Abstract
Our previous research showed large amounts of nitrous oxide (N2O) emission (>200 kg N ha−1 year−1) from agricultural peat soil. In this study, we investigated the factors influencing relatively large N2O fluxes and the source of nitrogen (N) substrate for N2O in a tropical peatland in central Kalimantan, Indonesia. Using a static chamber method, N2O and carbon dioxide (CO2) fluxes were measured in three conventionally cultivated croplands (conventional), an unplanted and unfertilized bare treatment (bare) in each cropland, and unfertilized grassland over a three-year period. Based on the difference in N2O emission from two treatments, contribution of the N source for N2O was calculated. Nitrous oxide concentrations at five depths (5–80 cm) were also measured for calculating net N2O production in soil. Annual N fertilizer application rates in the croplands ranged from 472 to 1607 kg N ha−1 year−1. There were no significant differences in between N2O fluxes in the two treatments at each site. Annual N2O emission in conventional and bare treatments varied from 10.9 to 698 and 6.55 to 858 kg N ha−1 year−1, respectively. However, there was also no significant difference between annual N2O emissions in the two treatments at each site. This suggests most of the emitted N2O was derived from the decomposition of peat. There were significant positive correlations between N2O and CO2 fluxes in bare treatment in two croplands where N2O flux was higher than at another cropland. Nitrous oxide concentration distribution in soil measured in the conventional treatment showed that N2O was mainly produced in the surface soil down to 15 cm in the soil. The logarithmic value of the ratio of N2O flux and nitrate concentration was positively correlated with water filled pore space (WEPS). These results suggest that large N2O emission in agricultural tropical peatland was caused by denitrification with high decomposition of peat. In addition, N2O was mainly produced by denitrification at high range of WFPS in surface soil.
Introduction
Tropical peatland in Indonesia comprise nearly 12% (27 Mha) of total global peatland (Maltby and Immirzi Citation1993). However, large areas of the tropical peatlands, have been damaged by forest fires or agricultural deforestation in Indonesia (Muhanmad and Rieley Citation2002; Page et al. Citation2002). In 1995, more than 1 Mha of tropical peat land in central Kalimantan in Indonesia was reclaimed for agricultural development by the “Mega Rice Project” (Muhanmad and Rieley Citation2002). Moreover, 28% of peat swamp forest was burned in central Kalimantan in 1997 (Page et al. Citation2002). In addition to the large-scale degradation and relatively large amount of carbon dioxide (CO2) release by drainage and associated peat fires (Hooijer et al. Citation2006; Page et al. Citation2002), cultivation in tropical peat soil has possibly led to increased nitrous oxide (N2O) emission (Terry et al. Citation1981; Takakai et al. Citation2006; Couwenberg et al. 2009). Increasing atmospheric N2O concentration appears to have been caused by human activities (IPCC Citation2007). Nitrous oxide is not only a greenhouse gas, but also one of the major ozone-depleting substances in the atmosphere (Ravishankara et al. Citation2009). Soil is an important source of atmospheric N2O (Mosier et al. Citation1998). Nitrous oxide emission from agricultural land has been estimated to be 21.6% (3.6 Tg N year−1) of total global emission of N2O (16.2 Tg N year−1) (IPCC Citation1995). There are, however, several studies documenting N2O emissions not only from natural tropical peatlands, but also those under cultivation (Terry et al. Citation1981; Inubushi et al. Citation2003; Hadi et al. Citation2005; Takakai et al. Citation2006; Melling et al. Citation2007).
An issue of the N2O emission study in agricultural tropical peatland is that factors influencing N2O emission and the sources of nitrogen (N) for N2O emissions have still been unclear. Several studies in agricultural boreal peatland (e.g. in Finland and Norway) have reported that N2O emissions from drained peatland ranged from 0.1 to 37 kg N ha−1 year−1 (Maljanen et al. Citation2003; Regina et al. Citation2004; Klemedtsson et al. Citation2005). These values were notably larger than the N2O emissions from agricultural fields on mineral soils (0.9–6.4 kg N ha−1 year−1, Bouwman et al. 2002). However, N2O emission from agricultural tropical peatland is reported to be much more variable. The values have been estimated to range from −1.1 to 259 kg N ha−1 year−1 (Terry et al. Citation1981; Inubushi et al. Citation2003; Hadi et al. Citation2005; Takakai et al. Citation2006; Melling et al. Citation2007). It is also reported N2O emission increased following change in land use from natural peat swamp forest to drained or burned peatland, and to agricultural peatland (Takakai et al. Citation2006; Melling et al. Citation2007).
In addition to the emission factor (EFF) induced by applied N fertilizer, N2O emission induced by cultivation of peatland is important for the calculation of annual N2O emission from agricultural fields on peatland. In the subarctic zone, Regina et al. (Citation2004) reported N2O emission from grass, barley and potato vegetation plots (2.6–24.1 kg N ha−1 year−1) were smaller relative to fallow plots (3.8–37 kg N ha−1 year−1) on boreal peatland in Finland. This means N source for N2O emission from soil organic matter (SOM) was larger than N2O emission induced by the application of N fertilizer. Moreover, Regina et al. (Citation2004) reported that average N2O emission induced by SOM was 10.4 kg N ha−1 year−1. Although, index of N2O emission induced by cultivation in tropical peatland was proposed by the Intergovernmental Panel on Climate Change (IPCC Citation2006) to be 16 kg N ha−1 year−1, this value was derived from data obtained from mid-latitude peatlands. However, some environmental conditions in peatland are notably different between boreal or temperate and tropical peatland. Mean annual air temperature is lower in boreal peatland (e.g. 5°C in Majnegarden, Sweden (Kasimir-Klemedtsson et al. Citation2009) than in tropical peatland (e.g. 26°C in central Kalimantan, Indonesia (Hirano et al. Citation2007). Peat is mainly derived from sphagnum or herbaceous plant species in boreal or temperate peatland and from woody plant species in tropical peatland (Andriesse Citation1988). These vegetational differences strongly influence the production and emission of N2O from peat soil because it is mainly produced by nitrification and denitrification in soil. Combined with warm temperatures and frequent rainfall in tropical climates, application of N fertilizer and high levels of organic matter in soil may enhance N2O production through nitrification and denitrification (Tiedje Citation1994; Bouwman Citation1996; Bremner Citation1997). Warm climates in tropical regions also allow multiple crop cultivations within a single year, which may cause rapid decomposition of peat soil due to plowing. Therefore, it is difficult to simply apply what is known regarding N2O dynamics in boreal or temperate peatland to tropical peatland.
Takakai et al. (Citation2006) reported large amount of annual N2O emission (at most 259 kg N ha−1 year−1) and high EFF value in the cultivated tropical peatland of this study located in central Kalimantan, Indonesia. For the rough calculation of EFF, N2O emission in unfertilized and unplowed grassland was assumed to be that derived from SOM decomposition of peat. As such, their reported EFF would be influenced by the N2O emission induced by the cultivation of peatland, and possibly be overestimated or underestimated. Understanding the mechanisms of such high N2O production and emission in agricultural tropical peatland will be essential for quantifying N2O emission and developing the mitigation method for N2O emission. The objectives of our study were to clarify the factors that influence N2O emission, identifying the source of N substrate for N2O, and to quantify annual N2O emission in tropical agricultural peatland in central Kalimantan, Indonesia.
Materials and Methods
Site description
This study was conducted in Kalampangan Village (2°17′S, 114°1′E) near Palangka Raya City (2°S, 114°E) in central Kalimantan, Indonesia, from March 2004 to March 2007. In this region, the dry season normally begins in June and ends in October (Takakai et al. Citation2006). Four adjacent study plots, which were designated as cropland A, B, and C (CL-A, CL-B, CL-C) and grassland (GL), were located in the center of the village and set up in March 2002 (Takakai et al. Citation2006). In these plots, cultivation practice was managed by owner farmer. Cultivation began in 1980 on CL-A, CL-B, and GL, and in 1996 on CL-C. After plowing, croplands were cultivated with cassava (Manihot esculenta Crants.), maize (Zea mays L.) or vegetables [e.g. egg plants (Solanum melongena), etc.].The vegetation in GL was turf grass which has been harvested or grazed. Crop cultivation and fertilization practice were managed by owner farmers. Average N fertilizer applied in CL-A, CL-B, and CL-C sites were 1607, 472 and 1113 kg N ha−1 year−1, respectively (). The soil classification according to USDA Soil Taxonomy at all the study sites was Histosols (Typic Tropofibrists, Takakai et al. Citation2006). Thickness of peat was 2.6–2.8 m. Bulk density and porosity of surface soil (0–10 cm) were approximately 0.4 g cm−3 and 73–77%, respectively. Total carbon (C) and N concentration in surface soil (0–10 cm) varied from 530 to 632 and 13 to 14.3 g kg−1, respectively. Detail information about soil chemical and physical characteristics and the method of soil analysis have been described in Takakai et al. (Citation2006).
Table 1 Amount of nitrogen (N) fertilizer application rate in cropland A (CL-A), cropland B (CL-B), cropland C (CL-C) from April 2002 to March 2007 in central Kalimantan, Indonesia
Treatments
Fields in CL-A, CL-B and CL-C were defined as conventional cultivation (conventional) treatments. Unplanted and unfertilized bare (bare) treatments, which were 0.5 m2 (1 m × 0.5 m) in area, were established in CL-A, CL-B, and CL-C in April 2004 for estimating N2O emission derived from SOM decomposition and were maintained as such for the duration of the study period. Thin woody plates were installed around the bare treatments down to 20 cm deep in the soil to prevent root intrusion and soil contamination. In the conventional treatment in CL-A, CL-B, and CL-C, chemical and organic fertilizers were applied by the farmer every cultivation at the point seeds were sown, which was based on common agricultural practices in the area. Organic fertilizer was produced from cattle manure kept by the farmer and grass. Fertilizer was not applied during the duration of the study period in GL.
Nitrous oxide and carbon dioxide flux measurements and soil nitrous oxide gas sampling
Nitrous oxide and CO2 fluxes were measured with a closed-chamber method with three replications at each site and treatment (Takakai et al. Citation2006). Gas fluxes were measured once a month from April 2004 to February 2006 and twice a month after March 2006 to March 2007. We followed the N2O and CO2 gas sampling method provided by Takakai et al. (Citation2006), Nakano et al. (Citation2004) and Toma and Hatano (Citation2007). Air temperature at a height of 1 m was measured with a thermometer at the same day of N2O and CO2 gas fluxes measurement.
We followed a protocol modified after the methods of Takakai et al. (Citation2006) and Morishita et al. (Citation2003) to sample soil gas sampling and calculate N2O production and consumption in soil.
Nitrous oxide and carbon dioxide concentration analysis, calculation of nitrous oxide and carbon dioxide fluxes, annual emission of nitrous oxide, and nitrogen fertilizer induced emission factor (EFF) of nitrous oxide
Gas samples stored in Tedlar bags for analysis of CO2 concentration and in vacuum vials for the analysis of N2O concentration were analyzed within 12 h and a month, respectively, after collecting these samples. Nitrous oxide and CO2 concentrations were analyzed with a gas chromatograph (GC-14B, Shimadzu, Kyoto, Japan) equipped with an electron-capture detector and CO2 analyzer (ZFP-9, Fuji Electric Systems, Tokyo, Japan), respectively. Gas fluxes were calculated following the method provided by Toma and Hatano (Citation2007).
The annual N2O emissions were calculated by linear integration of flux measurements during the measurement period (Toma and Hatano Citation2007; Toma et al. Citation2010).
Emission factor induced by N fertilizer (chemical and organic N) was calculated by the following equations for data collected in CL-A, CL-B, and CL-C:
Calculation of net nitrous oxide production rate in soil
Net N2O production rate is the difference between gross N2O production rate and gross N2O consumption rate. Net N2O production rate was calculated using the following equation based on Fick's Law (gradient method; Granli and Bøckman Citation1994):
Ancillary measurements
Soil temperature at a depth of 4 cm, volumetric soil water content from 0 to 6-cm depth, and water table depth were measured during the gas flux measurements. Soil temperature was measured in both conventional and bare treatments using a thermistor thermometer. Amplitude domain reflectometry (ADR, ML2 Theta Probe Delta-T Devices, Cambridge, UK) was used to measure the volumetric soil water content in both conventional and bare treatments. The resulting volumetric soil water content was converted into a value for water-filled pore space (WFPS), which represents the ratio of the volumetric water content to the total porosity of the soil, by assuming that the porosity measured by Takakai et al. (Citation2006) in February 2005 was consistent throughout the measurement period. There were three replications per chamber for the soil temperature and volumetric soil water content measurements. To measure the water table depth, perforated polyvinylchloride (PVC) pipes (1.57 inch in diameter) were inserted into the peat soil at each site. Air temperature and precipitation were measured every half hour using a 50 -m micrometeorological tower established inside the forest that was about 3 km from the study site (Hirano et al. Citation2005, Citation2007). Disturbed soil samples in conventional treatments were collected for soil ammonium () and nitrate (
) concentrations from 0–3-cm and 3–10-cm-depth between March 2004 and February 2006, and from 0 to 10-cm depth between March 2006 and March 2007 at the same day of gas flux measurement. In bare treatment at each site, soil samples were collected only two times in September 2006 (in the dry season) and February 2007 (in the rainy season). Collected soil samples were frozen until soil
and
concentrations of those samples were analyzed. Soil pH (H2O basis) was measured with a glass electrode pH meter (pH meter F-22, Horiba, Kyoto, Japan) in a 1:20 soil/deionized water mixture. Concentration of
in this suspension was also measured using ion chromatography (Dionex QIC Analyzer, Dionex Japan, Osaka, Japan). Ammonium in the soil was extracted with 2 mol L−1 potassium chloride solution (1:20 dried soil/water). Ammonium concentration was determined using colorimetry based on the indophenol-blue method with an ultraviolet-visible (UV-VIS) spectrophotometer (UV mini 1240, Shimadzu, Kyoto, Japan). Ammonium and
concentrations in soil samples, which were collected at the 0–10-cm depth from March 2004 to February 2006, were calculated by the weighted average of each concentration in soil samples from the 0–3-cm and 3–10-cm depth.
Statistical analysis
Comparisons of soil temperature and WFPS between conventional and bare treatments in CL-A, CL-B, and CL-C were analyzed with the Mann–Whitney's U-test (non-parametric). Comparisons of N2O fluxes or annual N2O emissions between conventional and bare treatments were performed using Student's t-test (parametric) based on studies that report N2O flux shows a log-normal distribution (Van-Cleemput et al. Citation1994; Velthof and Oenema Citation1995). Therefore, values of N2O flux or annual N2O emission were transformed logarithm values when student's t-test was carried out. The least significant difference test was used to determine significant differences (P < 0.05). One-sided 95% confidence interval of N2O emission data was calculated by using the following equation:
Results
Nitrous oxide fluxes in conventional and bare treatments in all plots increased from November to April (Figs ). Ranges of N2O fluxes during the study period in conventional treatments in CL-A, CL-B, and CL-C were 0–38, 0–1.46, and 0–9.27 mg N m−2 h−1, respectively. However, N2O fluxes in bare treatment in CL-A, CL-B, and CL-C were 0–43, 0–0.98, and 0–12 mg N m−2 h−1, respectively. Average N2O fluxes in conventional treatment from April to June, July to September, October to December and January to March significantly increased with increasing precipitation in each plot except for CL-C (CL-A y = 0.023x − 0.584, R = 0.55, P < 0.05; CL-B y = 0.0005x − 0.078, R = 0.64, P < 0.05; CL-Cy = 0.0019x − 0.037, R = 0.30, P = 0.18; GL y = 0.0011x − 0.134, R = 0.57, P < 0.05). There were no significant differences in N2O fluxes between conventional and bare treatments in CL-A, CL-B, and CL-C (). Nitrous oxide production rates were relatively high at 2.5–15-cm depth of soil compared to that below 15-cm depth in all plots ().
Figure 1 Seasonal variation in air temperature and precipitation (a), water table (b), soil temperature (c), water-filled pore space (WFPS) (d), ammonium (), nitrate (
) concentrations (e), and carbon dioxide (CO2) (f) and nitrous oxide (N2O) fluxes (g) in cropland A (CL-A) in central Kalimantan, Indonesia. Conventional and bare represents conventional cultivation treatment and bare treatment, respectively. Ammonium and
concentrations (e) are only reported in the conventional treatment. Error bars show standard deviation.
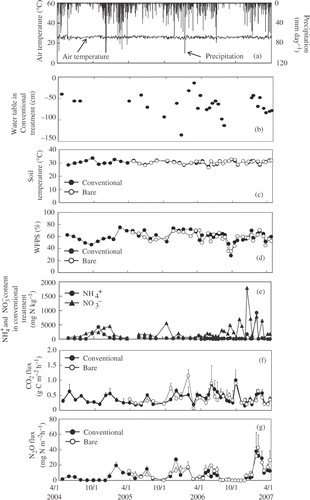
Figure 2 Seasonal variation in air temperature and precipitation (a), water table (b), soil temperature (c), water-filled pore space (WFPS) (d), ammonium (), nitrate (
) concentrations (e), and carbon dioxide (CO2) (f) and nitrous oxide (N2O) fluxes (g) in cropland B (CL-B) in central Kalimantan, Indonesia. Conventional and bare represents conventional cultivation treatment and bare treatment, respectively. Ammonium and
concentrations (e) are only reported in the conventional treatment. Error bars show standard deviation.
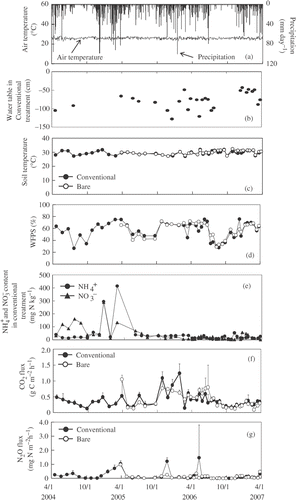
Figure 3 Seasonal variation in air temperature and precipitation (a), water table (b), soil temperature (c), water-filled pore space (WFPS) (d), ammonium (), nitrate (
) concentrations (e), and carbon dioxide (CO2) (f) and nitrous oxide (N2O) fluxes (g) in cropland C (CL-C) in central Kalimantan, Indonesia. Conventional and bare represents conventional cultivation treatment and bare treatment, respectively. Ammonium and
concentrations (e) are only reported in the conventional treatment. Error bars show standard deviation.
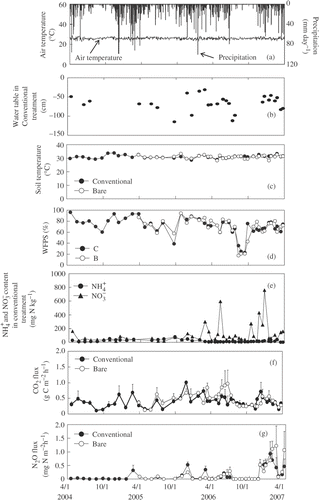
Figure 4 Seasonal variation in air temperature and precipitation (a), water table (b), soil temperature (c), water-filled pore space (WFPS) (d), ammonium (), nitrate (
) concentrations (e), and carbon dioxide (CO2) (f) and nitrous oxide (N2O) fluxes (g) in grassland (GL) in central Kalimantan, Indonesia. Error bars show standard deviation.
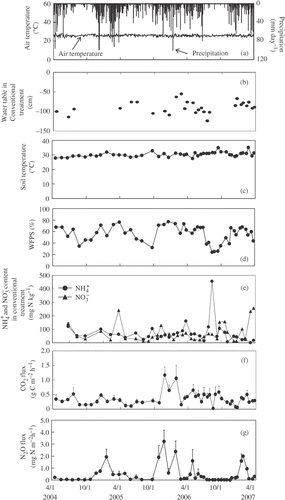
Table 2 Statistical analysis of the difference in nitrous oxide (N2O) flux, soil temperature, and water-filled pore space (WFPS) between conventional and bare treatments in cropland A (CL-A), cropland B (CL-B), and cropland C (CL-C) from April 2004 to March 2007 in central Kalimantan, Indonesia
Table 3 Net nitrous oxide (N2O) production rate in soil in conventional treatments in cropland A (CL-A), cropland B (CL-B), cropland C (CL-C), and grassland (GL) from April 2004 to March 2007 in central Kalimantan, Indonesia
Although the seasonal trend of water table depth was not clear, average water table depths in conventional treatments in CL-A, CL-B, CL-C, and GL were 67.2, 77.1, 67.8, and 87.7 cm, respectively (, , and ). In all plots, soil temperature was stable around 30°C (, 2c, 3c and 4c). There were no significant differences in soil temperature between conventional and bare treatments for the duration of the study (). Water-filled pore space in each plot tended to be high (around 80%) from November to April in all years (Figs ). There were no significant differences in WFPS between conventional and bare treatments in CL-A, CL-B, and CL-C (). Clear seasonal trends of soil and
concentrations in the plots were not observed (, , and ). The average
concentrations in conventional treatments in CL-A, CL-B, CL-C, and GL were 59.4, 35.2, 18.5, and 65.8 mg N kg−1, respectively. Average
concentrations in conventional treatments in CL-A, CL-B, CL-C, and GL were 249, 40.8, 111, and 63.7 mg N kg−1, respectively. Nitrate concentrations in CL-A and CL-C tended to be higher than in CL-B and GL. Average
concentrations in bare treatments in CL-A, CL-B and CL-C were 10.8, 10.8, and 13.6 mg N kg−1, respectively. Average
concentrations in bare treatments in CL-A, CL-B, and CL-C were 99.9, 2.6, and 12.5 mg N kg−1, respectively. Values of
and
concentrations in soil in bare treatment were lower than the values in conventional treatments in CL-A, CL-B, and CL-C (, and ). Water table depth and WFPS were almost equivalent between CL-A and CL-C ( and ). There were no consistent relationships at any of the plots between N2O flux and water table depth, soil temperature,
or
contents (). However, N2O flux in CL-A, CL-B, and GL significantly increased with increasing WFPS. In addition, N2O fluxes in CL-A and CL-C, in which N2O fluxes were relatively high compared with CL-B and GL, were significantly and positively correlated with CO2 fluxes (). Although not significant, N2O increased with increasing CO2 flux in bare treatment in CL-A and CL-B (CL-A y = 17.7x − 5.65, R = 0.36, P = 0.06; CL-B y = 0.46x − 0.04, R = 0.53, P < 0.01; CL-C y = 0.35x − 2.40, R = 0.02, P = 0.93). In all plots, the ratio of N2O flux and soil
concentration at log scale [ln(N2O/
)] were significantly correlated with WFPS (). However, the slope of ln(N2O/
) against WFPS was higher in CL-A (0.13) compared with other plots (0.05–0.08). Carbon dioxide flux in bare treatment tended to increase in the rainy season in CL-A, CL-B, and CL-C (, and ).
Figure 5 Relationships between logarithmic value of the ratio of nitrous oxide (N2O) flux and soil nitrate () concentration [ln(N2O flux/
)] against water-filled pore space (WFPS) in conventional treatment in cropland A (CL-A), cropland B (CL-B), cropland C (CL-C), and grassland (GL) in central Kalimantan, Indonesia.
![Figure 5 Relationships between logarithmic value of the ratio of nitrous oxide (N2O) flux and soil nitrate () concentration [ln(N2O flux/)] against water-filled pore space (WFPS) in conventional treatment in cropland A (CL-A), cropland B (CL-B), cropland C (CL-C), and grassland (GL) in central Kalimantan, Indonesia.](/cms/asset/24c41387-d279-4d49-a455-ab1d4bd39612/tssp_a_587203_o_f0005g.gif)
Table 4 Spearman's rank correlation coefficients of nitrous oxide (N2O) flux and water table depth, soil temperature, water-filled pore space (WFPS), ammonium ( ) and nitrate (
) and nitrate ( ) concentrations, or carbon dioxide (CO2) flux in conventional treatments from April 2004 to March 2007 in cropland A (CL-A), cropland B (CL-B), and cropland C (CL-C) and grassland (GL) in central Kalimantan, Indonesia
) concentrations, or carbon dioxide (CO2) flux in conventional treatments from April 2004 to March 2007 in cropland A (CL-A), cropland B (CL-B), and cropland C (CL-C) and grassland (GL) in central Kalimantan, Indonesia
Average annual N2O emissions during the study period in conventional treatment in CL-A, CL-B, CL-C, and GL were 580, 25.1, 92.5 and 43.1 kg N ha−1 year−1, respectively (). Significant linear correlations between annual N2O emission in conventional treatment in all plots and annual mean air temperature or annual precipitation were not observed (mean air temperature, P = 0.29; annual precipitation, P = 0.47). In CL-A and CL-C, in which the average N fertilizer application rate was more than 1000 kg N ha−1 year−1 (), annual N2O emissions in conventional treatments were larger than of those in CL-B or GL. Annual N2O emissions in conventional treatments significantly increased with N fertilizer application rate (y = 17.1 exp(1.00x), R = 0.70, P < 0.001). Even though N fertilizer was not applied in GL, annual N2O emissions were generally greater than 20 kg N ha−1 year−1 (). Average annual N2O emissions in bare treatment in CL-A, CL-B, and CL-C were 733, 6.96 and 126 kg N ha−1 year−1, respectively (). Significant differences in annual N2O emissions between conventional and bare treatments were observed in CL-B and CL-C during April 2005 to March 2006. Annual N2O, however, emissions in bare treatments in CL-A, CL-B, and CL-C were parallel to annual N2O emission in the conventional treatments (). The EFF was calculated only during April 2005 to March 2006 in CL-B and CL-C site, where annual N2O emissions between the two treatments were significantly different (). The EFF in CL-B and CL-C were 0.81 and 3.59%, respectively. At another site and period, the EFF was 0% because there were no significant differences in annual N2O emission between the conventional and bare treatments. From these values, annual N2O emission induced by N fertilizer and derived from N in SOM were estimated to 5.21 and 7.38 kg N ha−1 year−1 in CL-B and 38.3 and 35.4 kg N ha−1 year−1 in CL-C, respectively, during April 2005 to March 2006. In addition, the proportion of annual N2O emission derived from SOM N to annual N2O emission was 58.6 and 48.0% in CL-B and CL-C during April 2005 to March 2006, respectively.
Table 5 Annual nitrous oxide (N2O) emission in conventional and bare treatments from 2002 to 2007 in cropland A (CL-A), cropland B (CL-B), cropland C (CL-C), and grassland (GL) in central Kalimantan, Indonesia
Discussion
Main process of nitrous oxide production
Increases in N2O fluxes from October to May (, , and ) and significant correlation between three-month average N2O flux and precipitation suggest that N2O was actively produced during the rainy season. Wanner-Riddle et al. (Citation2007) reported large pulse fluxes of N2O in the wet season in tropical rain forests on mineral soil (e.g. Inceptisols, Oxisols) in Kenya (00°8′N–00°23′N, 34°46′–34°58′E). Takakai et al. (Citation2006) reported N2O flux was significantly correlated with soil concentration at or above 60–70% WFPS in the same field of this study. While clear relationships between N2O flux and water table depth, soil temperature,
or
concentrations in all sites were not observed (), significant correlations between N2O flux and WFPS in CL-A, CL-B, GL or between ln(N2O/
) and WFPS in all sites indicated that N2O was mainly produced by denitrification (, ). Nitrous oxide is generally produced in the processes of nitrification and denitrification (Bouwman Citation1996; Bremner Citation1997; Tiedje Citation1994). In the denitrification process, N2O is mostly reduced to N2 when WFPS is greater than 70% (Davidson et al. Citation2000). However, linear relationships between ln(N2O/
) and WFPS in the conventional treatment in each site suggested that N2O was not substantially reduced at high WFPS (). This indicates that N2O reductase (nos) in N2O-producing microbes in soil might be inactive even at high WFPS (i.e. above 70%). In acidic soils, higher N2O:N2 fraction for denitrification were reported (Alexander Citation1977). Dannenmann et al. (Citation2008) reported N2:N2O ratio increased exponentially with increasing pH in the soil Ah horizon between pH values of 6.2 and 7.3 in Germany. This means that N2O:N2 ratio increased with decreasing soil pH. In our study site, pH in surface soil was lower than 6.0. Thus, the reaction of N2O to N2 in denitrification process might be small due to low soil pH. Hashidoko et al. (Citation2008) found denitrifying bacteria (Janthinobacterium spp.) in the surface soil in CL-A, and suggested the possibility of inactivity of nos of Janthinobacterium spp. Furthermore, Yanai et al. (Citation2007) reported N2O-producing fungi Fusarium oxysporum and Neocosmospora vasinfecta were isolated in CL-A. Because the inactivity of nos in some fungal species were reported (Shoun et al. Citation2006), nos of the fungi in our study sites was possibly inactive. Nitrous oxide-producing bacteria and fungi without nos may potentially contribute to the high N2O flux and emission at the study site.
Origin of the substrate for nitrous oxide productions
Nitrous oxide flux was not always measured just after N fertilizer application and was measured randomly at most two times a month. However, seasonal variation of N2O flux in both conventional and bare treatments clearly showed the increase in N2O flux in the rainy season. In addition, differences in N2O fluxes between conventional and bare treatments were not observed (). These results suggest that N2O flux induced by applied N fertilizer was unknown or small compared with that by other form of N in soil such as soil organic N. Nitrate and C are required for denitrification as an electron accepter and donor, respectively (Bouwman Citation1996; Bremner Citation1997). Except for in CL-B and CL-C from April 2005 to March 2006, differences in annual N2O emission between conventional and bare treatments in CL-A, CL-B, and CL-C also were not observed. Net N2O production rate was mostly high in the soil depth of 2.5 to 15 cm (). Root barriers also were installed around bare treatments at 20-cm depth. This suggests N2O produced in conventional treatment probably did not influence N2O flux in the bare treatments. Therefore, this indicated that the source of N for N2O production was mainly derived from SOM in peat. Ammonium and concentrations in soil in bare treatments were lower than in the conventional treatments. In addition, CO2 flux in conventional treatment in CL-A and CL-C, in which high N2O fluxes were observed, was significantly correlated with N2O flux (). Therefore, the limiting factor of N2O production is the decomposition of SOM in peat. After decomposition of SOM, mineralized organic N was possibly nitrified and denitrified quickly to N2O. Takakai et al. (Citation2007) reported CO2 flux in conventional treatment during the rainy season in the same plot as our study field was significantly higher than during the dry season. In addition, there were no significant differences in CO2 emission between conventional and bare treatments in all plots due to the high decomposition rate of SOM (Takakai et al. Citation2007). This indicated that decomposition of SOM in peat was accelerated in the rainy season and production of CO2 was mainly induced by the decomposition of SOM, but not root respiration. Nitrous oxide flux tended to increase with increasing CO2 flux in bare treatment in CL-A and CL-B. Thus, N2O production might be closely influenced by the decomposition of SOM in peat. Also, N2O-producing bacteria or fungi (e.g. Janthinobacterium spp., Fusarium oxysporum, and Neocosmospora vasinfecta) probably were important decomposers of peat during the rainy season (Yanai et al. Citation2007; Hashidoko et al. Citation2008).
Nitrous oxide emission in agricultural peat land
Because there were no significant differences in N2O flux between conventional and bare treatments in CL-A, CL-B, and CL-C (), increase in annual N2O emission could not be due to increase in annual N fertilizer application rate. Thus, the correlation between annual N2O emission and annual N fertilizer application rate might indicate the influence of long-term management on annual N2O emission. Highest mean total N application in conventional treatment in CL-A among the study plots indicated large amount of N fertilizer might have been applied for a long time in CL-A compared with those in other plots. Management or history of cultivation prior to this study would change the quality of peat and characteristics of microorganisms. Though there was no referable study on tropical agricultural peatland, study on boreal organic soil in Finland, Maljanen et al. (Citation2003) reported difference in annual N2O emission between adjusted forested and cultivated peatlands. In addition, application of organic and chemical fertilizer improved the nutrient (N, phosphorous, and potassium) levels in soil over a 23-year period under a wheat–wheat–maize cropping system on a silt loam soil in China (Su et al. Citation2006). Zhong et al. (Citation2010) reported long-term application of chemical and organic fertilizer to mineral soil collected in Jiangxi Province, China changed soil quality and microbial community and diversity. Klemedtsson et al. (Citation2005) reported that when soil C:N ratio of peat in boreal forests in Sweden decreased from 90 to 13, mean annual N2O emission increased from 0.05 to 30 kg N ha−1 year−1. There were few differences in soil C:N ratio and soil pH among our study sites (Takakai et al. Citation2006). Thus, the influence of soil C:N ratio and soil pH on N2O emission were not clear. However, as reported by Klemedtsson et al. (Citation2005), quality of peat and cultivation history may be a good indicator for the estimation of N2O emission from agricultural peat soil in tropical region.
Annual N2O emissions from conventional treatment in CL-A, CL-B, and CL-C, in which fertilizers were applied, ranged from 12.6 to 698 kg N ha−1 year−1 (). These values of annual N2O emissions were greater than annual N2O emission (0.1–56 kg N ha−1 year−1) from boreal and temperate peat soils (Kasimir-Klemedtsson et al. Citation1997; Maljanen et al. Citation2003, Citation2004; Regina et al. Citation2004), and other N2O emission values (0.9–6.4 kg N ha−1 year−1) from mineral soil reviewed and summarized by Bouwman et al. (Citation2002). Furthermore, annual N2O emissions in this study were also greater than N2O emissions from tropical agricultural peat soil in south Kalimantan, Indonesia (−1.1–2.03 kg N ha−1 year−1, Hadi et al. Citation2005; Inubushi et al. Citation2003) and Sarawak, Malaysia (1.2–3.3 kg N ha−1 year−1, Melling et al. Citation2007). Therefore, annual N2O emission from agricultural fields in our study field might be the highest value reported to date. Absence of the difference in annual N2O emission between conventional and bare treatment plots, except for in CL-B and CL-C from April 2005 to March 2006, suggested that most of the emitted N2O in conventional treatment derived from the decomposition of SOM. Therefore, larger annual N2O emission compared with N2O emissions in those studies might be affected by factors other than the amount of N fertilizer application. The agricultural peat lands in our study sites are cultivated generally three to four times a year. Fertilizer was applied in each cultivation. Thus, cultivation practices in our study site were different than that reported of tropical peatland where oil palms (Melling et al. Citation2007) and sago palm (Hadi et al. Citation2005) were cultivated. Intensive cultivation with high N application on drained peat soil may change the quality of peat suitable for N2O production by denitrification. Kasimir-Klemedtsson et al. (Citation2009) reported N2O emission peaked after soil cultivation, plowing and harrowing in grassland on drained peatland in Sweden (58°20’N, 13°30’E). The period of cultivation on drained peat land possibly affects the N2O emission because the quality of peat and microbial community changes gradually after drainage (Su et al. Citation2006; Zhong et al. Citation2010). Hence, differences in history and management of cultivation may cause large variation of N2O emission from tropical agricultural peat soil. Currently, there are not many studies discussing N2O emission in agricultural fields on tropical peat soil. Additional research regarding N2O emission from agricultural fields on tropical peat soil, therefore, is needed because it is likely that large amounts of N2O have been emitted and will continue to be emitted after deforestation and establishment of arable land on peat soil.
In our study site, most N2O was derived from the decomposition of SOM. Even when the difference in annual N2O emission was detected, the ratio of N2O emission induced by N fertilizer and derived from SOM decomposition was almost equal (48–58.6%). Since annual N2O emission in agricultural tropical peat land was higher than that in a natural forest, a regenerated forest after burning, and a burned forest located close to our study site, N2O emission derived from SOM might be influenced by cultivation or land-use change from natural forest to agricultural field. Indeed, the N2O emission factor induced by the cultivation in tropical peat soil (16 kg N ha−1 year−1) estimated by IPCC (Citation2006) was calculated using the data in boreal peat soil (8 kg N ha−1 year−1). However, estimated N2O emission originated from decomposition of SOM exceeded the value provided from IPCC (Citation2007), whereas N2O emission derived from SOM in other agricultural peat soil reported by Melling et al. (Citation2007) and Hadi et al. (Citation2005) might be lower because N2O emissions from those study sites were lower than 4 kg N ha−1 year−1 despite application of N fertilizer at those sites. This indicated that the N2O emission derived from decomposition of SOM in tropical agricultural peat soil might vary widely. Thus, additional research on N2O emission in tropical agricultural peat soil is needed to provide a more accurate value of EFF for the cultivation in tropical peat soil, its uncertainty, and to determine the factors influencing its variation.
The EFF value of this study (0–3.59%) was smaller than the range reported by Takakai et al. (Citation2006) (1.8–36%) in our study field. Because Takakai et al. (Citation2006) used N2O emission values from GL as the estimate for N2O emission in CL-A, CL-B, and CL-C, EFF may have been overestimated due to spatial variation of N2O emission among the sites. However, the EFF values in this study were similar or higher than the 1.00% value reported by IPCC (Citation2006). Akiyama et al. (Citation2006) summarized published data and reported that EFF in well-drained and poorly drained soil were 0.32 and 1.40%, respectively. Bouwman (Citation1996) reported EFF of chemical and organic fertilizer ranged from 0.1 to 1.6% on mineral soil. Thus, EFF values in our study were similar or slightly higher than other agricultural fields on mineral soil. However, EFF, on dry and wet Histosols in the Netherlands were 4.21 and 1.38%, respectively (Van Beek et al. Citation2010). Although more work is needed to normalize EFF values in agricultural peat land in tropical regions, EFF value in Histosols may be higher than in mineral soils.
Acknowledgments
The authors would like to thank Dr Hanny Wijaya (Bogor Agricultural University) and the staffs at the University of Palangka Raya (Tony Wahyudi, Ledy, Logah, Paty, Ube) for their support during the research. Also, the authors’ appreciation goes to Professor Takashi Hirano (Hokkaido University) for providing climatic data. This study was partly supported by the JSPS-LIPI Core University Program and a Japanese Grant-in-Aid for Science Research from the Ministry of Education, Culture, Sports, Science and Technology (No. 13574012). Also, this study was financially supported by the Global Environmental Research Program of the Ministry of the Environment of Japan (No. S-2).
References
- Akiyama , H , Yan , X and Yagi , K . 2006 . Estimations of emission factors for fertilizer-induced direct N2O emissions from agricultural soils in Japan: summary of available data . Soil Sci. Plant Nutr. , 52 : 774 – 787 .
- Alexander , M . 1977 . Introduction to Soil Microbiology , 2nd , New York : John Wiley & Sons .
- Andriesse JP 1988: Nature and Management of Tropical Peat Soils, FAO Soils Bulletin 59. Food and Agriculture Organization of the United Nations, Rome
- Bouwman , AF . 1996 . Direct emission of nitrous oxide from agricultural soils . Nutr. Cycling Agroecosys. , 46 : 53 – 70 .
- Bouwman AF, Boumans LJM, Batjes NH 2002: Emissions of N2O and NO from fertilized fields: summary of available measurement data. Glob. Biogeochem. Cycles, 16, 1058–1070, doi: 10.1029/2001GB001811
- Bremner , JM . 1997 . Sources of nitrous oxide in soils . Nutr. Cycling Agroecosys. , 49 : 7 – 16 .
- Couwenberg , J , Dommain , R and Joosten , H . 2009 . Greenhouse gas fluxes from tropical peatlands in south-east Asia . Glob. Change Biol. , 16 : 1715 – 1732 .
- Dannenmann , M , Butterbach-Bahl , K , Gasche , R , Willibald , G and Papen , H . 2008 . Dinitrogen emissions and the N2:N2O emission ratio of a Rendzic Leptosol as influenced by pH and forest thinning . Soil Biol. Biochem. , 40 : 2317 – 2323 .
- Davidson , EA , Keller , M , Erickson , HE , Verchot , LV and Veldkamp , E . 2000 . Testing a conceptual model of soil emissions of nitrous and nitric oxides . BioScience , 50 : 667 – 680 .
- Granli , T and Bøckman , OC . 1994 . The experimental basis, nitrous oxide from agriculture . Nor. J. Agric. Sci. , 12 : 22 – 29 .
- Hadi , A , Inubushi , K , Furukawa , Y , Purnomo , E , Rasmadi , M and Tsuruta , H . 2005 . Greenhouse gas emissions from tropical peatlands of Kalimantan, Indonesia . Nutr. Cycling Agroecosys. , 71 : 73 – 80 .
- Hashidoko , Y , Takakai , F , Toma , Y , Darung , U , Melling , L , Tahara , S and Hatano , R . 2008 . Emergence and behaviors of acid-tolerant Janthinobacterium sp. that evolves N2O from deforested tropical peatland . Soil Biol. Biochem. , 40 : 116 – 125 .
- Hirano , T , Segah , H , Harada , T , Limin , S , June , T , Hirata , R and Osaki , M . 2007 . Carbon dioxide balance of a tropical peat swamp forest in Kalimantan, Indonesia . Glob. Change Biol. , 13 : 412 – 425 .
- Hirano , T , Segah , H , Limin , S , June , T , Tuah , SJ , Kusin , K , Hirata , R and Osaki , M . 2005 . Energy balance of a tropical peat swamp forest in central Kalimantan, Indonesia . Phyton , 45 : 67 – 71 .
- Hooijer A, Silvius M, Wösten H, Page S 2006: PEAT–CO2 – Assessment of CO2 Emissions from Drained Peatlands in SE Asia, Delft Hydraulics Report Q3943, p. 36. Delft Hydraulics, Delft
- Intergovernmental Panel on Climate Change (IPCC) 1995: Climate Change 1995: The Science of Climate Change. Contribution of Working Group I to the Second Assessment Report of the Intergovernmental Panel on Climate Change, Houghton JT, Meira Filho LG, Callander BA, Harris N, Kattenberg A, Maskell K (eds). Cambridge University Press, Cambridge
- Intergovernmental Panel on Climate Change (IPCC) 2006: N2O emissions from managed soils, and CO2 emissions from lime and urea application. In: 2006 IPCC Guidelines for National Greenhouse Gas Inventories. Vol. 4. Agriculture, Forestry and Other Land Use. http://www.ipcc-nggip.iges.or.jp/public/2006gl/vol4.htm (accessed 15 July 2010)
- Intergovernmental Panel on Climate Change (IPCC) . 2007 . Climate Change 2007: The Physical Science Basis , Cambridge : Cambridge University Press .
- Inubushi , K , Furukawa , Y , Hadi , A , Purnomo , E and Tsuruta , H . 2003 . Seasonal changes of CO2, CH4 and N2O fluxes in relation to land-use change in tropical peatlands located in coastal area of south Kalimantan . Chemosphere , 52 : 603 – 608 .
- Kasimir-Klemedtsson , Å , Klemedtsson , L , Berglung , K , Martikainen , P , Silvola , L and Oenema , O . 1997 . Greenhouse gas emissions from farmed organic soils: a review . Soil Use Manag. , 13 : 245 – 250 .
- Kasimir-Klemedtsson , Å , Weslien , P and Klemedtsson , L . 2009 . Methane and nitrous oxide fluxes from a farmed Swedish Histsol . Europ. J. Soil Sci. , 60 : 321 – 331 .
- Klemedtsson , L , Arnold , KV , Weslien , P and Gundersen , P . 2005 . Soil CN ratio as a scalar parameter to predict nitrous oxide emissions . Glob. Change Biol. , 11 : 1142 – 1147 .
- Kusa , K , Sawamoto , T , Hu , R and Hatano , R . 2008 . Comparison of the closed-chamber and gas concentration gradient methods for measurement of CO2 and N2O fluxes in two upland field soils . Soil Sci. Plant Nutr. , 54 : 777 – 785 .
- Maljanen , M , Komulainen , VM , Hytönen , L , Martikaine , PJ and Laine , J . 2004 . Carbon dioxide, nitrous oxide and methane dynamics in boreal organic agricultural soils with different soil management . Soil Biol. Biochem. , 36 : 1801 – 1808 .
- Maljanen , M , Liikanen , A , Silvola , J and Martikaine , PJ . 2003 . Nitrous oxide emissions from boreal organic soil under different land-use . Soil Biol. Biochem. , 35 : 689 – 700 .
- Maltby , E and Immirzi , P . 1993 . Carbon dynamics in peatlands and other wetland soils. Regional and global perspectives . Chemosphere , 27 : 999 – 1023 .
- Melling , L , Hatano , R and Goh , KJ . 2007 . Nitrous oxide emissions from three ecosystems in tropical peatland of Sarawak, Malaysia . Soil Sci. Plant Nutr. , 53 : 792 – 805 .
- Morishita , T , Hatano , R and Desyatkin , RV . 2003 . CH4 flux in an alas ecosystem formed by forest disturbance near Yakutsk, eastern Siberia, Russia . Soil Sci. Plant Nutr. , 49 : 369 – 377 .
- Mosier , AR , Kroeze , C , Nevison , C , Oenema , O , Seitzunger , S and Van Cleemput , O . 1998 . Closing the global N2O budget: nitrous oxide emissions through the agricultural nitrogen cycle. OECD/IPCC/IEA phase development of IPCC guidelines for national greenhouse gas inventory methodology . Nutr. Cycling Agroecosys. , 52 : 225 – 248 .
- Muhanmad NZ, Rieley JO 2002: Management of tropical peatlands in Indonesia: mega reclamation project in central Kalimantan. In: Rieley JO, Page SE (eds) Peatlands for People, Natural Resources Function and Sustainable Management, proceedings of the International Symposium on Tropical Peatlands, August 22–23, 2001, Jakarta, Indonesia, pp. 155–160. BPPT and Indonesian Peat Association, Jakarta
- Nakano , T , Sawamoto , T , Morishita , T , Inoue , G and Hatano , R . 2004 . A comparison of regression methods for estimating soil–atmosphere diffusion gas fluxes by a closed-chamber technique . Soil Biol. Biochem. , 36 : 107 – 113 .
- Page , SE , Slegert , F , Rieley , JO , Boehm , HDV , Zaya , A and Limin , S . 2002 . The amount of carbon released from peat and forest fires in Indonesia during (1997) . Nature , 420 : 61 – 65 .
- Ravishankara , AR , Daniel , JS and Portmann , RW . 2009 . Nitrous oxide (N2O): the dominanat ozone-depleting substance emitted in the 21st century . Nature , 326 : 123 – 125 .
- Regina , K , Syvasalo , E , Hannukkala , A and Esala , M . 2004 . Flux of N2O from farmed peat soils in Finland . Eur. J. Soil Sci. , 55 : 591 – 599 .
- Shoun , H , Kim , DH , Uchiyama , H and Sugiyama , J . 2006 . Denitrification by fungi . FEMS Microbio. Lett. , 94 : 277 – 281 .
- Su , YZ , Wnag , F , Suo , DR , Zhang , ZH and Du , MW . 2006 . Long-term effect of fertilizer and manure application on soil–carbon sequestration and soil fertility under the wheat–wheat–maize cropping system in northwest China . Nutr. Cycling Agroecosys. , 75 : 285 – 295 .
- Takakai , F , Morishita , T , Hashidoko , Y , Darung , U , Kuramochi , K , Dohong , S , Limin , SH and Hatano , R . 2006 . Effects of agricultural land-use change and forest fire on N2O emission from tropical peatlands, central Kalimantan, Indonesia . Soil Sci. Plant Nutr. , 52 : 662 – 674 .
- Takakai F, Toma Y, Morishita T, Darung U, Dohong S, Limin SH., Hatano R 2007: Contribution of organic matter decomposition and root respiration to CO2 emissions from cultivated tropical peatland in central Kalimantan, Indonesia. In: Iswandi A, Wijaya HC, Guhardja S, Segah H, Iwakuma T, Osaki M (eds) Proceedings of the International Workshop on Human Dimension of Tropical Peatland under Global Environmental Changes, December 8–9, 2004, Bogor, Indonesia, pp. 101–111. Bogor Agricultural University/Hokkaido University, Bogor/Hokkaido
- Terry , RE , Tate , RL and Duxbury , JM . 1981 . Nitrous oxide emissions from drained, cultivated organic soils of South Florida . J. Air Pollut. Contr. Assoc. , 31 : 1173 – 1176 .
- Tiedje JM 1994: Denitrifiers. In: Weaver RW (ed) Methods of Soil Analysis. Part 2. SSSA Book Series 5, pp.245–257 SSSA, Madison, MI
- Toma , Y and Hatano , R . 2007 . Effect of crop residue C:N ratio on N2O emissions from Gray Lowland soil in Mikasa, Hokkaido, Japan . Soil Sci. Plant Nutr. , 53 : 198 – 205 .
- Toma , Y , Kimura , SD , Yamada , H , Hirose , Y , Fujiwara , K , Kusa , K and Hatano , R . 2010 . Effects of environmental factors on temporal variation in annual carbon dioxide and nitrous oxide emissions from an unfertilized bare field on Gray Lowland soil in Mikasa, Hokkaido, Japan . Soil Sci. Plant Nutr. , 56 : 663 – 675 .
- Van Beek , CL , Pleijiter , M , Jacpbs , CMJ , Velthof , GL , van Groenigen , JW and Kuikman , PJ . 2010 . Emissions of N2O from fertilized and grazed grassland on organic soil in relation to groundwater level . Nutr. Cycling Agroecosys. , 86 : 331 – 340 .
- Van-Cleemput , O , Vermoesen , A , Degroot , CJ and Vanryckeghem , K . 1994 . Nitrous oxide emission out of grassland . Environ. Monit. Assess. , 31 : 145 – 152 .
- Velthof , GL and Oenema , O . 1995 . Nitrous oxide fluxes from grassland in the Netherlands. 1. Statistical analysis of flux-chamber measurements . Eur. J. Soil Sci. , 46 : 533 – 540 .
- Wanner-Riddle , C , Furon , A , Mclaughlin , NL , Lee , I , Barbeau , J , Jayasundara , S , Parkin , G , Bertoldi , P and Warland , J . 2007 . Intensive measurement of nitrous oxide emissions from a corn–soybean–wheat rotation under two contrasting management systems over 5 years . Glob. Change Biol. , 13 : 1722 – 1736 .
- Yanai , Y , Toyota , K , Morishita , T , Takakai , F , Hatano , R , Limin , SH , Darung , U and Dohong , S . 2007 . Fungal N2O production in an arable peat soil in central Kalimantan, Indonesia . Soil Sci. Plant Nutr. , 53 : 806 – 811 .
- Zhong , W , Gu , I , Wnag , W , Zhang , B , Lin , X , Huang , Q and Shen , W . 2010 . The effects of mineral fertilizer and organic manure on soil microbial community and diversity . Plant Soil , 326 : 511 – 522 .