Abstract
Boron (B) deficiency is widespread and serious in navel orange orchards in southern Jiangxi in China and has been considered an important soil constraint to citrus yield and quality. It has been observed that there was great variation in responses to different B supply levels between “Newhall” navel orange grafted on citrange and trifoliate orange. The aim of this work was to compare the different responses of the two scion-rootstock combinations under different B stresses (low and high) in young citrus trees. “Newhall” navel orange grafted on citrange or trifoliate orange were incubated in nutrient solution containing different levels of B (0, 0.5 and 5.0 mg L−1), of which 0 and 5.0 mg L−1 were considered as deficient and excessive B and 0.5 mg L−1 was considered as an appropriate level of B. The experiment was carried out in a greenhouse for 117 days. The results showed that, under low B or high B conditions, better growth performance was observed in “Newhall” plants grafted on citrange than in those grafted on trifoliate orange, suggesting that citrange-grafted plants were more tolerant to deficient and excessive B than the plants grafted on trifoliate orange. Boron seemed to be preferentially translocated to the new leaves (leaves from current year), and resulted in normal growth of young leaves under limited B supply. Boron distribution in different plant parts indicated that there was a restriction in translocation of B from roots to scion tissues (stems and leaves of scion) under limited B availability. The relatively high B concentration observed in plants grafted on trifoliate orange suggested higher demand for B than those grafted on citrange. There existed a balance within the B forms to maintain normal growth. Thus, R value (R = semi-bound B/free B) was much higher in citrange-grafted plants than in trifoliate orange-grafted plants under the same B supply level. This explained why citrange-grafted plants had less B but produced relatively more dry mass at cellular level. These results suggested that the utilization efficiency of B of citrange-grafted plants was probably greater than trifoliate orange-grafted plants.
Introduction
Boron (B) is an essential micronutrient element required for growth and development of higher plants (Loomis and Durst Citation1992). It plays important roles in stability of cell walls (Matoh Citation1997) and cellular activities (Cakmak and Römheld Citation1997). Boron deficiency in plants may restrain root elongation through limiting cell enlargement and cell division in the growing zone of root tips, and possibly impede leaf expansion due to the decrease of the photosynthetic capacity (Dell and Huang Citation1997). Factors affecting B availability to plants are solution pH, texture, moisture, temperature, organic matter, and clay mineralogical characteristics (Shorrocks Citation1997). According to the soil B maps of China (Liu et al. Citation1980, Citation1989), large areas in south and east of China contain very low level of hot water soluble B (<0.25 mg kg−1).
Ganzhou city in Jiangxi province in south China is a predominant region of naval orange production, and plays an important role in citrus production in China. However, leaf yellowing a symptom of B deficiency occurs in main local cultivar “Newhall” navel orange (Citrus sinensis Osb.), which accounts for over 90% of citrus cultivation area. This symptom is especially prevalent in the period of fruit enlargement (Jiang et al. Citation2009). Some more serious symptoms include deformation in immature leaves and corky split veins in mature leaves as well as early leaf senescence. As a result, the tree vigour declines rapidly after fruit set, and therefore affects fruit yield and quality in the coming years (Xiao et al. Citation2007). Interestingly, the majority of orange plants with B deficiency symptoms are trifoliate orange, while citrange in the same region have little or no B deficiency symptoms. The different performance to B indicates the difference of B efficiency of “Newhall” navel orange plants grafted on the two rootstocks. A wide range of genotypic variations in B efficiency has been found in numerous species of crops and other domesticated plants (Rerkasem and Jamjod Citation1997). Variation in B efficiency in plant species and genotypes may be due to their different mechanisms and ability to acquire B from the soil and to distribute and utilize B in the plant (Marschner Citation1995). Taylor and Dimsey (Citation1993) found a strong effect of rootstocks of navel citrus on B concentration in orange leaves. Boaretto et al. (Citation2008) studied the variation of Valencia sweet orange trees budded on Rangpur lime or Swingle citrumelo in response to B and suggested B concentration in Swingle was higher than that in Rangpur. And rootstock can also greatly affect the scion's tolerance to B toxicity (Papadakis et al. Citation2004) and deficiency (Boaretto et al. Citation2008). In addition, interaction of scion-rootstock combination was also reported in other plants, such as melon (Edelstein et al. Citation2005), apple (Wojcik et al. Citation2003), olive (Chatzissavvidis et al. Citation2008), and pear (Sotiropoulos et al. Citation2006).
Trifoliate orange [Poncirus trifoliata (L.) Raf.] and citrange [Citrus sinensis (L.) Osb. × P. trifoliata (L.) Raf.] are considered two important rootstock resources (Forner-Giner et al. Citation2003). However, little is known about their adaptability to different B levels. The objectives of this work were: (a) to evaluate the effects of different B levels on plant growth and the distribution, accumulation and forms of B in Newhall navel orange plants grafted on trifoliate orange and citrange rootstocks; (b) to compare the differential responses of the two scion-rootstock combinations under high and low B stress.
Materials and Methods
Plant material and culture
In this study, we used “Newhall” navel orange (Citrus sinensis Osb.) grafted on rootstocks of trifoliate orange [Poncirus trifoliata (L.) Raf.] and citrange [C. sinensis (L.) Osb. × P. trifoliata (L.) Raf.]. At budding time, one-year-old seedlings were selected from trifoliate orange and citrange rootstocks with uniform stem diameter of 4–5 mm. Both virus-free seedlings and bud sticks used in the present experiment were harvested from the Fruit Bureau of Ganzhou city, Jiangxi Province, China. The rootstock-scion combination plants had cultured for 14 months at the same nutrient matrix before being used in this study.
Nutrient solution culture method was used in this study. All the plants were washed with distilled water to remove surface contaminants after two-day starvation of tap water, followed by transplantation to black pots (one plant per pot), each containing 8-L of nutrient solution. The black pots were immersed in 1 mol L−1 HCl, and washed with distilled water prior to the experiment. The composition and content of salts of the culture solution were as follows (in mg L−1): KNO3, 202; Ca(NO3)2 · 4H2O, 578; MgSO4 · 7H2O, 500; Na2HPO4 · 12H2O, 100; NaH2PO4 · 2H2O, 100; MnCl2 · 4H2O, 1.81; ZnSO4 · 7H2O, 0.22; CuSO4 · 5H2O, 0.08; Na2MoO4, 0.09; EDTA-Fe, 48.5. Two B concentrations were supplied at 0.5 mg L−1 (B05, control treatment) and 5.0 mg L−1 (B50, excessive B) using boric acid, accompanied with a treatment without B (B0, deficient B). The culture solution was aerated for 10 minutes one day, and renewed every eight to 10 days. The nutrient solution was half the strength at the first 16 days, and then was subsequently changed to full-strength. The pH of the solution was adjusted to about six with 0.5 M H2SO4 or 1 M NaOH.
The plants were placed in a greenhouse under natural conditions at Huazhong Agricultural University, Wuhan, China. The treatments started at the end of April in 2009 and the experiment was terminated after 117 days.
Determination of boron forms
Boron forms were extracted by the method described by Du et al. (Citation2002). In this method, a batch of 3 g of fully expanded fresh spring-flush leaves (after treatments for 90 days) was cut into pieces of approximately 1 mm2, and was put into a clean and dry plastic bottle with 15 mL distilled water. The bottle was shaken at 100 rpm at 25°C in a water bath for 24 h. The mixture was then filtered through 0.15 mm gauze and quantitative filter paper, respectively. The supernatant was collected to determine B concentration as free B form. The residue was added with 10 mL 1 mol L−1 NaCl, and shaken at 100 rpm at 25°C in the water bath for 24 h. After filtration, the supernatant was determined for B as semi-bound B form. The residue was then added with 10 mL 1 mol L−1 HCl, and shaken at 100 rpm at 25°C in the water bath for 24 h. The B in the supernatant was bound B.
The ratio of semi-bound B/free B (R) represents the ability of B from apoplast pass membrane to cytoplasm, and thus the ratio can be considered as an indicator of B utilization efficiency in cells (Du et al. Citation2000).
Sampling, plant analysis, and boron determination
When the plants were harvested, the leaves and stem of scion and root and stem of rootstock were separately sampled. The leaves were further separated into mature leaves (fully expanded at the beginning of the experiment) and young leaves (fully expanded at harvest, but not existing at the beginning of the experiment). All the samples were washed initially with tap water, and afterwards with double deionized water three times. The samples were oven dried at 70°C for three days. Each dry sample was ground to fine powder and stored in an air-tight glass container for subsequent analyses.
For measurement of B concentration, 0.12 g of the powdered sample was put into a polyethylene bottle containing 20 mL 1 mol L−1 HCl, then shaken for 2 h at the rate of 180 rpm to extract B. The concentration of B in the digested solution was determined using the colorimetric curcumin method (Bao Citation1999).
Boron distribution was expressed as percentage of B content (concentration × dry weight) in one plant part to the total plant B content.
Boron utilization index (in g mg−1) represents B utilization ability at whole plant level, and it is calculated by the amount of biomass per unit B, i.e. whole plant biomass (in grams)/total B accumulation (in milligrams) (Siddiqi and Glanss Citation1981).
Experimental design and statistical analysis
A completely randomized 2 × 3 factorial design was used for the two rootstocks (trifoliate orange and citrange) and three B treatments (0, 0.5, and 5.0 mg L−1), each containing three replications (one plant in each replicate). In total, 18 pots (or plants) were used in this experiment.
The data was subjected to two-way analysis of variance (ANOVA) using the SAS (SAS Institute Inc. Citation1996), and the differences were compared by employing the Tukey test with a significance level of P < 0.05.
Results
Plant growth and symptoms
Water-soaked spots and unequal leaf expansion were observed in young leaves (leaves that were not fully expanded) of the plants grafted on both rootstocks of trifoliate orange and citrange under deficient boron (B0). However, these symptoms were gradually eased with the growth of leaves. At the same B0 condition, the symptoms of curling of the leaves, corking of the veins and extensive defoliation were observed in old leaves (the basal leaves) of the plants grafted on trifoliate orange, but not on citrange (). High B supply (B50), however, resulted in B toxicity, symbolized by leaf tip and margin burn in leaves of plants grafted on trifoliate orange, of which the symptoms appeared to be more serious than those on citrange (). None of the above symptoms was observed in the plants supplied with normal quantity of B (B05).
Figure 1. Performance of “Newhall” navel orange plants grafted on trifoliate orange and citrange at the treatment B0 (no boron) after 117 days. Left: citrange, right: trifoliate orange.
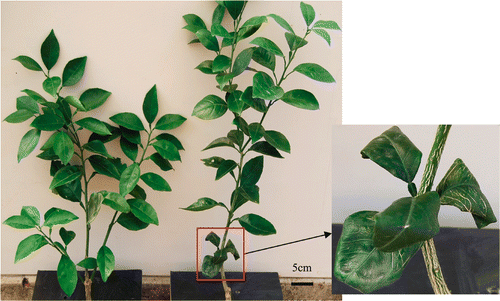
Figure 2. Performance of “Newhall” navel orange plants grafted on trifoliate orange and citrange at the treatment B50 (excessive boron) after 117 days. Left: citrange, right: trifoliate orange.

There was no significant difference in dry mass in various parts of the young “Newhall” navel orange (Citrus sinensis Osb.) plants between the no-B (B0) treatment and the normal-B (B05) treatment, regardless of types of rootstock (). In comparison, the dry mass of various parts of plants decreased under high-B (B50) supply compared to normal-B (B05) supply grafted on both the two rootstocks (). As shown in , the dry mass of rootstock parts and scion parts did not decrease significantly for B0, but the dry mass of scion parts decreased to a larger extent than that at B50. And the dry weight of mature leaves grafted on trifoliate orange showed a larger decrease than those grafted on citrange in response to excessive boron (B50).
Table 1. Dry mass (grams per plant) of different parts of “Newhall’ navel orange plants grafted on trifoliate orange and citrange with different boron treatments [treatment without boron (B0, no boron), boron concentrations at 0.5 mg L−1 (B05, control treatment) and 5.0 mg L−1 (B50, excessive boron) using boric acid]
Boron concentration in different plant parts
Leaves contained more B than the other plant parts, but they also showed greater reduction than the other plant parts as B supply was decreased (). Boron concentration in new leaves was much higher than in mature leaves, regardless of the types of rootstock or B treatments. When 5.0 mg L−1 B was supplied, B concentrations in various parts in citrange-grafted plants were higher than those when 0.5 or 0 mg L−1 B was supplied. There was no significant difference between B0 and B05 treatments, except for the mature and young leaves. Different B supply greatly affected B concentrations in various parts in trifoliate orange-grafted plants (), which were higher than those in citrange-grafted plants at the same B supply level, except for the roots at the B0 treatment, stem of scion at B0 and B05 treatments, mature leaves at the B0 treatment and new leaves at the B50 treatment ().
Table 2. Boron concentration (in mg kg−1) of different parts of “Newhall” navel orange plants grafted on trifoliate orange and citrange with different boron treatments [treatment without boron (B0, no boron), boron concentrations at 0.5 mg L−1 (B05, control treatment) and 5.0 mg L−1 (B50, excessive boron) using boric acid]
Compared to the B concentration in trifoliate orange-grafted plants, the B concentration in various parts in citrange-grafted plants decreased slightly from B05 to B0, but was increased greatly from B05 to B50 ().
Boron accumulation, distribution and utilization index
Boron contents (concentration × dry weight) in various parts of both grafted plants increased significantly as B supply elevated, except for the no difference in root and rootstock's parts (root and stem of rootstock) of citrange between B05 and B0 (). Leaves retained as the dominant organs of B accumulation, regardless of the types of rootstock and B treatments (). In general, plants grafted on trifoliate orange contained a relatively higher B content than trees grafted on citrange (). shows the effects of B treatments and rootstocks on the distribution of B in different parts of “Newhall” plants. As B supply was increased from 0 to 5.0 mg L−1, the fractions of total plant B content in leaves and scion's parts increased, while the total B content in roots and rootstock's parts decreased. Under B deficiency (B0), leaves and scion's parts of citrange-grafted plants contained 60% and 71% of the total B, which were less than those of trifoliate orange-grafted plants (70% and 77%). However, plants grafted on citrange rootstock contained more B in roots (14%) and rootstock's parts (29%) than those grafted on trifoliate orange (13% in roots and 23% in rootstock's parts). There was no significantly difference between the two rootstocks at the high B treatment.
Figure 3. Boron accumulation (in milligrams per plant) in (A) root, (B) rootstock's parts (root and stem of rootstock), (C) leaves, (D) scion's parts (leaves and stems of scion) and (E) whole plant of “Newhall” navel orange plants grafted on trifoliate orange and citrange with different boron treatments [B0 (no boron), B05 (control treatment) and B50 (excessive boron)]. Values are means of three replicates ± standard error. Bars with different letters are significantly different at P < 0.05.
![Figure 3. Boron accumulation (in milligrams per plant) in (A) root, (B) rootstock's parts (root and stem of rootstock), (C) leaves, (D) scion's parts (leaves and stems of scion) and (E) whole plant of “Newhall” navel orange plants grafted on trifoliate orange and citrange with different boron treatments [B0 (no boron), B05 (control treatment) and B50 (excessive boron)]. Values are means of three replicates ± standard error. Bars with different letters are significantly different at P < 0.05.](/cms/asset/0612526e-fe96-44cf-9e78-a090d7f6111f/tssp_a_551299_o_f0003g.gif)
Figure 4. Fractions (in percentages) of total plant boron content in (A) root, (B) rootstock's parts (root and stem of rootstock), (C) leaves and (D) scion's parts (leaves and stems of scion) of “Newhall” navel orange plants grafted on trifoliate orange and citrange with different boron treatments [B0 (no boron), B05 (control treatment) and B50 (excessive boron)]. Values are means of three replicates ± standard error. Bars with different letters are significantly different at P < 0.05.
![Figure 4. Fractions (in percentages) of total plant boron content in (A) root, (B) rootstock's parts (root and stem of rootstock), (C) leaves and (D) scion's parts (leaves and stems of scion) of “Newhall” navel orange plants grafted on trifoliate orange and citrange with different boron treatments [B0 (no boron), B05 (control treatment) and B50 (excessive boron)]. Values are means of three replicates ± standard error. Bars with different letters are significantly different at P < 0.05.](/cms/asset/4d13cc32-4b87-4d4d-9477-8c4641318135/tssp_a_551299_o_f0004g.gif)
As shown in , B utilization index of young orange plants grafted on both rootstocks declined significantly as B supply increased. And B utilization index of citrange-grafted plants was generally higher than that of trifoliate orange-grafted plants at the same B treatment.
Figure 5. Boron utilization index (in g mg−1) of “Newhall” navel orange plants grafted on trifoliate orange and citrange with different boron treatments ([B0 (no boron), B05 (control treatment) and B50 (excessive boron)]. Values are means of three replicates ± standard error. Bars with different letters are significantly different at P < 0.05.
![Figure 5. Boron utilization index (in g mg−1) of “Newhall” navel orange plants grafted on trifoliate orange and citrange with different boron treatments ([B0 (no boron), B05 (control treatment) and B50 (excessive boron)]. Values are means of three replicates ± standard error. Bars with different letters are significantly different at P < 0.05.](/cms/asset/af0e0911-aea3-47e4-b235-0c3588d11e2b/tssp_a_551299_o_f0005g.gif)
Leaf boron forms
Free B content increased significantly as B supply was increased, regardless of rootstocks. For treatments B0 and B05, the free B content of citrange-grafted plants was lower than that of trifoliate orange-grafted plants, but at the treatment B50, the content was higher than that of trifoliate orange-grafted plants (). Semi-bound B content was relatively stable at the treatment B0 and B05 (). Citrange-grafted plants had lowest bound B content under low B supply (B0), whereas there was no significant difference between the treatments B05 and B50. Bound B content of trifoliate orange-grafted plants increased 39.9% from B0 to B05 treatment, and the content increased 103.5% from B05 to B50 treatment (). From , we calculated R values (semi-bound B/free B) of citrange-grafted plants, and the values were 2.31, 1.23 and 1.06 for the treatments B0, B05 and B50, respectively, while the R values of trifoliate orange-grafted plants were 1.80, 0.57 and 0.82 for the treatments B0, B05 and B50, respectively.
Table 3. Spring-flush leaves boron (B) forms (free B, semi-bound B and bound B) of “Newhall” navel orange plants grafted on trifoliate orange and citrange with different B treatments [treatment without B (B0, no B), boron concentrations at 0.5 mg L−1 (B05, control treatment) and 5.0 mg L−1 (B50, excessive B) using boric acid]
The relative content of semi-bound B of both rootstocks was greater at B0 treatment than that at B05 treatment. The relative content of bound B of citrange-grafted plants was the lowest, while that of trifoliate orange-grafted plants was the highest at B50 treatment ().
Discussion
Boron deficiency and toxicity in plant growth
It was observed from our experiment that water-soaked spots and unequal leaf expansion occurred in young leaves (not fully expanded) of both grafted plants under B deficiency (data not shown), implying that B deficiency took place primarily in the growing regions. This conclusion was consistent with the results by Marschner (Citation1995). Similar results were also obtained in citrus [Citrus sinensis (L.) Osbeck cv.Xuegan] (Han et al. Citation2009). The symptoms of B deficiency observed in this study could be attributed to the theory that B plays an important role in cell wall structure and plasticity (Hu and Brown Citation1994). Boron cross-links pectin in cell walls, and the cross-linking appeared to be essential for normal expansion of leaves (O’Neill et al. Citation2001). In addition, the other symptoms of B deficiency, curling of the leaves, corking of the veins and extensive defoliation in the mature leaves, only occurred in trifoliate orange-grafted plants at the conditions of B deficiency (). This result was in accordance with published researches (Roy Citation1943; Jiang et al. Citation2009). This may be caused by the translocation of B from mature leaves to growing tissue under B deficient condition, so these B-deficiency mature leaves accrued the symptoms due to abundance accumulation of carbohydrates (Camacho-Cristóbal and González-Fontes Citation1999; Han et al. Citation2008). Boron was always considered as inefficient in remobilization in many plants (Brown and Shelp Citation1997). However, more and more researches demonstrated that plants that could produce sugar alcohols containing cis-hydroxyl groups could readily form poly-B complex and was likely to allow B to be transported through phloem (Brown and Hu Citation1996; Jiang et al. Citation2008). The cis-polyols are present in many species (Brown and Hu Citation1996). However, they are not synthesized in citrus plants. In this species, sucrose is the predominate forms (Boaretto et al. Citation2008). More recently, increasing evidence showed that non-sugar alcohol-producing plants could transport boric acid preferentially to young tissues only under B deficiency (Matoh and Ochiai Citation2005; Huang et al. Citation2008), suggesting that plants are capable of sensing B level and regulating B distribution within the plant. This regulation is essential for plant survival under conditions of limited B supply (Tanaka and Fujiwara Citation2008). The fact that citrange-grafted naval orange plants did not exhibit the symptom in the mature leaves might be due to the lower B demands.
Boron toxicity in mature leaves appeared at the end of the transpiration stream (Brown and Shelp Citation1997), and this symptom was in agreement with the results described by Turan et al. (Citation2009). However, trifoliate orange-grafted plants seemed to be more sensitive to B toxicity than citrange-grafted plants (), attesting that different rootstocks can influence plants’ tolerance to B toxicity (Edelstein et al. Citation2005) possibly through different B distribution in leaves. For some species of plants, B was mostly concentrated at the tips and margins, even though the majority of leaf blades had similar concentrations (Nable et al. Citation1997).
There was no difference in dry mass in various parts of young “Newhall” navel orange (Citrus sinensis Osb.) plants between B0 and B05 (). Similar results were observed in “Valencia” sweet orange [C. sinensis (L.) Osb.] trees budded on Rangpur lime [C. limonia (L.) Osb.] and Swingle citrumelo [C. paradisi Macfad. cv. ‘Duncan’ × Poncirus trifoliata (L.) Raf.] as well as sour orange (C. sinensis Osb.) (Papadakis et al. Citation2003; Boaretto et al. Citation2008). Nevertheless, the root growth defects were observed and could be ascribed to the inhibition of root elongation through limiting cell enlargement and cell division in the growing zone of root tips (Cakmak and Römheld Citation1997). Grown in nutrient solution for a long term, the B-deficient citrus trees exhibited reduction in shoot and root development (Haas and Klotz Citation1931). In naval orange orchards, B-deficiency inhibited tree growth and development (Jiang et al. Citation2009). The data on plant growth suggested that scion's parts were more sensitive to high B than rootstock's parts. In addition, the inhibitory effect on the growth was more pronounced in the stem of scion and the new leaf (), implying that these two parts were more sensitive to high B than the other tissues. Boron toxicity exerts different effects on very diverse processes in vascular plants, and these effects includes altered metabolism, reduced root cell division, lowered leaf chlorophyll content and photosynthetic rate, and decreased lignin and suberin level (Nable et al. Citation1997; Reid Citation2007). Accordingly, a reduced growth of shoots and roots is typical in plants exposed to high B level. Furthermore, the inhibitory effect on the growth was more serious in the mature leaf and root of trifoliate orange-grafted plants than those of citrange-grafted plants (), and this trend was in accordance with observed B toxicity symptoms ().
Boron concentration, distribution and utilization
Our results indicated that B concentration in leaves was generally higher than all other plant parts, such as roots and stems (rootstock and scion) (), hinting that the leaf is the dominant sites of B accumulation (). These results are in agreement with those reported for “Newhall” (Citrus sinensis Osb.) and “Skagg's Bonanza” (Citrus sinensis Osb.) navel orange (Sheng et al. Citation2009) and melon (Cucumis melo L.) (Edelstein et al. Citation2005). Boron concentration in new leaves was much higher than mature leaves, regardless of rootstocks or B treatments (), indicating that B might be preferentially transported to new leaves of “Newhall” navel orange (Citrus sinensis Osb.). The observation that B is predominately distributed to new leaves rather than old leaves in navel orange may be caused by two mechanisms: one is that newly taken up B mainly distributed to new leaves; the other is that the B in phloem partly translocated to new leaves. This preference could be responsible for the normal development of young leaves and the reduction of physiological functions of mature leaves. This explains why the dry mass reduction of new leaves under low B was less significant than the dry mass reduction of new leaves under high B (). Except for new and mature leaves, there was no significant difference in B concentrations in other parts of citrange-grafted plants between B0 and B05, whereas B concentration in various parts in trifoliate orange-grafted plants was greatly affected by B supply (). Compared to B05, B0 only slightly decreased B concentration in various parts of citrange-grafted plants, and the reduction was much larger that of trifoliate orange-grafted plants. These results suggest that “Newhall” navel orange (Citrus sinensis Osb.) grafted on trifoliate orange was more susceptible under B deficiency than that grafted on citrange.
Results of B distribution in different parts of “Newhall” plants showed that decreasing B supply in the nutrient solution resulted in reduction of B in leaves and scion's parts and enhancement of B in roots and rootstock's parts (). These findings suggest that the translocation of B from roots to scion tissues (stems and leaves of scion) may be limited under low B condition. This is consistent with the findings for Norway spruce (Picea abies) (Möttönen et al. Citation2001) and canola (Brassica napus L.) (Asad et al. Citation2002). This phenomenon may be ascribed partly to the impairments of vascular tissues induced by B deficiency (Dell and Huang Citation1997). Our results also indicated that more B was distributed to the roots of citrange-grafted plants than those of trifoliate orange-grafted plants at the end of this experiment ().This allowed the citrange-grafted plants to grow in a relatively normal status under the B deficiency condition.
The influence of rootstock on the mineral composition of leaves in the scion is well known in citrus plants (Eaton and Blair Citation1935). In the present study, plants grafted on trifoliate orange had a higher B concentration than plants grafted on citrange (). Taylor and Dimsey (Citation1993), who found a strong effect of rootstocks on B concentration of “Navel” orange leaves, observed similar results to ours. The total amount of B was also higher in trifoliate orange, but there was no statistical difference in dry mass between the two rootstocks. This indicated that “Newhall” plants grafted on citrange had lower B concentration, but produced higher dry mass, as compared to those grafted on trifoliate orange, suggesting a higher B utilization index of citrange-grafted plants (). Boron utilization index (in g mg−1) is defined as the amount of synthesized biomass per unit B, i.e. whole plant biomass (in grams)/total B accumulation (in milligrams) (Siddiqi and Glanss Citation1981). Our results revealed that citrange was apparently more efficient in B utilization than trifoliate orange. These results were in agreement with field observations, where trees grafted on trifoliate orange showed symptoms of B deficiency, whereas plants grafted on citrange in the same area did not. Although B concentration of trifoliate orange-grafted plants was higher () than that of citrange-grafted plants, the symptoms of B deficiency were more serious in the trifoliate orange-grafted plants (). Accordingly, the present results inferred that the B concentrations in leaves and shoots were not likely to be directly related to B tolerance in citrus plants, which needs to be re-investigated by further studies.
Boron forms
Dannel et al. (Citation1998) developed a method to distinguish between two B pools of different solubility and physiological behavior. The soluble B pool (cell sap) is a mixture of intracellular and apoplastic fluids, whereas the insoluble B pool [water-insoluble residue (WIR)] mainly comprises B that is closely bound to cell wall structures. Goldbach et al. (Citation2000) suggested that there might be a minimum or a critical level of soluble B (i.e. not tightly bound to rhamno-galacturonan II) that was needed in the most B requiring tissues to avoid deficiency reactions. However, the method does not allow differentiation between apoplast and cytoplasm. Therefore, in this study, we used the continuous extraction method of Du et al. (Citation2002) to fractionate B in plant tissues into free B (B in apoplast), semi-bound B (B in cytoplasm), and bound B (B in cell wall). Free B was water soluble, and could pass the cell membrane freely. The B concentration in cytoplasm could show the capability of B utilization (Du et al. Citation2002). Because the normal cell wall can not form without B (Matoh Citation1997) and new cell wall is formed in cytoplasm, B must travel into cytoplasm as a material to synthesize cell wall (Du et al. Citation2002). Boron in the cytoplasm could be also involved in a variety of metabolic activities (Pfeffer et al. Citation2001). In our results, citrange-grafted plants had lower free B content in B0 and B05, and higher free B at B50 than trifoliate orange-grafted plants (). These results could be explained by several reasons: (1) the critical level of free B in citrange was lower than in trifoliate orange (Goldbach et al. Citation2000); (2) the ability of B transmembrane was stronger in citrange, resulting in relatively more B transported into the cytoplasm (Raven Citation1980); (3) xylem lording of B in citrange-grafted plants was easier in citrange-grafted plant than trifoliate orange-grafted plants under high B (Dannel et al. Citation2002). Semi-bound B was relatively stable under B0 and B05 (), further implying that B in cytoplasm is not only as structural material, but also involved in metabolism. Similar results were observed in sunflower (Pfeffer et al. Citation2001). Du et al. (Citation2002) suggested that the content of free B in B-inefficient cultivars was significantly higher than that in B-efficient cultivars, while the semi-bound B content in B-efficient cultivars was significantly higher than that in B-inefficient cultivars. In other words, higher free B concentrations indicated lower utilization of B, and lower semi-bound B might also indicate lower utilization. Based on these results, authors propose a concept of R value (R = semi-bound B/free B), and proved its availability (Du et al. Citation2000). The larger the R value, the greater the B utilization efficiency. In the present study, the R value of citrange-grafted plants was much greater than that of trifoliate orange-grafted plants under the same B supply level (leaf B forms). This indicated high B utilization efficiency of “Newhall” naval orange grafted on citrange, and this also explained why citrange-grafted plants had less B, but produced relatively more dry mass.
Bound B is B bound on the cell wall probably with pectin polysaccharides, and is a critical B form in plants for the stability of cell walls (Hu and Brown Citation1994). Hu et al. (Citation1996) claimed that cell wall pectin content was correlated to the variability in B requirements between plant species. The relative proportion of cell wall-bound B varies with species, organs and B supply levels. In our study, there was no significant difference in bound B of citrange-grafted plants between B05 and B50 (). Similar results were observed in sunflower by Dannel et al. (Citation1999), who considered that the cell walls of sunflower possessed a limited number of B binding sites and behaved like a chemical adsorbent for B. However, bound B of trifoliate orange-grafted plants was much higher in B50 than in B05 (). Dannel et al. (Citation1998) suggested that the external B supply increased from 0.1 to 1600 μmol L−1, B concentration in root and leaf walls increased by a factor of 2.8 and 22, respectively. Different species have lipid vesicles composed of a variety of different phospholipids with different head groups and fatty acyl chains. Depending on the nature of the head group, on the chemical structure of side chains and on the type of chain linkage, the permeability of B may be different from one to another (Jansen and Blume Citation1995; Hu and Brown Citation1997). The R value in this study suggested that B in citrange-grafted plants was easy to transmembrane transport. Possibly, cell walls have a number of B binding sites in trifoliate orange-grafted plants, however, B in these plants was hard to tansmenbrane transport, which induced low B contents in cytoplasm. Accordingly, B and cell wall binding sites may be not fully integrated at the B05 treatment. So the “bound B” in trifoliate orange increased from B05 to B50. These results suggested that cell walls of citrange-grafted plants had combined enough B under relatively low B supply level, but trifoliate orange-grafted plants did not. In addition, because of their low B utilization efficiency and high B requirement, trifoliate orange-grafted plants were more vulnerable to B deficiency and could more easily develop the symptom than citrange-grafted plants under a low B condition.
Furthermore, previous research in radish (Raphanus sativus) and squash (Curcurbita pepo L.) (Matoh et al. Citation1993; Hu and Brown Citation1994) indicated that under low B condition, cell wall B represented most of cellular B. However, the results in the present study were not consistent with this (). Boron was not only involved in the synthesis of the cell wall (Matoh Citation1997), but also involved in lots of physiology metabolism (Dannel et al. Citation2002; Han et al. Citation2009). Species, plant organ and B supply could influence the proportion of B in the cell wall of higher plants (Goldbach et al. Citation2000; Dannel et al. Citation2002). We speculated that some plants may need more B to synthesis the cell wall, while the other need more B to be involved in metabolism in cytoplasm. And navel orange belongs to the latter.
Conclusions
Under both low B and high B conditions, “Newhall” plants grafted on citrange grew better than in those grafted on trifoliate orange, suggesting that citrange-grafted plants were more tolerant to B stresses (low and high) than the plants grafted on trifoliate orange. Boron seemed to be preferentially translocated to new leaves (leaves from current year), resulting in normal growth of young leaves under limited B supply. The distribution of B in different plant parts indicated that there was a restriction in translocation of B from roots to scion tissues (stems and leaves of scion) under limited B availability. The relatively high B concentration observed in plants grafted on trifoliate orange suggested their demand for B was greater than those grafted on citrange. There existed a balance within B forms to maintain normal growth. The R value was much higher in citrange-grafted plants than in trifoliate orange-grafted plants under the same B supply level. This explained why citrange-grafted plants had less B but produced relatively more dry mass. These results suggested that the utilization efficiency of B of citrange-grafted plants was probably greater than trifoliate orange-grafted plants.
Acknowledgments
The authors want to take this opportunity to thank Professor Peng Shuang and Deng Xiuxin of Huazhong Agricultural University for their great support. This research project was financial supported by the State Scientific Support Programs (2007BAD61B01) and (Citation2008BADA4B05).
References
- Asad , A , Blamey , FPC and Edwards , DG . 2002 . Dry matter production and boron concentration of vegetative and reproductive tissues of canola and sunflower plants grown in nutrition solution . Plant Soil , 243 : 243 – 252 .
- Bao , SD . 1999 . Soil and Agricultural Chemistry Analysis , 276 Beijing : Chinese Agriculture Publishing House .
- Boaretto , RM , Quaggio , JA , Filho , F , Gine , MF and Boaretto , AE . 2008 . Absorption and mobility of boron in young citrus plants . Commun. Soil Sci. Plan. , 39 : 2501 – 2514 . doi: DOI: 10.1080/00103620802358383
- Brown , PH and Hu , H . 1996 . Phloem mobility of boron is species dependent: evidence for phloem mobility in sorbitol-rich species . Ann. Bot. , 77 : 497 – 505 .
- Brown , PH and Shelp , BJ . 1997 . Boron mobility in plants . Plant Soil , 193 : 85 – 101 .
- Cakmak , I and Römheld , V . 1997 . Boron deficiency-induced impairments of cellular functions in plants . Plant Soil , 193 : 71 – 83 .
- Camacho-Cristóbal , JJ and González-Fontes , A . 1999 . Boron deficiency causes a drastic decrease in nitrate content and nitrate reductase activity, and increases the content of carbohydrates in leaves from tobacco plants . Planta , 209 : 528 – 536 .
- Chatzissavvidis , C , Therios , L , Antonopoulou , C and Dimassi , K . 2008 . Effects of high boron concentration and scion-rootstock combination on growth and nutritional status of olive plants . J. Plant Nutr. , 31 ( 4 ) : 638 – 658 .
- Dannel , F , Pfeffer , H and Römheld , V . 1998 . Compartmentation of boron in roots and leaves of sunflower as affected by boron supply . J. Plant Physiol. , 153 : 615 – 622 .
- Dannel , F , Pfeffer , H and Römheld , V . 1999 . Distribution within the plant or compartmentation does not contribute substantially to the detoxification of excess boron in sunflower (Helianthus annuus) . Aust. J. Plant Physiol. , 26 : 95 – 99 .
- Dannel , F , Pfeffer , H and Römheld , V . 2002 . Update on boron in higher plants—uptake, primary translocation and compartmentation . Plant Biol. , 4 : 193 – 204 .
- Dell , B and Huang , L . 1997 . Physiological response of plants to low boron . Plant Soil , 193 : 103 – 120 .
- Du , CW , Wang , YH , Xu , FS and Wang , HY . 2000 . Preliminary study on establishment of one boron nutrition genetic index and its application in rape . Plant Nutr. Fert. Sci. , 6 ( 3 ) : 349 – 352 .
- Du , CW , Wang , YH , Xu , FS , Yang , YH and Wang , HY . 2002 . Study on the physiological mechanism of boron utilization efficiency in rape cultivars . J. Plant Nutr. , 25 ( 2 ) : 231 – 244 .
- Eaton , FM and Blair , GY . 1935 . Accumulation of boron by reciprocally grafted plants . Plant Physiol. , 10 ( 3 ) : 411 – 424 .
- Edelstein , M , Ben-Hur , M , Cohen , R , Burger , Y and Ravina , I . 2005 . Boron and salinity effects on grafted and non-grafted melon plants . Plant Soil , 269 : 273 – 284 .
- Forner-Giner , MA , Alcaide , A , Primo-Millo , E and Forner , JB . 2003 . Performance of ‘Navelina’ orange on 14 rootstocks in Northern Valencia(Spain) . Sci. Hort. , 98 : 223 – 232 .
- Goldbach , HE , Wimmer , MA and Findeklee , P . 2000 . Boron – How can the critical level be defined? . J. Plant Nutr. Soil Sci. , 163 : 115 – 121 .
- Haas , ARC and Klotz , LJ . 1931 . Further evidence on the necessity of boron for health in citrus . Botanical Gazette , 92 : 94 – 100 .
- Han , S , Chen , LS , Jiang , HX , Smith , BR , Yang , LT and Xie , CY . 2008 . Boron deficiency decreases growth and photosynthesis, and increases starch and hexoses in leaves of citrus seedlings . J. Plant Physiol. , 165 : 1331 – 1341 .
- Han , S , Tang , N , Jiang , HX , Yang , LT , Li , Y and Chen , LS . 2009 . CO2 assimilation, photosystem II photochemistry, carbohydrate metabolism and antioxidant system of citrus leaves in response to boron stress . Plant Sci. , 176 : 143 – 153 .
- Hu , H and Brown , PH . 1994 . Localization of boron in cell walls of squash and tobacco and its association with pectin . Plant Physiol. , 105 : 681 – 689 .
- Hu , H , Brown , PH and Labavitch , JM . 1996 . Species variability in boron requirement is correlated with cell wall pectin . J. Exp. Bot. , 47 : 227 – 232 .
- Hu , H and Brown , PH . 1997 . Absorption of boron by plant roots . Plant Soil. , 193 : 49 – 58 .
- Huang , L , Bell , RW and Dell , B . 2008 . Evidence of phloem boron transport in response to interrupted boron supply in white lupin (Lupinus albus L. cv. Kiev Mutant) at the reproductive stage . J. Exp. Bot. , 59 : 575 – 583 .
- Jansen , M and Blume , A . 1995 . A comparative study of diffusive and osmotic water permeation across bilayers composed of phospholipids with different head groups and fatty acyl chains . Biophysical J. , 68 : 997 – 1008 .
- Jiang , CC , Wang , YH , Liu , GD , Xia , Y , Peng , SA , Zhong , BL and Zeng , QL . 2009 . Effect of boron on the leaves etiolation and fruit fallen of Newhall Navel Orange . Plant Nutr. Fert. Sci. , 15 ( 3 ) : 656 – 661 .
- Jiang , F , Jeschke , WD , Hartung , W and Cameron , DD . 2008 . Mobility of boron–polyol complexes in the hemiparasitic association between Rhinanthus minor and Hordeum vulgare: the effects of nitrogen nutrition . Physiol. Plantarum , 134 : 13 – 21 . doi: DOI: 10.1111/j.1399-3054.(2008).01116.x
- Liu , Z , Zhu , QQ and Tong , LH . 1980 . Boron deficient soils and their distribution in China . Acta Ped. Sin. , 17 : 228 – 239 .
- Liu , Z , Zhu , QQ and Tong , LH . 1989 . Regularities of content and distribution of boron in soils . Acta Ped. Sin. , 26 : 353 – 361 .
- Loomis , WD and Durst , RW . 1992 . Chemistry and biology of boron . Biofactors , 3 : 229 – 239 .
- Marschner , H . 1995 . Mineral Nutrition of Higher Plants , 2nd , London : Academic Press .
- Matoh , T . 1997 . Boron in plant cell walls . Plant Soil , 193 : 59 – 70 .
- Matoh , T , Ishigaki , K , Ohno , K and Azuma , J . 1993 . Isolation and characterization of a boron–polysaccharide complex from radish roots . Plant Cell Physiol. , 34 : 639 – 642 .
- Matoh , T and Ochiai , K . 2005 . Distribution and partitioning of newly take-up boron in sunflower . Plant Soil , 278 : 351 – 360 .
- Möttönen , M , Lehto , T and Aphalo , PJ . 2001 . Growth dynamics and mycorrhizas of Norway spruce (Picea abies) seedlings in relation to boron supply . Trees , 15 : 319 – 326 .
- Nable , RO , Baňuelos , GS and Paull , JG . 1997 . Boron toxicity . Plant Soil , 193 : 181 – 198 .
- O’Neill , MA , Eberhard , MAS , Albersheim , P and Darvill , AG . 2001 . Requirement of borate cross-linking of cell wall rhamnogalacturonan II for Arabidopsis growth . Science , 294 : 846 – 849 .
- Papadakis , IE , Dimassi , KN and Therios , IN . 2003 . Response of two citrus genotypes to six boron concentrations: concentration and distribution of nutrients, total absorption, and nutrient use efficiency . Aust. J. Agr. Res. , 54 ( 6 ) : 571 – 580 .
- Papadakis , IE , Dimassi , KN , Bosabalidis , AM , Therios , IN , Patakas , A and Giannakoula , A . 2004 . Boron toxicity in ‘Clementine’ mandarin plants grafted on two rootstocks . Plant Sci. , 166 ( 2 ) : 539 – 547 .
- Pfeffer , H , Dannel , F and Römheld , V . 2001 . Boron compartmentation in roots of sunflower plants of different boron status: a study using the stable isotopes 10B and 11B adopting two independent approaches . Physiol. Plantarum , 113 : 346 – 351 .
- Raven , JA . 1980 . Short- and long-distance transport of boric acid in plants . New Phytol. , 84 : 231 – 249 .
- Reid , R . 2007 . “ Update on boron toxicity and tolerance in plants ” . In Advances in Plant and Animal Boron Nutrition , Edited by: Xu , F , Goldbach , HE , Brown , PH , Bell , RW , Fujiwara , T , Hunt , CD , Goldberg , S and Shi , L . 83 – 90 . Dordrecht : Springer .
- Rerkasem , B and Jamjod , S . 1997 . Genotypic variation in plant response to low boron and implications for plant breeding . Plant Soil , 193 : 169 – 180 .
- Roy , WR . 1943 . Studies of boron deficiency in grapefruit . Proc. Florida State Hort. Soc. , 56 : 38 – 43 .
- SAS Institute Inc. 1996 . Analysis of Covariance , Cary, NC : SAS Institute Inc. .
- Sheng , O , Song , SW , Chen , YJ , Peng , SA and Deng , XX . 2009 . Effects of exogenous B supply on growth, B accumulation and distribution of two navel orange cultivars . Trees , 23 : 59 – 68 .
- Shorrocks , VM . 1997 . The occurrence and correction of boron deficiency . Plant Soil , 193 : 121 – 148 .
- Siddiqi , MY and Glanss , ADM . 1981 . Utilization index: a modified approach to the estimation and comparison of nutrient utilization efficiency in plants . J. Plant Nutr. , 4 ( 3 ) : 289 – 302 .
- Sotiropoulos , TE , Fotopoulos , S , Dimassi , KN , Tsirakoglou , V and Therios , IN . 2006 . Response of the pear rootstock to boron and salinity in vitro . Biol. Plantarum , 50 ( 4 ) : 779 – 781 .
- Tanaka , M and Fujiwara , T . 2008 . Physiological roles and transport mechanisms of boron: perspectives from plants . Eur. J. Physiol. , 456 : 671 – 677 .
- Taylor , BK and Dimsey , RT . 1993 . Rootstock and scion effects on the leaf nutrient composition of citrus trees . Aust. J. Exp. Agr. , 33 ( 3 ) : 363 – 371 .
- Turan , MA , Taban , N and Taban , S . 2009 . Effect of calcium on the alleviation of boron toxicity and localization of boron and calcium in cell wall of wheat . Not. Bot. Hort. Agrobot. Cluj , 37 ( 2 ) : 99 – 103 .
- Wojcik , P , Wojcik , M and Treder , W . 2003 . Boron absorption and translocation in apple rootstocks under conditions of low medium boron . J. Plant Nutr. , 26 : 961 – 968 .
- Xiao , JX , Yang , X , Peng , SA and Fang , YW . 2007 . Seasonal changes of mineral nutrients in fruit and leaves of ‘Newhall’ and ‘Skagg's Bonanza’ navel oranges . J. Plant Nutr. , 30 : 671 – 690 .
![Figure 6. Relative content of boron forms of “Newhall” navel orange plants grafted on trifoliate orange and citrange with different boron treatments [B0 (no boron), B05 (control treatment) and B50 (excessive boron)]. Values are means of three replicates.](/cms/asset/e7ce2a22-1b19-4dde-af96-ffe655880952/tssp_a_551299_o_f0006g.gif)