the dissolved oxygen (DO) varies daily and reaches a supersaturated condition during the day. These events are caused by photosynthesis and respiration of cyanobacteria and algae in the water (Saito and Watanabe Citation1978; Ito and Masujima Citation1980, Citation1984; Ito Citation1987; Mowjood and Kasubuchi Citation1998a,b; Kishida et al. Citation2001). Steemann (Citation1960) reported that in the daytime, absorption of carbon dioxide (CO2) by aquatic plants for photosynthesis and the discharge of OH− ions led to an increase in water pH. The pH in flood water increased with a decrease in dissolved CO2 by photosynthesizing plants and aquatic biota activity during the day, and the pH decreased by respiration at night (Mikkelsen et al. Citation1978). Some reports have examined the change in pH in ponded paddy fields (Ito and Masujima Citation1980, Citation1984; Kishida et al. Citation2001; Usui et al. Citation2003). Usui et al. (Citation2003) reported that paddy-field ponded water absorbed CO2 from the air above the water surface in the daytime by the increase in the pH. Thus, the ponded water and its surrounding environment comprise a variety of physical, chemical, and biological phenomena. It seems that these phenomena are influenced by the activities of cyanobacteria and microalgae that inhabit the ponded paddy field. To understand multiple functions of the ponded paddy field, it is indispensable to make clear the mechanism of the changes in physical and physicochemical conditions in ponded water resulting from the activities of cyanobacteria and microalgae.
Herbicides have remarkably reduced farming labor and contributed to an increase in labor productivity; at the same time, they may be influencing cyanobacteria and microalgae in the ponded water of paddy fields. It is known that farming methods and agricultural chemicals affect organisms inhabiting paddy fields (Ishibashi et al. Citation1983; Roger et al. Citation1994; Simpson et al. Citation1994; Abdullah et al. Citation1997). It has been reported that differences in farming methods affected algal communities in paddy soil (Fujita and Nakahara Citation1999). Thus, herbicide application could influence not only weeds, but also cyanobacteria and microalgae in the ponded water. Kai et al. (Citation1986) reported a reduction in actinomycetes and bacteria after herbicide application. Kamata et al. (Citation1987) reported the populations of actinomycetes, bacteria, and blue-green algae in the herbicide application plot were smaller than those in the control plot during their experiment. The effects of agrochemical applications on aquatic organisms were reviewed by Kimura (Citation2005). In light of these studies, it is possible that the herbicide application influences the environment, which is determined, in part, by activities of living organisms inhabiting ponded paddy fields. One study compared DO in a NaN3-added paddy field with DO in a field without chemicals. The DO in the treated field did not change, but the DO in the field without chemicals varied daily (Mowjood and Kasubuchi Citation1998a). Considering these facts, herbicide application likely influences CO2, DO, pH, and RpH (the pH at which the CO2 concentration of the water is in equilibrium with that of the air). However, we are not aware of any studies on these effects that use successive measurements in paddy fields. Such studies have not been carried out due to a lack of the proper instrumentation until recently. However, information-processing technology and measurement instrumentation have been developed in recent years, and it is now possible to conduct successive measurements of CO2, DO, pH, and RpH in the field. Therefore, the objective of this study was to analyze the effects of herbicide application on CO2, DO, pH, and RpH in ponded water.
Materials and Methods
Experiment site
Experiments were conducted in a paddy field at the experimental farm of Yamagata University, Tsuruoka, Japan. The experimental plot was prepared using conventional practices: tillage, pre-saturation, puddling, and leveling. After preparation, a 2 m × 2 m plot was surrounded by a plastic fence to maintain a water depth of approximately 7.5–9.0 cm throughout the experimental period (September 1–5, 2004). To confirm basic functions in the ponded paddy field, we needed to investigate physical phenomena in the field without rice seedlings; therefore, experiments were conducted in a bare paddy field. We chose this time period mainly in consideration of a continuous five days in September. Before the experiments, no pesticides, germicides, or herbicides were applied. The soil type of this plot is fine-textured gray lowland soils (gray-brown) and soil texture is LiC (light clay).
Herbicide applications
We used two types of granular herbicide that are available on the market and are commonly used: HA [ACN, (2-amino-3-chloro-1,4-naphthoquinon) 9%, granular] and HB (Daimuron 4.5%, Cafenstrole 2.1%, Azimsulfuron 0.06%, Cyhalofop-butyl 1.5%, Bensulfuron-methyl 0.3%, granular) at a ratio of three to one, as used at the Yamagata University experimental farm. The mixed herbicides were applied at 4 g m−2 (40 kg ha−1), which was based on manufacturer instructions (HA 30 kg ha−1 and HB 10 kg ha−1), on the surface of the experimental plot (16 g per 4 m2 plot) at noon on September 2, 2004. The following is the mechanism of herbicidal action for each herbicide: ACN, inhibition of porphyrin biosynthesis (Koura et al. Citation1994); Daimuron, inhibition of seminal root elongation (Ogasawara et al. Citation1991); Cafenstrole, inhibition of elongation at the growing point (Kanzaki et al. Citation2001); Azimsulfuron, inhibition of acetolactate synthase (ALS), which is involved in biosynthesis of branched-chain amino acids (Shirakura et al. Citation1995); Cyhalofop-butyl, inhibition of fatty acid synthesis by activity inhibition of acetyl-CoA carboxylase (ACCase) (Ito et al. Citation1998); Bensulfuron-methyl: inhibition of ALS, which is involved in biosynthesis of branched-chain amino acids (Yuyama et al. Citation1987).
Field measurement of the ponded water
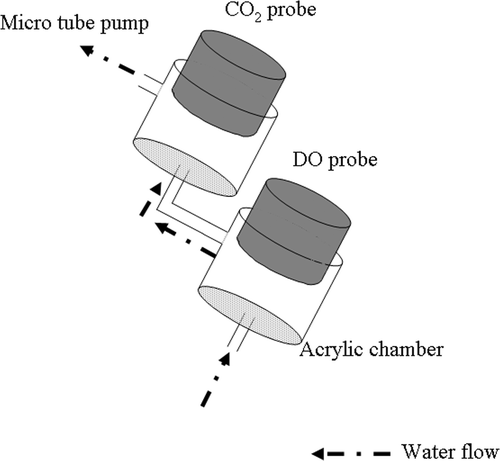
shows a schematic diagram of the system for measuring dissolved CO2, DO, pH, and RpH. For measurement of dissolved CO2 and DO, a CO2 probe (CE-331, DKK · TOA Co., Ltd., Tokyo, Japan) and a DO probe (OE-350AM, DKK · TOA Co., Ltd.) were connected to CO2 (CGP-1, DKK · TOA Co., Ltd.) and DO (DDIC-7, DKK · TOA Co., Ltd.) meters, respectively. Each instrument was connected to a data-acquisition system (GK-88, ESD Co., Ltd., Tokyo, Japan), and each data point was input into a personal computer (PC) at 15-minute intervals. To successively measure dissolved CO2 and DO in the field, we applied a tube-pump sampling method (Mowjood and Kasubuchi Citation1998a) () and sampled water from 2 cm above the soil surface. Mowjood and Kasubuchi (Citation1998a) reported that the DO concentration was uniform throughout the water layer. Therefore, the DO concentration in sampled water does not depend on the sampling point. The sampled water from the field was introduced continuously into acrylic chambers by a micro-tube pump (MP-3, EYELA Co. Ltd., Tokyo, Japan). The sampled water passed through the DO probe and the CO2 probe.
Figure 1. Schematic diagram of the system for measurement of dissolved carbon dioxide (CO2), dissolved oxygen (DO), pH, and RpH. PC, personal computer.
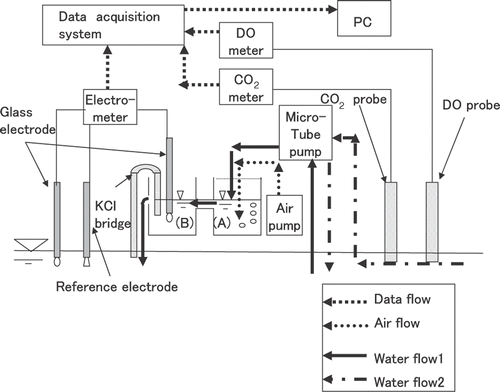
Figure 2. Schematic diagram of the tube-pump sampling method. CO2, carbon dioxide; DO, dissolved oxygen.
RpH is defined as the pH at which the CO2 concentration of the water is in equilibrium with that of the air (e.g. Hanya and Ogura Citation1995). Usui et al. (Citation2003) reported a simultaneous measurement system for pH and RpH in the field to determine whether the water is supersaturated or unsaturated with atmospheric CO2 by a comparison of the pH and RpH values. To measure pH and RpH simultaneously in the field, we adopted the method by Usui et al. (Citation2003). For RpH, the water was drawn into a small chamber (chamber A) through a micro-tube pump (MP-3, EYELA Co., Ltd.). Simultaneously, air drawn from just above the water surface was aerated in the water of chamber A. The aerated water was then introduced into the adjacent chamber (chamber B) through a connecting tube. An agar bridge saturated with potassium chloride (KCl) maintained an electrical connection between the water in chamber B and the ponded water. The RpH was measured with a glass electrode (HGS-2005, DKK · TOA. CO., Ltd.) in chamber B. A second glass electrode was set in the ponded water to measure the pH. The reference electrode (HS-205C, DKK · TOA. CO., Ltd.) was also set in the ponded water connected to an electrometer (ODIC-7, DKK · TOA. CO., Ltd.). The RpH and pH were measured continuously and averaged every 15 minutes. The data were stored in the PC ().
The temperature of the ponded water was measured with a thermocouple installed 2 cm above the soil surface. This temperature was used to estimate the saturated DO concentration (sat DO) using a conversion table (e.g. Hanya and Ogura Citation1995), which showed the relationship between temperature and sat DO.
Other measurements
Solar radiation was measured with a pyranometer (CM3 Pyranometer, Kipp and Zonen Inc., Delft, Netherlands) and recorded with a data logger (CR10X, Campbell Scientific Inc., Logan, UT, USA).
Results and Discussion
Effect on diurnal variations in DO
shows diurnal variations and changes in DO and sat DO in the ponded water before and after herbicide application. Before the herbicide application (12:00, September 1, September 2), DO concentration in daytime exceeded sat DO (20°C, 0.276 mM). Therefore, the ponded water was supersaturated with respect to DO, and DO decreased to a minimum at night. Thus, before the herbicide application, diurnal variation of DO in the ponded water corresponded to variation in solar radiation. These results indicate photosynthesis and respiration by cyanobacteria and algae (Saito and Watanabe Citation1978; Ito and Masujima Citation1980, Citation1984; Ito Citation1987; Mowjood and Kasubuchi Citation1998a,b; Kishida et al. Citation2001).
Figure 3. Diurnal variations and changes in dissolved oxygen (DO), saturated (sat) DO, carbon dioxide (CO2), pH, RpH, and solar radiation before and after the herbicide application.
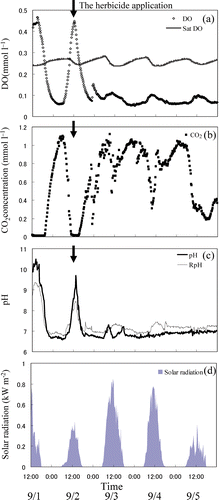
After the herbicide application on September 3, as this was a day with higher solar radiation (), DO should have increased during the day. However, DO on September 3 did not increase beyond sat DO in the daytime and had a smaller decrease corresponding to lack of solar radiation at night than before the herbicide application. Thus, DO in the ponded water did not vary as much after the herbicide application. If the herbicide application directly influences DO in ponded water, DO in ponded water must be decreased with the herbicide application. Therefore, these facts indicated that the herbicide application inhibited the photosynthesis and reduced the population of cyanobacteria and microalgae. It was reported that a number of actinomycetes, bacteria, and blue-green algae in the herbicide-applied area decreased compared with non-treated fields (Kai et al. Citation1986; Kamata et al. Citation1987). Yamazaki et al. (Citation2003) suggested that the growth of Chlorococcales (phytoplankton) and Lemnaceae (macrophyte) would be inhibited by herbicide during the early flooding period. Thus, herbicide application could also reduce the population of cyanobacteria and microalgae.
Herbicide application could affect not only DO diurnal variation in ponded water, but also O2 exchange across the air–water interface. Mowjood and Kasubuchi (Citation1998a) reported that water supersaturated with O2 released O2 due to convection. However, as the DO of the ponded water did not reach saturation after the herbicide application throughout the day, it should absorb O2 from the air throughout the day. Mowjood and Kasubuchi (Citation2002) reported that unsaturated DO in ponded water could absorb O2 from the air in the vicinity of the ponded water surface, and O2 re-aeration and de-aeration coefficients for the field ranged from 2.1 to 2.4 and from 5.7 to 6.3 d−1, respectively. Because of the unsaturated DO state throughout the day, the ponded water could absorb O2 during the day. The daily change in DO after herbicide application was small, probably because of photosynthesis inhibition by the herbicide application and absorption of O2 from air above the water surface. The failure of DO in the ponded water to reach 0 mM was believed to be caused by absorption of O2 from air.
Effect on the diurnal variation in dissolved CO2
shows the diurnal variation and the change in CO2. Before the herbicide application, the CO2 concentration in the ponded water was almost 0 mM in the daytime. With a decrease in solar radiation, CO2 concentration in the ponded water increased and reached a maximum at night. Thus, diurnal variation in CO2 responded to changes in solar radiation before the herbicide application. After the herbicide application, the diurnal variation in CO2 concentration was reduced, and the minimum concentration of CO2 increased. It seems that the high CO2 concentration during the daytime and the reduction in variation after herbicide application were caused by a decrease in CO2 consumption owing to the inhibition of photosynthesis. The herbicide application could lead to changes in CO2 concentration by the reduction of cyanobacteria and microalgae. Therefore, CO2 concentration in the ponded water may increase during the daytime. However, it seems that the decreasing efficiency of the herbicide application toward the end of the experimental period could lead to a reduction in the minimum CO2 concentration in the daytime.
Effect on pH and RpH in ponded water
Usui et al. (Citation2003) reported that CO2 concentration is related to pH in ponded water and that comparing pH with RpH confirmed whether the water had absorbed or emitted CO2. Thus, we investigated the pH and RpH of ponded water before and after the herbicide application.
shows diurnal variations and changes in pH, RpH, and response to solar radiation in ponded water before and after herbicide application. Before herbicide application, diurnal variations in pH and RpH corresponded to the daily change in solar radiation, i.e., pH increased in the daytime and decreased at night, so that the water was alkaline during the day and weakly acidic at night. RpH of the ponded water increased during the day and decreased at night, and it was lower than pH during the day and higher at night. This indicates that, before the herbicide application, the ponded water could absorb CO2 from the air during the day and emit CO2 to the air at night.
However, after the herbicide application, the pH in the ponded water did not increase during the daytime, and notwithstanding the increase in solar radiation, the water was weakly acidic throughout the day. This indicated that the decrease in pH was caused by the depression of CO2 consumption owing to the inhibition of photosynthesis. Because CO2 concentration in ponded water was influenced by the herbicide application, the decrease in pH, as well as DO and CO2, might be indirectly influenced by the herbicide application. Moreover, the RpH did not change significantly throughout the day and remained neutral during the experimental period after the herbicide application. Both pH and RpH gradually increased with time. Considering these facts, it is concluded that the application of herbicide affected not only DO and CO2 in the ponded water, but also atmospheric O2 and CO2 in the vicinity of the water surface.
Further studies should be carried out to evaluate the population of cyanobacteria and microalgae and to quantify the flux and balance of CO2 at the surface of the ponded water and their association with herbicide application.
Conclusion
The physical phenomena in the ponded paddy field were influenced by the herbicide application. Diurnal variation in DO, CO2, pH, and RpH in the ponded water varied diurnally before the herbicide application. However, after the herbicide application, the variations became smaller. The ponded water emitted O2 in the daytime and absorbed O2 at night before the herbicide application, but after the herbicide application, DO did not increase, the water did not become supersaturated, and the ponded water absorbed O2 throughout the day.
In the case of CO2, after the herbicide application, CO2 concentration in ponded water increased, and comparisons between pH and RpH indicated that the ponded water constantly emitted CO2 to the air.
These results were caused by reduction in the activities of cyanobacteria and microalgae in the water due to the herbicide.
Acknowledgments
The authors thank staff members of the experimental farm of Yamagata University for their maintenance of the paddy field. They also thank two anonymous reviewers for their helpful comments on improving the paper.
References
- Abdullah , AR , Bajet , CR , Martin , MA , Nhan , DD and Sulaiman , AH . 1997 . Ecotoxicology of pesticide in the tropical paddy field ecosystem . Environ. Toxicol. Chem. , 16 : 59 – 70 .
- Fujita , Y and Nakahara , H . 1999 . Effects of cultivation conditions on algal communities in paddy soils . Jpn. J. Limnol. , 60 : 77 – 86 . (in Japanese with English summary)
- Hanya , T and Ogura , N . (1995): Investigating Methods for Water Quality, third edition, pp. 188–192, 215. Maruzen, Tokyo (in Japanese)
- Ishibashi , N , Kondo , E and Ito , S . 1983 . Effects of application of certain herbicides on soil nematodes and aquatic invertebrates in rice paddy fields in Japan . Crop. Prot. , 2 : 289 – 304 .
- Ito , A . 1987 . Change of water temperature, pH, dissolved oxygen, inorganic nitrogen and phosphorus concentrations in flowing irrigation water on paddy surface . Soil Sci. Plant Nutr. , 33 : 449 – 459 .
- Ito , M , Kawahara , H and Asai , M . 1998 . Selectivity of Cyhalofop-butyl in Poaceae species . J. Weed Sci. Tech. , 43 : 122 – 128 .
- Ito , A and Masujima , H . 1980 . Studies on the purification of polluted water in paddy field: 1. The change of N and P concentration in the surface flow . J. Sci. Soil Manure. Jpn. , 51 : 478 – 486 . (in Japanese)
- Ito , A and Masujima , H . 1984 . The change of dissolved inorganic nitrogen and phosphorus concentrations in running water on the surface of planting and fallow paddy field . J. Sci. Soil Manure. Jpn. , 55 : 123 – 128 . (in Japanese)
- Kai , H , Kamata , M , Kawaguchi , S and Kanayama , H . 1986 . Effects of herbicide applications on microbial ecosystem and fertility of paddy soil. I. Seasonal changes of Microflora affected by repeated applications of herbicides in paddy soil . Sci. Soil Manure. Jpn. , 57 : 535 – 543 . (in Japanese)
- Kamata , M , Kai , H , Kawaguchi , S and Kawachino , K . 1987 . Effect of herbicide application on microbial ecosystem and fertility of paddy soil (Part 2): Seasonal changes of the microbial N2-fixing activities and the responsible Miroflora affected by the repeated applications of herbicide in paddy soil . Sci. Soil Manure Jpn. , 58 : 517 – 527 . (in Japanese)
- Kanzaki , M , Takeuchi , M and Shirakawa , N . 2001 . Factors influencing the herbicidal activity of Cafenstrole against Echinochloa oryzicola vasing in paddy fields . J. Weed Sci. Tech. , 46 : 25 – 30 . (in Japanese with English summary)
- Kimura , M . 2005 . Populations, community composition and biomass of aquatic organisms in the floodwater of rice fields and effects of field management . Soil Sci. Plant Nutr. , 51 : 159 – 181 .
- Kishida , T , Iwata , T , Miura , T , Ohtaki , E , Nishimura , K , Higuchi , Y , Ohtou , A and Miyata , A . 2001 . Factors affecting the diurnal variation of carbon dioxide concentration in standing water in a rice field . J. Agric. Meteorol. , 57 : 117 – 126 .
- Koura , S , Takasuka , K , Katsuyama , N , Ogino , C , Sato , Y and Wakabayashi , K . 1994 . Mode for herbicide action 2-amino-3-chloro-1,4-naphthoquinone (ACN) . Biosci. Biotech. Biochem. , 58 : 1210 – 1212 .
- Mikkelsen , DS , De Datta , SK and Obcemea , WN . 1978 . Ammonia volatilization losses from flooded rice soil . Soil Sci. Soc. Am. J. , 42 : 725 – 730 .
- Mowjood , MIM , Ishiguro , K and Kasubuchi , T . 1997 . Effect of convection ponded water on the thermal regime of a paddy field . Soil Sci. , 162 : 583 – 587 .
- Mowjood , MIM and Kasubuchi , T . 1998a . Dynamics of dissolved oxygen (DO) in the ponded water of a paddy field . Soil Sci. Plant Nutr. , 44 : 405 – 413 .
- Mowjood , MIM and Kasubuchi , T . 1998b . Heat and dissolved oxygen (DO) transfer phenomenon in ponded paddy field . Soil Phys. Cond. Plant Growth, Jpn. , 79 : 41 – 47 .
- Mowjood , MIM and Kasubuchi , T . 2002 . Effect of convection on the exchange coefficient of oxygen and estimation of net production rate of oxygen in ponded water of a paddy field . Soil Sci. Plant Nutr. , 48 : 673 – 678 .
- Ogasawara , M , Ogawa , S , Takeuchi , Y and Konnai , M . 1991 . Effects of Dimepiperate and Daimuron on inhibition of seminal root elongation by chloroacetanilide herbicides . Weed Res., Jpn. , 36 : 294 – 298 .
- Roger , PA , Simpson , IC , Oficial , R , Ardales , S and Jimenez , R . 1994 . Effects of pesticide on soil and water microflora and mesofauna in wetland ricefields: A summary of current knowledge and extrapolation to temperature environments . Aust. J. Exp. Agric. , 34 : 1057 – 1068 .
- Rose , CW and Chapman , AL . 1968 . A physical analysis of diurnal temperature regimes in clear and turbid water layer: A problem in rice culture . Agric. Meteorol. , 5 : 391 – 409 .
- Saito , M and Watanabe , I . 1978 . Organic matter production in rice field flood water . Soil Sci. Plant Nutr. , 24 : 427 – 440 .
- Shirakura , S , Ito , K , Barefoot , AC , Aizawa , H and Ishizuka , K . 1995 . Differences in absorption, translocation and metabolism of Azimsulfuron between rice (Oryza sativa) and flat sedge (Cyperus serotinus) . Weed Res., Jpn. , 40 : 299 – 307 .
- Simpson , IC , Roger , PA , Oficial , R and Grant , IF . 1994 . Effects of nitrogen fertilizer and pesticide management on floodwater ecology in a wetland ricefield . Biol. Fertil. Soils , 17 : 129 – 137 .
- Steemann , NE . 1960: Uptake of CO2 by the plant. In: Handbuch der Pflanzenphysiologie 5, pp. 70–84, Springer-verlag, Berlin
- Usui , Y , Mowjood , MIM and Kasubuchi , T . 2003 . Absorption and emission of CO2 by ponded water of a paddy field . Soil Sci. Plant Nutr. , 49 : 853 – 857 .
- Yamazaki , M , Hamada , Y , Kamimoto , N , Momii , T , Aiba , Y , Yasuda , N , Mizuno , S , Yoshida , S and Kimura , M . 2003 . Changes in the community structure of aquatic organisms after midseason drainage in the floodwater of Japanese paddy fields . Soil Sci. Plant Nutr. , 49 : 125 – 135 .
- Yuyama , T , Ackerson , RC and Takeda , S . 1987 . Uptake and distribution of Bensulfuron methyl (DPX F5381) in rice . Weed Res., Jpn. , 32 : 173 – 179 .