Abstract
The nitrate/nitrite response, which occurs within minutes for the gene expression of the primary nitrogen metabolism in higher-plant roots, has almost been described, but the intermediate signaling molecule that exerts in signal transduction remains to be elucidated. It is hypothesized that the constitutively present signaling molecule may bind quickly to exogenously applied nitrate/nitrite to transduce the nitrate/nitrite signal. To examine the quick binding of nitrate/nitrite to high-molecular compounds, which may include intermediate signaling molecules, in the roots of the rice plant (Oryza sativa L.), the segmented roots of rice seedlings were exposed to nitrogen-13 (13N)-labeled nitrate/nitrite for either 2 or 5 min at 25°C. The supernatant of the Tris-buffer root extract was separated by a desalting pre-packed column. The incorporation of 13N into the high-molecular fraction was identified by 13N feeding for 2 min, and by 13N feeding for 5 min, however, the amount of 13N incorporation was not increased. Although the findings are preliminary, it was clearly demonstrated that 13N-labeled nitrate/nitrite was quickly incorporated into a limited amount of the soluble high-molecular compound(s). The formation of NO x -bound proteins, which may play a role in nitrate/nitrite signal transduction, is discussed.
Introduction
Higher plants can utilize nitrate via its reduction to nitrite and ammonia by nitrate reductase (NR, EC 1.6.6.1) and nitrite reductase (NiR, EC 1.7.7.1), respectively, and then by incorporation of the ammonia into glutamate, resulting in the formation of glutamine by glutamine synthetase (GS, EC 6.3.1.2) (Miflin and Lea Citation1980). The adaptive formation of NR protein by feeding of nitrate, which is a direct substrate for NR, was first reported in rice seedlings by Tang and Wu (Citation1957). Nitrate reductase activity in rice seedlings can be induced by the addition of not only nitrate but also nitrite, the direct product of NR (Shen Citation1972). Nitrate ion () uptake activity, presumed by nitrate transporter (NRT), NR activity and NR mRNA were induced by a 10-min treatment of µM-level
(Tischner et al.
Citation1993). Recently, Wang et al. (Citation2007) demonstrated that treatment for 5 to 20 min by nM-level nitrite induces the expression of the NRT, NR and NiR genes, as nitrate treatment does. Thus, nitrate/nitrite added to roots may act not only as a substrate for the nitrate reduction after hours but also as a signal, inducing the gene expression of nitrate absorption and assimilation within minutes (Crawford Citation1995; Wang et al.
Citation2007).
The specific expressions of genes for NRT1.1, NR, NiR, GS, glutamate synthase (GOGAT), ferredoxin (Fd), and non-symbiotic hemoglobin (Ohwaki et al. Citation2005) start shortly (∼20 min) after feeding of low concentrations of nitrate/nitrite (Wang et al. Citation2007). Such a short-term expression suggests that the specific intermediates that carry the nitrate/nitrite signal may be present constitutively (Hwang et al. Citation1997) without de novo synthesis of proteins for this purpose (Guo et al. Citation2000) and that upon feeding of nitrate/nitrite, they may form complexes with nitrate/nitrite. The constitutively present proteins can not be pursued from the gene expression upon short-term feeding of nitrate/nitrite. Thus it is hypothesized that the constitutively present signaling molecule may bind quickly to nitrate/nitrite to transduce the nitrate/nitrite signal. To the best of our knowledge, there have been no reports of any evidence that shows the presence of such nitrate/nitrite combined high-molecular compound (HMC) in higher plants.
One way to demonstrate the formation of such nitrate/nitrite-binding molecules is the use of isotopes of nitrogen and/or oxygen. Nitrogen-15 (15N), a stable isotope for nitrogen, and oxygen-18 (18O), a stable isotope for oxygen, are candidates for such tracing tools. However, in order to detect significant incorporation of 15N or 18O from 15N- or 18O-labeled nitrate/nitrite into the protein fraction requires more than 30 min (Yoneyama and Kumazawa Citation1975), since a significant excess over the natural abundances of 15N and 18O (0.37% and 0.204%, respectively) is required. In the present study, we chose to use nitrogen-13 (13N), a positron-emitting radioisotope, which causes easily-detectable gamma rays, to trace binding of nitrate/nitrite with HMC within a few minutes. Although its half-life is short (10.0 min), cyclotron can produce 13N with high specific radioactivity. The use of oxygen-15 (15O), a radioisotope of oxygen, was abandoned due to its very short half-life (2.0 min).
Materials and Methods
Plant culture and treatment with nitrogen-13 (13N)
Rice seedlings (Oryza sativa L. cultivar Nipponbare) were grown on a floating net in 1 L deionized water containing only 0.5 mM calcium sulfate (CaSO4) (pH 5.5) for two weeks in a growth chamber with a day/night temperature of 30°C/25°C (12 h/12 h). Five-millimeter root segments (7.1 g for Experiment 1 and 5.8 g for Experiment 2) excised from 3-cm root tips of 150 plants were first collected into 50-mL tubes containing 0.5 mM CaSO4 solution at 25°C. The solution was discarded and the root segments in the tubes were then exposed to a new 0.5 mM CaSO4 solution (5 mL) containing 4.9 nmol N (Experiment 1) or 4.1 nmol N (Experiment 2) of 13N-labeled /
, which was prepared as described later, and incubated for either 2 (Experiment 1) or 5 min (Experiment 2) at 25°C. After removal of the 13N-labeled
/
-containing solution bathing the root segments by syringes, the root segments were washed three times with ice-cooled Tris buffer containing 50 mM Tris-hydrogen chloride (HCl) and 150 mM sodium chloride (NaCl) at pH 7.4, and finally dried with paper towels. The root segments were transferred to cooled porcelain mortars, and liquid nitrogen was added to crush them. We then mixed with the Tris buffer (pH 7.4). The homogenized root-containing solution was centrifuged at 7500 g for 1 min and the supernatant was passed through a double layer of filters (0.45 µm and then 0.22 µm). The final filtrate (root extract) was placed on a HiTrap Sephadex™ G-25 desalting column (GE Healthcare Japan, Tokyo, Japan) to be separated into high molecules (proteins) and low molecules. Elutes of approximately every 0.25 and 0.5 mL for the first 5 mL in Experiments 1 and 2, respectively, and those of 0.5 mL in both experiments during elution after the first 5-mL elution were assayed for radioactivity by a gamma well counter (Aloka, Tokyo, Japan). The exact volumes of the elute fractions were measured after measurement of the radioactivity. Decay correction for radioactivity was properly performed to estimate the amount of 13N distributed into each fraction.
Production and use of nitrogen-13 (13N)-labelled nitrate/nitrite
Nitrogen-13 (13N) was produced with an azimuthally varying field (AVF) cyclotron at the Takasaki Ion accelerator for Advanced Radiation Application (TIARA) of Japan Atomic Energy Agency (JAEA) by a reaction of 16O(p,α)13N. Water was bombarded with an 18.5-MeV energetic proton beam for 2 min at a current of 2 µA. These substances were purified by a cation-exchange resin and subsequently by aluminum column (Fujimaki et al.
Citation2010). An aliquot of this purified solution was added to the feeding solution containing 114 MBq (Experiment 1) and 92 MBq (Experiment 2) of 13
/13
(corresponding to 0.16 pmol and 0.13 pmol, respectively) at the collected time and also non-radioactive
/
(estimated to be 4.9 nmol and 4.1 nmol, respectively). To accurately measure the radioactivity of 13N, which has an extremely short half-life (10 min), in the elute fractions, all the experiments were designed to terminate within 60 min.
Results and Discussion
Under 13N feeding for 2 min (Experiment 1), the first 13N peak was found at around 2.5 mL elution, clearly demonstrating 13N incorporation from exogenously added nitrate/nitrite into the HMC (the protein fraction) (). Increasing the period of 13N feeding to 5 min did not increase the amount of 13N in the HMC fraction (). The amounts of total /
(radioactive and non-radioactive) bound to the HMC were estimated to be 1.1 pmol and 0.9 pmol per grams fresh weight root for Experiments 1 and 2, respectively. The results indicate that the amount of
/
-binding HMC in the root tissue is very limited and the binding is quickly completed.
Figure 1 Nitrogen-13 (13N) specific activities in the factions separated by a desalting column from the nitrate/nitrite ()-fed root extract. The first peak (also shown in a magnified view) indicates 13N-bound HMC(s).
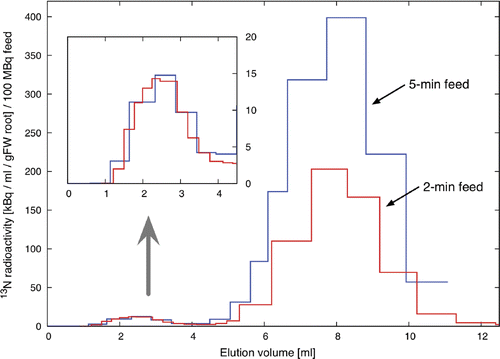
Table 1 Nitrogen-13 (13N) radioactivity in the root extract, and high and low molecules by 2 and 5-min 13N feeding
The nature of this 13N incorporation from nitrate/nitrite into the HMC should be characterized. Maeda and Omata (Citation1997) isolated a lipoprotein that may bind to nitrate/nitrite in Synechococcus sp. strain PPC 7942, however, such lipoproteins have not been found in higher plants. Protein nitration (the introduction of a nitro group into a chemical compound) at tyrosine and occasionally tryptophan residues may be induced by the addition of nitrite (Amoresano et al.
Citation2007). Possible nitrite-dependent hemoglobin nitration has been demonstrated in Arabidopsis (Sakamoto et al.
Citation2004). Nitrite oxide (NO) is produced by NR from the nitrite ion () (Yamazaki et al.
Citation1999); such NO may also bind to non-symbiotic hemoglobin, which is localized in the nucleus of alfalfa culture cells (Seregélyes et al.
Citation2000). Nitrite oxide may also bind to protein cysteine residues or to the transition metals (iron and copper) of metalloproteins: such reactions are known as nitrosylation (Wang et al.
Citation2006). Both the nitration and nitrosylation of proteins are post-translational, and may regulate the activity of a wide variety of proteins from iron channels to transcription factors (Wang et al.
Citation2006; Rinalducci et al.
Citation2008). Techniques to identify nitrated and nitrosylated proteins must be developed (Camerini et al.
Citation2007), and a method of identifying specific 13N-labelled HMC(s) is essential in order to search for the protein(s) that participate in signal transduction in the nitrate/nitrite response.
Acknowledgment
The authors thank Mr H. Suto for his technical assistance in irradiation for 13
/13
production.
References
- Amoresano , A , Chiappetta , G , Pucci , P , D’lschia , M and Marino , G . 2007 . Bidimentional tandem mass spectrometry for selective identification of nitration sites in proteins . Anal. Chem. , 79 : 2109 – 2117 .
- Camerini , A , Polci , ML , Restuccia , U , Usuelli , V , Malgaroli , A and Bachi , A . 2007 . A novel approach to identify proteins modified by nitric oxide: the His-TAG switch method . J. Proteome Res. , 6 : 3224 – 3231 .
- Crawford , NM . 1995 . Nitrate: nutrient and signal for plant growth . Plant Cell , 7 : 859 – 868 .
- Fujimaki , S , Ishii , S and Ishioka , NS . 2010 . “ Non invasive imaging and analyses of transport of nitrogen nutrition in intact plants using a position-emitting tracer imaging system (PETIS) ” . In Nitrogen Assimilation in Plants , Edited by: Ohyama , T and Sueyoshi , K . 119 – 132 . Kerala : Research Signpost .
- Guo , F-Q , Wang , R and Crawford , NM . 2000 . The Arabidopsis dual-affinity transporter gene AtNRT1.1 (CHL1) is regulated by auxin in both shoots and roots . J. Exp. Bot. , 53 : 835 – 844 .
- Hwang , C-F , Lin , Y , D’Souza , T and Cheng , C-L . 1997 . Sequences necessary for nitrate-dependent transcription of Arabidopsis nitrate reductase genes . Plant Physiol. , 113 : 853 – 862 .
- Maeda , S and Omata , T . 1997 . Substrate-binding lipoprotein of the cyanobacterium Synechococcus sp. strain PCC 7942 involved in the transport of nitrate and nitrite. . J. Biol. Chem. , 272 : 3036 – 3041 .
- Miflin , BJ and Lea , PJ . 1980 . “ Ammonia assimilation ” . In The Biochemistry of Plants , Edited by: Miflin , BJ . 169 – 202 . New York : Academic Press .
- Ohwaki , Y , Kawagishi-Kobayashi , M , Wakasa , K , Fujihara , S and Yoneyama , T . 2005 . Induction of class-1 type non-symbiotic hemoglobin genes by nitrate, nitrite and nitric oxide in cultured cells . Plant Cell Physiol. , 46 : 324 – 331 .
- Rinalducci , S , Murglano , L and Zolla , L . 2008 . Redox proteomics: basic principles and future perspectives for the detection of protein oxidation in plants . J. Exp. Bot. , 59 : 3781 – 3801 .
- Sakamoto , A , Sakurao , S , Fukunaga , K , Matsubara , T , Ueda-Hashimoto , M , Tsukamoto , S , Takahashi , M and Morikawa , H . 2004 . Three distinct Arabidopsis hemoglobins exhibit peroxidase-like activity and differentially mediate nitrite-dependent protein nitration . FEBS Lett. , 572 : 27 – 32 .
- Seregélyes , C , Mustardy , L , Ayaydin , F , Sass , L , Kovacs , L , Endre , G , Lukacs , N , Kovacs , I , Vass , I , Kiss , GB , Horvath , GV and Dudits , D . 2000 . Nuclear localization of a hypoxia-inducible novel non-symbiotic hemoglobin in cultured alfalfa cells . FEBS Lett. , 482 : 125 – 130 .
- Shen , TC . 1972 . Nitrate reductase of rice seedlings and its induction by organic nitro compounds . Plant Physiol. , 49 : 546 – 549 .
- Tang , PS and Wu , HY . 1957 . Adaptive formation of nitrate reductase in rice seedlings . Nature , 179 : 1355 – 1356 .
- Tischner , R , Waldeck , B , Goyal , S and Rains , WD . 1993 . Effect of nitrate pulses on the nitrate-uptake rate, synthesis of mRNA coding for nitrate reductase and nitrate-reductase activity in the roots of barley seedlings . Planta , 189 : 533 – 537 .
- Wang , R , Xing , X and Crawford , N . 2007 . Nitrite acts as a transcriptome signal at micromolar concentrations in Arabidopsis roots . Plant Physiol. , 145 : 1735 – 1745 .
- Wang , Y , Yun , B-W , Kwon , E , Hong , JK , Yoon , J and Loake , GJ . 2006 . S-Nitosylation: an emerging redox-based post-transcriptional modification in plants . J. Exp. Bot. , 57 : 1777 – 1784 .
- Yamazaki , H , Sakihama , Y and Takahashi , S . 1999 . An alternative pathway for nitric oxide production in plants: new feature of an old enzyme . Treads Plant Sci. , 4 : 128 – 129 .
- Yoneyama , T and Kumazawa , K . 1975 . A kinetic study of the assimilation of 15N-labelled nitrate in rice seedlings . Plant Cell Physiol. , 16 : 21 – 26 .