Abstract
Soil respiration is a carbon flux that is indispensable for determining carbon balance despite variations over time and space in forest ecosystems. In Kanchanaburi, western Thailand, we measured the soil respiration rates at different slope positions—ridge (plot R), upper slope (plot U), and lower slope (plot L)—on a hill in a seasonal tropical forest [mixed deciduous forest (MDF)] to determine the seasonal and spatial variations in soil respiration on the slope. The heterotrophic (organic layer and soil) and autotrophic (root) respiration was differentiated by trenching. Soil respiration rates showed clear seasonal patterns: high and low rates in rainy and dry seasons respectively, at all plots, and tended to decrease up the slope. Soil respiration rates responded significantly to soil water content in the 0–30 cm layer, but the response patterns differed between the lower slope (plot L) and the upper slope (plots R and U): a linear model could be applied to the lower slope but exponential quadratic models to the upper slope. The annual carbon dioxide (CO2) efflux from the forest floor was also associated with the slope position and ranged from 1908 gC m−2 year−1 in plot L to 1199 gC m−2 year−1 in plot R. With ascending position from plot L to R, the contribution of autotrophic respiration increased from 19.4 to 36.6% of total soil respiration, while that of the organic layer decreased from 26.2 to 9.4%. Mineral soil contributed to 46.3 to 54.4% of the total soil respiration. Soil water content was the key factor in controlling the soil respiration rate and the contribution of the respiration sources. However, the variable responses of soil respiration to soil water content create a complex distribution of soil respiration at the watershed scale.
Introduction
Carbon dynamics in a forest ecosystem are of great concern in relation to the influence of global warming and the function of forest ecosystems in mitigating atmospheric carbon dioxide (CO2). In terrestrial ecosystems, soil is the largest carbon reservoir so their fluctuation may considerably affect the atmospheric CO2 concentration (Lal Citation2004; Knorr et al. Citation2005). With this in mind, soil respiration is a crucial parameter for evaluating the carbon balance in forest ecosystems (Raich and Schlesinger Citation1992; Raich and Tufekciogul Citation2000; Yang et al. Citation2007).
Previous reviews of soil respiration in global forest ecosystems did not distinguish dry and moist forests in the tropical biomes (Raich and Schlesinger Citation1992; Raich and Tufekciogul Citation2000; Subke et al. Citation2006). In monsoonal Asia, seasonal dry forests, where continuous drought periods occur, are widely distributed from the Indochina peninsula to India (Bullock et al. Citation1995). Following the climate pattern, soil respiration in a forest ecosystem shows seasonal fluctuation, whereby high and low soil respiration is recorded in the rainy and dry seasons respectively (Tulaphitak et al. Citation1985; Cuevas Citation1995; Hashimoto et al. Citation2004; Adachi et al. Citation2009). In contrast to the soil respiration in moist temperate forests, where the soil temperature mostly determines the soil respiration rate (Ishizuka et al. Citation2006; Shinjo et al. Citation2006), soil moisture is the most influential factor in controlling the soil respiration rate in tropical seasonal forests (Hashimoto Citation2005; Takahashi et al. Citation2009).
To scale up carbon balance from the stand level to the watershed level, spatial variation in soil respiration in the watershed must be examined. Because topography regulates soil water movement and drainage, lower slopes tended to be moist and upper slopes drier, which created a biogeochemical gradient on the slope (Swanson et al. Citation1988; Hirobe et al. Citation1999; Tsui et al. Citation2004). Likewise, soil respiration varies according to the topographical position on the hill slope. For instance, Nakane et al. (Citation1984) reported that the lower slope had higher soil respiration rates than the mid-slope in a Japanese red pine forest. Shimada et al. (Citation1998) also reported that the annual carbon efflux from the forest floor was 13.4 MgC ha−1 year−1 on the lower slope and 9.9 MgC ha−1 year−1 on the upper slope in a Japanese coniferous plantation. However, no correlation (Fang et al. Citation2009), and the opposite trend, namely lower soil respiration rates on the lower slope and at the valley bottom than the upper slope (Chambers et al. Citation2004; Tsutsumi et al. Citation1985; Epron et al. Citation2006; Mitani et al. Citation2006; Kosugi et al. Citation2007) have been reported in several forest ecosystems. Although many reports have suggested that soil respiration varies with slope position, no general principle concerning the relationship between slope position and soil respiration has yet been established.
Soil respiration comprises heterotrophic respiration by soil biota and autotrophic respiration by living plant roots hence separating these respiration sources is important to understand the carbon balance in an ecosystem (Hamilton et al. Citation2002; Bond-Lamberty et al. Citation2004; Cisneros-Dozal et al. Citation2006; Sakata et al. Citation2007; Yang et al. Citation2007). Although there are methodological difficulties in separating respiration sources (Hanson et al. Citation2000; Subke et al. Citation2006), one practical method to estimate root respiration is to dig a trench around the respiration monitoring chamber in order to exclude root respiration from the soil (Katagiri Citation1988; Lee et al. Citation2003; Sulzman et al. Citation2005; Vogel et al. Citation2005, Wang et al. Citation2006; Schaefer et al. Citation2009). The root trenching method in particular is often used in the forest ecosystem because of its high applicability to remote areas despite criticisms that disturbance by trenching affects root decomposition, soil moisture, and the soil microbial community (Hanson et al. Citation2000; Kuzyakov and Larionova Citation2005). Review articles have been written on the separation of autotrophic and heterotrophic respiration in forest ecosystems worldwide (Hanson et al. Citation2000; Bond-Lamberty et al. Citation2004; Subke et al. Citation2006), but few studies have yet been conducted on tropical forests, especially in a tropical monsoon climate (Schaefer et al. Citation2009; Takahashi et al. Citation2009; Yi et al. Citation2007). In addition, variations in heterotrophic and autotrophic respiration in different topographies have never been reported.
The purpose of this study is to (1) understand the spatial variation in soil respiration rates on a slope, and (2) estimate the contribution of CO2 sources, i.e. organic layer, roots, and soil, in terms of the total soil respiration. We measured the soil respiration rates at three different positions on a hill, and analyzed the spatial variation in the soil respiration rates in a watershed of a tropical seasonal forest in western Thailand. Carbon dioxide sources were separated by removing the organic layer and roots by trenching. The annual carbon efflux from the forest floor over two years was also estimated.
Materials and Methods
Study sites
The study was conducted in the Mae Klong Watershed Research Station (14°35′N, 98°52′E), Lintin, Thong Pha Phum, Kanchanaburi province, western Thailand (). The average air temperature was about 25°C, ranging from 9.3 to 42.2°C, and the annual precipitation was 1650 mm, mostly falling during the rainy season from April to October (Suksawang Citation1995). Tributary streams dry up in the dry season. The monthly rainfall and temperature during the study period from 1997 to 1999 have been reported elsewhere (Takahashi et al. Citation2009). Rainfall sometimes fluctuates because of the El Niño Southern Oscillation (Nounmusig et al. Citation2006): the annual rainfall at the station was 1927 mm in 1997, 1243 mm in 1998, and 1733 mm in 1999 respectively. The forest in the watershed was mainly covered by mixed deciduous forest (MDF) (Rundel and Boonpragob Citation1995). Detailed descriptions of the vegetation and eco-physiological characteristics were given by Marod et al. (Citation1999, Citation2002) and Ishida et al. (Citation2006). The dominant tree species in the study area were Shorea siamensis, Vitex peduncularis, Dillenia parviflora var. Keruii, and Xylia xylocarpa var. Keruii. The tree density and basal area, DBH > 5 cm, of the forest were 171 trees ha−1 and 17.3 m2 ha−1 respectively. Four bamboo species were distributed mosaically under the tree canopy (Takahashi et al. Citation2007). Although the forest is called a natural forest, there may have been selective felling of particular trees by local people. The dry weight of the annual litterfall on the upper part of the study slope (plots R and U, see later) was 5.91 Mg ha−1 in 1997, 5.88 Mg ha−1 in 1998, and 3.75 Mg ha−1 in 1999, and leaf litter accounted for 55% of the total litterfall on average (Marod, unpublished data, 2000). The soil at the study sites was classified as Alfisols (Soil Survey Staff Citation2006) derived from sedimentary rock, gneiss, and limestone (Suksawang Citation1995). The chemical and physical characteristics of the soil at the pits close to the plots for soil respiration measurement are shown in . In the Mae Klong Watershed Research Station, the net ecosystem CO2 and water exchange of the forest have been monitored by a flux tower (Huete et al. Citation2008; Fisher et al. Citation2009).
Figure 1 Map of the study site in Thailand. Plots R, U, and L denote the ridge, upper, and lower slopes, respectively.
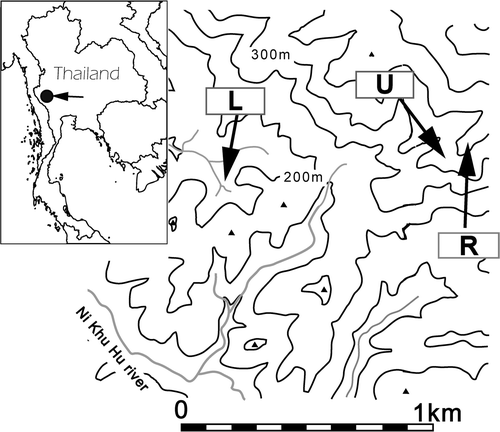
Table 1 Chemical and physical properties of the representative soil profiles at the Mae Klong Watershed Research Station
The watershed was mountainous, with an incline of about 40° in some places. To evaluate variation in the soil respiration rate according to slope positions in the watershed, three positions were selected: ridge (R), upper slope (U), and lower slope (L) (). The altitudes of the study plots ranged from 150 to 350 m above sea level (a.s.l.). The tree species composition and soil properties at the study plots varied slightly but were classified as the same, MDF and Alfisols, respectively.
Measurement of soil respiration
The soil respiration rate was measured by a static closed chamber (Takahashi et al. Citation2009), with a steel chamber 30 cm in diameter and 30 cm in height. The bottom rim of the chamber was inserted about 3 cm into the surface soil. Its top cover was sealed and about 0.5 L of the air in the headspace was collected at a 20-minute interval to determine the CO2 concentration by an infrared gas analyzer (IRGA) (ZFP5, Fuji Electronics Co. Ltd, Japan). Measurements were performed mostly from 9 a.m. to 2 p.m. We confirmed the diurnal variation of soil respiration was insignificant, similar to the other sites in Thailand (Adachi et al. Citation2009; Hanpattanakit et al. Citation2009). Soil respiration rates were calculated from the rate of increase of CO2 concentration using a linear model with temperature correction as follows: Sr = ρ × V/A × ΔC/ΔT × 273/(273 + T) × 12/44, where Sr is the CO2 flux (in gC m−2 h−1), ρ is the CO2 density (1.96 × 103 g m−3), V is the chamber volume (in m3), A is the cross-sectional area (in m2), ΔC/ΔT is the CO2 concentration increase rate (in m3 m−3 h−1) during time ΔT (in h−1) and T is the temperature (in degrees centigrade) inside the chamber (Hu et al. Citation2004).
In 1997, the soil respiration rate in plot U was measured monthly to identify seasonal variation in the soil respiration in the study forest. Subsequently, in 1998 and 1999, the soil respiration of the different slope positions, i.e. plots R, U, and L, was compared by bimonthly measurement.
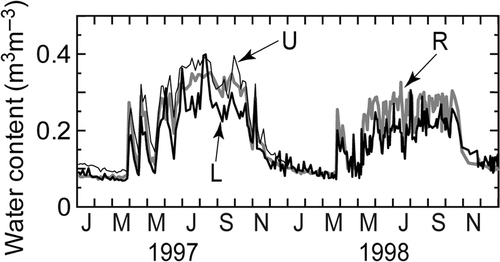
When the soil respiration rate was measured, the surface soil (0–5 cm) and organic layer samples around the chambers were collected to determine the moisture content. The sample was dried at 105°C in an oven and the water content was expressed on a fresh weight basis. The air and soil temperature at a depth of 10 cm were also measured at the plots. The volume metric soil moisture in the 0–15 and 15–30 cm layers were monitored every one to three days by TDR sensors (Moisture Point TM Model MP-917, Environmental Sensors Inc., Victoria, BC, Canada). The TDR measurement was performed from 7 a.m. to 10 a.m. by manual inspection.
Estimating the annual carbon efflux from the forest floor
Using the relationship between the soil respiration rate and soil water content, empirical models (Wen et al. Citation2006) were examined to estimate the former at each study plot. To estimate the annual carbon efflux from the surface of the forest floor, the cumulative amount of carbon was calculated by multiplying the soil respiration rate obtained by using the empirical model by the interval of days of soil moisture monitoring during that year. Variation in the annual soil carbon efflux in 1997 and 1998 was calculated from the soil moisture data over two years in the same manner, although the soil respiration rates in plots R and L were not measured in 1997. There were no annual estimates in 1999 due to TDR sensor troubles at certain periods of the year.
Separation of carbon dioxide sources
The CO2 sources in soil respiration were separated by trenching as described previously (Takahashi et al. Citation2009). In short, four sets of three chambers were installed at each site. The first chamber (chamber A) was for measuring the total soil respiration. The second chamber (chamber B) was for measuring soil respiration without CO2 respiration from the organic layer, and was prepared by removing the organic layer from the chamber. The third chamber (chamber C) was for measuring mineral soil only, and was prepared by removing the organic layer and cutting the roots with a 30 cm-deep trench around the chamber, which was treated for two months before measurement commenced. The difference in soil respiration between chambers A and B indicated CO2 respiration from the organic layer. The difference between chambers B and C indicated CO2 respiration from the roots at a depth of 30 cm.
The annual contribution of roots, organic layer, and soil to total soil respiration was estimated in the same way as for the calculation of the annual carbon efflux, i.e. using empirical models derived from the relationships between soil respiration rates in chambers B and C, soil water content in a 0–30 cm layer, and data from monitoring soil water content. Root respiration from soil layers deeper than 30 cm was corrected by the proportion of root biomass in the 0–30 cm layer to that in the deeper rooting zone. In this study we assumed that the rooting zone was 1.2 m deep and that root respiration was related to the fine root biomass (<3 mm).
Carbon stocks in the soil and organic layer and root biomass
The carbon stock in the soil was determined by the bulk density of the fine soil (<2 mm) measured by soil cylinder core (400 mL, 100 cm2 × 4 cm) and the carbon content in the fine soil, which was collected from soil profiles of the pits located close to the plots for soil respiration monitoring (). The organic layer was collected using a 0.5 m × 0.5 m frame with four replications in March, July, September, and December in 1998, and weighed after oven-drying at 70°C. The carbon content was analyzed by dry combustion using an NC analyzer (NC-800, Sumitomo Chemical Co., Japan).
Root biomass (<2 cm in diameter) was measured at 30 cm intervals up to a final depth of 1.2 m via a 15 cm × 15 cm soil column. The sampling was performed in triplicate in November 1998, with dead roots eliminated by visible inspection. Roots of diameter finer than 3 mm were classified as fine roots. All roots, including trees, bamboo, and herbs, were washed to remove the soil, and weighted after oven-drying at 70°C.
Statistics
Differences in soil respiration and related parameters among plots were tested by repeated measures of analysis of variance (ANOVA). Regression analysis was also performed to analyze the soil respiration control factors. Models of linear and quadratic functions were selected to estimate the soil respiration rate from the soil water content. All the statistics were generated by STATISTICA (Stat Japan, Co., Ltd.).
Results
Carbon stocks and root biomass along with slope position
The carbon stock in the organic layer, which mainly comprised fresh litter (Oi layer), was relatively larger on the upper slope (plots R and U) than the lower slope (plot L) (). For the surface soil 0–0.3 m deep, carbon stock increased up the slope. About half the carbon stock was concentrated in the surface soil layer (A/B in ).
Table 2 Carbon stock (in kgC m−2) in the organic layer (O-layer), soil at a depth of 0 to 0.3 m, and soil at a depth of 0 to 1.2 m
The total root biomass (<2 cm in diameter) increased up the slope from plots L to R (), and plot R had 3.2 times more root biomass than plot L. Fine roots (<3 mm) were mainly distributed in the surface soil 0–0.3 m deep, and the biomass in plot R was 1.8 times larger than in L.
Table 3 Total root biomass (<2 cm) and fine-root biomass (<3 mm) in soil up to 1.2m deep and fine-root biomass 30 cm deep at the study plots
Soil respiration rate, soil temperature, and water content
The soil respiration rates in all the plots showed a seasonal pattern: high and low in the rainy and dry seasons respectively (). The plot L tended to have higher soil respiration rates (CO2-C g m−2 d−1) than plots U and R in both the dry and rainy seasons: 5.49 in the dry season and 7.35 during the rainy season in plot L, compared to 2.98 in the dry season and 6.35 during the rainy season in plot R of the average field measurements (). The dry season significantly reduced the soil respiration rate in plot R, when compared to plot L. The soil respiration rates in plot R decreased to 86 and 54% of that in plot L for the rainy and dry seasons.
Figure 2 Seasonal changes in the soil carbon dioxide (CO2) efflux at the ridge (R), upper slope (U), and lower (L) slope positions, soil water content, organic layer water content, and soil temperature at a depth of 10 cm. Symbols in the uppermost figure (R), i.e. soil respiration of chamber A (dot, total soil respiration), chamber B (open triangle, without organic layer respiration), and chamber C (circle, without root and organic layer respiration) can be applied to slope positions U and L. The vertical bar denotes standard error (n = 4). In the lowest figure, no data were available in October 1999 (the arrow mark).
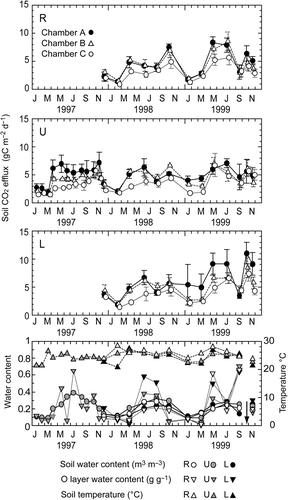
Table 4 Comparison of average air temperature, soil temperature, water content in the organic layer (O-layer) and soil, and soil respiration rate during the dry and rainy seasons for each plot
The soil temperature and moisture regimes also differed depending on the slope position. The average soil temperature increased, but the soil water content decreased up the slope. In the dry season, the soil was severely dry in plot R, whereas during the rainy season, the soil temperature and water content did not differ significantly among the plots. For the organic layer, the water content was high, with significant fluctuation during the rainy season, and low in the dry season ( and ).
The soil water content showed a clear seasonal pattern for every plot. During the rainy season, monitoring by a TDR sensor showed that the volumetric soil water content in the 0–30 cm layer was usually higher in the upper rather than lower slope (), although the surface soil layer (0–5 cm) in the upper slope was drier than that in the lower slope. During the dry season, the water content in the surface soil layer was low, especially in plot R, but no clear difference among the plots was found in the 0–30 cm layer.
Factors controlling soil respiration
To examine the factors for determining soil respiration, the relationship between total soil respiration and soil temperature at 10 cm depth, air temperature, water content in the organic layer, water content in the surface soil layer (0–5 cm), and the volumetric water content in the 0–30 cm soil layer, which was calculated by averaging the content in the 0–15 and 15–30 cm layers, were analyzed (). The factor most strongly correlated to the total soil respiration was water content in the 0–30 cm soil layer. In plot R, water content in the surface soil (0–5 cm) was also significantly related to the soil respiration rate. The soil temperature showed a positive correlation to soil respiration, although there was slight annual variation. Air temperature was negatively but not significantly correlated with soil respiration. Consequently, soil and air temperature had no significant correlations with the soil respiration rate.
Table 5 Correlation coefficient between total soil respiration and water content in soil layer 0–30 cm deep, water content in soil layer 0–5 cm deep, water content in organic layer, soil temperature at depth of 10 cm, and air temperature
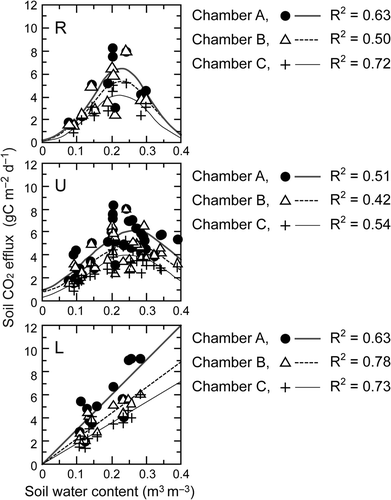
An empirical model with a high coefficient of determination was selected to estimate the soil respiration rate by soil water content for each plot (). The simple linear function Sr = a × WC + c was selected for plot L, and the exponential quadratic function Sr = exp (a × WC2 + b × WC + c) was selected for plots R and U, where WC was the volumetric water content in the 0–30 cm layer, and a, b, and c were the constants at each site. This means that the soil respiration rate increased almost linearly with the soil water content in plot L. In plots R and U, soil respiration increased with soil water content to peak: at 0.261 and 0.205 for plots U and R respectively, then decreased with increasing soil water content. High soil water content depressed the soil respiration rate on the upper slope. Compared with plot L, the response of the soil respiration rate to the soil water content was sharp before the rate peaked.
Figure 4 The relationships between soil carbon dioxide (CO2) effluxes and water contents of soil layer (0–30 cm) at ridge (R), upper (U) and lower (L) slope positions. Soil respiration of chamber A (dot, total soil respiration), chamber B (open triangle, without root respiration) and chamber C (cross, without root and organic layer respiration) are denoted in the figures. Emprical equations; y = exp(ax 2 + bx + c) for plots R and L and y = ax + b for plot L, are applied to each dataset of the chambers and R-squared values are also shown.
Separation of carbon dioxide sources
The total soil respiration (chamber A) was usually the highest, followed by the soil respiration from the chamber without litter (chamber B), and the soil respiration from the chamber without litter and the roots respiration (chamber C), although these trends were sometimes disturbed in every plot (). Using empirical models () and data from monitoring the soil water content (), the percentage contributions of fine roots, organic layer, and soil respiration to the total soil respiration were calculated on an annual basis (). The contribution of autotrophic (roots) respiration ranged from 19.4 to 36.6% of the total soil respiration and was higher in plots R and U than in plot L. For heterotrophic respiration, the proportion of the organic layer decreased up the slope from 26.6% (plot L) to 9.4% (plot R). Mineral soil contributed 46.4 to 54.4% of the total soil respiration. Using these proportions, annual respiration from roots, organic layer and soil was calculated from total soil respiration in 1997 ().
Table 6 Contribution (%) of autotrophic (roots) and heterotrophic (organic layer and soil organic matter decomposition) respiration to total soil respiration and annual respiration from roots, organic layer and soil in 1997
Estimation of carbon dioxide efflux from the forest floor
The carbon efflux from the forest floor varied with the sites and years, ranging from 1199 to 1908 gC m−2 year−1 in the watershed (). The annual precipitation in 1998 was 35% lower than in 1997, and consequently, soil water content was low in 1998. Applying the model of each site, the annual carbon efflux was reduced to 90% in plot L but increased to 119% in plot R in 1998. As a result, annual carbon efflux in plot R was 63% that of plot L in 1997 and 83% in 1998. Although the lower slope usually had a higher soil respiration rate than the upper slope, the distribution pattern of soil carbon efflux in the watershed varied in 1997 and 1998.
Table 7 Annual soil carbon dioxide (CO2) effluxes at the ridge (R), upper slope (U) and lower slope (L) positions in 1997 and 1998 (in gC m−2 year−1)
Discussion
Spatial variation in soil respiration rate on the slope
The total soil respiration rates decreased up the slope in the study watershed (), a trend which is similar to the results obtained by Nakane et al. (Citation1984) and Shimada (Citation1998) in temperate forests in Japan. As we reviewed in the introduction, however, opposite results, whereby lower slopes had a lower soil respiration rate than upper slopes were reported in several forest ecosystems (e.g. Chambers et al. Citation2004; Kosugi et al. Citation2007). The discrepancy of these reports may result from the different characteristics of the study sites, such as vegetation-soil association, soil microbes, the physical and chemical properties of the soil, and soil water legumes on the slope.
One aspect we should consider is the distribution of soil-vegetation associations on the slope. Some reports compared the soil respiration rates of sites with different soil-vegetation associations that existed on a hill slope (Chambers et al. Citation2004; Epron et al. Citation2006) but others compared sites within the range of the same soil-vegetation association (Nakane et al. Citation1984). In this study we focused on the variation in soil respiration within a soil-vegetation association, i.e. Alfisols-MDF and considered that the main process and response of soil respiration would be relatively similar among the sites. As the principal factor, we assumed that the soil moisture gradient in the watershed would determine the soil respiration rate.
The results showed that the lower slope (plot L) usually showed higher soil respiration rates than the upper slope (plots U and R) were within our expectation. However, it was unexpected to see for the soil water gradient that the soil water content in the 0–30 cm layer was higher in the upper slope than in the lower slope during the rainy season () because rainwater usually runs off from the upper to the lower slope. Despite the surface soil being drier in the upper than the lower slope, which might be related to the concentration of fine roots in the surface soil, the subsurface soil layer was actually relatively moist during the rainy season. We have no data on the soil porosity of the monitoring plots, and the available data on the soil physical properties () do not explain the reason for the trend in water content. However, the responses of soil respiration to soil water condition, as expressed by the exponential quadratic functions, seem to be reasonable for the upper slope because under a soil water condition of near-saturation, soil respiration is often depressed by the increase in the water-filled pore space (Doff sotta et al. Citation2004; Kang et al. Citation2003) or by the decline in microbial activity due to the oxygen deficit (McGroddy and Silver Citation2004; Skopp et al. Citation1990). Similar results were found in the other areas of Thailand; depression of soil respiration occurred under high soil moisture content over 0.21 (Adachi et al. Citation2009). Meir et al. (Citation2008) suggested that an accurate relationship between soil respiration and soil moisture is an important key to specify when constructing a spatially consistent model of soil respiration.
Beside the physiological responses of the soil microbe to soil moisture, the quality of the organic matter would differ among the sites. A lower contribution of heterotrophic respiration in the upper slope, indicating the slow decomposition of organic matter, might result in the accumulation of labile organic matter at the site, which is suggested by the fact that soil organic matter on the upper slope has a relatively high carbon/nitrogen (C:N) ratio (). In addition, root exudates from the dense fine-roots on the upper slope could have been a source of labile organic matter (Chapin et al. Citation2009), which might have resulted in a sharp increase in soil respiration rates under moderate soil water conditions on the upper slope ().
In contrast, soil respiration on the lower slope showed no limits in terms of high water content although we did not observe the soil respiration rate when the soil water content exceeded 0.3. This type of linear response is the same as the observation for teak plantations (Takahashi et al. Citation2009) that were located close to plot L in the watershed. Because plot L was well-drained, probably due to its stand position (over 2 m high) from the bed of the brook and water uptake by roots, soil gas exchange would be smooth, even during the rainy season. Therefore, we concluded that the soil water gradient formed on the slope basically controlled the spatial variation in soil respiration rates in the watershed. In addition, the quality of the organic matter, physical soil properties, and the physiological responses of the soil microbes should be examined, which would create a complex spatial distribution of soil respiration rates in the watershed.
Variation of carbon dioxide sources in different slope positions
The reviewed data from forest biomes worldwide indicated that autotrophic respiration occupied about 50% of the total soil respiration (Hanson et al. Citation2000; Bond-Lamberty et al. Citation2004; Subke et al. Citation2006). Subke et al. (Citation2006) found that the contribution of autotrophic respiration increased with increasing total soil respiration on a global scale, which suggests that autotrophic respiration is related to the biomass production of forests. However, Asian seasonal tropical and sub-tropical forests showed relatively lower autotrophic respiration, although the separation methodology for autotrophic respiration differed among researchers (). Autotrophic respiration ranged from 14.6 to 50.5% of the total soil respiration and averaged 31.7% (standard deviation, 10.8). Although unconfirmed, this might be responsible for the swift decomposition of litter and soil organic matter under a moist and warm environment during the rainy season and low autotrophic respiration during the dry season. Schaefer et al. (Citation2009) also demonstrated that autotrophic respiration occurred in a small proportion of a monsoon forest in China by their sophisticated manipulation experiment for separating root respiration. However, we may need to wait before drawing a conclusion because root trenching may have side effects such as root decomposition and changes in soil microbial community (Díaz-Pinés et al. Citation2010).
Table 8 Comparison of autotrophic and heterotrophic respiration in total soil respiration among Asian monsoon tropical and subtropical seasonal forests
Regarding the variation of the slope position, root respiration may be correlated to root biomass. The root biomass regression was used to estimate root respiration in some studies (e.g. Beheraa et al. Citation1990; Rodeghiero and Cescatti Citation2006; Wang et al. 2008). High root density would result in a high contribution of root respiration to the upper slope (). Many studies have reported that dry conditions tended to enhance the growth of fine roots in the surface soil (e.g. Noguchi et al. Citation2007), which would result from a preferable allocation of photosynthesis assimilates to belowground parts in drier soil on the upper slope (Tateno et al. Citation2004). In tropical seasonal forests in Mexico, root systems were densely distributed in the top 20 cm soil layer (Castellanos et al. Citation1991). Thus, the large contribution of autotrophic respiration would be reasonable in dry soil conditions on the upper slope position.
As for heterotrophic respiration, soil respiration contributed in relatively stable proportions, comprising 46.3–54.4% of total soil respiration, meaning respiration from litter was a key factor in variation of heterotrophic respiration. The contribution of the organic layer respiration decreased up the slope. Although the amounts of organic layer were relatively large on the upper slope (), they were still smaller than the annual litterfall input: 3.75 to 5.91 Mg ha−1. Somrithipol (Citation1997) reported that the decomposition of leaf-litter was quick in this watershed: 85% of bamboo leaves and 95% of Shorea siamensis leaves had disappeared within a year. Despite the lack of comparisons of litterfall among the plots, however, significant litterfall may occur in plot L due to the greater biomass production on the lower slope. These results indicate that the litterfall may be an indicator of the heterotrophic respiration rate from the organic layer and its contribution to total soil respiration.
Seasonal variation in soil respiration
The overall seasonal pattern of soil respiration rates; high and low rates during the rainy and dry seasons respectively, was the same as other observations in Thailand (Tulaphitak et al. Citation1985; Hashimoto et al. 2004; Adachi et al. Citation2009; Takahashi et al. Citation2009). The moist soil condition enhanced the physiological activities of soil microbes and the root respiration to increase the soil respiration rate. Regarding temperature, the small year-round fluctuation in soil temperature resulted in no clear statistical correlation with the soil respiration rate. Conversely, an increase in temperature sometime depressed the soil respiration rate. Adachi et al. (Citation2009) also reported that high temperatures in the dry season led even to a midday depression of the soil respiration rate in a forest of Thailand. Thus, in seasonal tropical forests, soil water content would be the most influential factor for seasonal and temporal fluctuations of the soil respiration, but high temperature may sometimes also have an effect as a stress factor for the respiration.
The organic layer is also a source of heterotrophic respiration and its water content would certainly be a significant factor for decomposing litter. However, no close relationship between the soil respiration rate and the moisture in the organic layer was detected in our observation. This is probably because of the lower contribution of the organic layer to total soil respiration in plots R and U () and significant fluctuations in its water content even during the rainy season (). Chambers et al. (Citation2004) also pointed out that the organic layer and deadwood were very sensitive to moisture stress, thereby affecting CO2 efflux from the forest floor. The development of a monitoring system for water content in the organic layer may improve the precision of soil respiration rate estimates, especially during the rainy season.
Annual soil respiration
Variation in annual carbon efflux was considerable in the watershed, from 1199 to 1908 gC m−2 year−1 in 1997 (), although the range is comparable to the review data by Raich and Tufekciogul (Citation2000). Similar to the soil respiration rate, the efflux decreased up the slope. However, the differences between plots L and R were minor in 1998 when rainfall declined because moderate soil water content during the rainy season resulted in a high soil respiration rate on the upper slope while efflux on the lower slope decreased. This suggests that scaling up the carbon balance from a stand scale to a watershed scale could not be achieved from soil water distribution data in the watershed alone. It is noted that annual variation in rainfall did not linearly determine the annual soil carbon efflux.
Carbon stock and soil respiration
There was no apparent relationship between the total carbon stock in soil and soil respiration rates (r = 0.34, p = 0.66). As we discussed, the labile fraction of soil carbon may be responsible for short-term heterotrophic soil respiration (Gu et al. Citation2004; Knorr et al. 2005). The reasons for the high soil carbon stock on the ridge would be the slow decomposition of soil organic matter and concentration of fine roots (Santantonio and Hermann Citation1985; Tsutsumi et al. Citation1985). The sheet erosion of surface soil should also be a factor in reducing the accumulation of soil carbon stock on steep slopes. Therefore, carbon stock is not likely to be a key parameter to estimate the soil respiration rate in the short run.
Conclusion
Seasonal and spatial variations in soil respiration in the watershed under a tropical seasonal forest in Thailand were significantly dictated by changes in the soil moisture regime. Seasonal variation, whereby the soil respiration rate was high and low during the rainy and dry seasons respectively was observed. For spatial variation, the soil respiration rate was usually higher on the lower slope (plot L) than on the upper slope (plots R and U). However, the response patterns of soil respiration to soil water content differed between the lower and upper slopes. Annual CO2 efflux from the forest floor ranged from 1199 gC m−2 year−1 in plot R to 1908 gC m−2 year−1 in plot L. The contribution of autotrophic respiration to the total soil respiration increased and the contribution of organic layer respiration decreased up the slope. We concluded that the soil respiration rate and the contribution of CO2 sources was influenced by the soil water content formed on the sloping topography. The varying responses of the soil respiration rate to soil water content at different slope positions create complex spatial variation in the soil respiration rate at a watershed scale.
Acknowledgments
The field study was supported by funds from the Science Technology Agency Japan and the National Research Council Thailand. The authors were also funded by the Global Environment Research Account for National Institutes (Advancement of East Asia forest dynamics plot network), Ministry of the Environment Japan while writing this paper. The authors would like to thank Drs U. Kutintara and C. Yarwudhi at Kasetsart University, Ms V. Sunantapongsuk and Ms P. Tummakate in the Land Development Department Thailand, Drs K. Ishizuka, N. Tanaka and H. Tanaka in the Forestry and Forest Products Research Institute, Professors S. Kobayashi and A. Ishida at Kyoto University, and T. Nakashizuka at Tohoku University for their valuable suggestions and supporting research activities.
References
- Adachi , M , Ishida , A , Bunyavejchewin , S , Okuda , T and Koizumi , H . 2009 . Spatial and temporal variation in soil respiration in a seasonally dry tropical forest, Thailand . J. Trop. Ecol. , 25 : 531 – 539 .
- Beheraa , N , Joshi , SK and Pati , DP . 1990 . Root contribution to total soil metabolism in a tropical forest soil from Orissa, India . For. Ecol. Manage. , 36 : 125 – 134 .
- Bond-Lamberty , B , Wang , C and Gower , ST . 2004 . A global relationship between the heterotrophic and autotrophic components of soil respiration? . Global Change Biol. , 10 : 1756 – 1766 .
- Bullock , SH , Mooney , HA and Medina , E . 1995 . Seasonally Dry Tropical Forests , New York : Cambridge University Press .
- Castellanos , J , Maass , M and Kummerow , J . 1991 . Root biomass of a dry deciduous tropical forest in Mexico . Plant Soil. , 131 : 225 – 228 .
- Chapin , FS III , McFarland , J , McGuire , AD , Euskirchen , ES , Ruess , RW and Kielland , K . 2009 . The changing global carbon cycle: linking plant–soil carbon dynamics to global consequences . J. Ecol. , 97 : 840 – 850 .
- Chambers JQ, Tribuzy ES, Toledo LC, Crispim BF, Higuchi N, dos Santos J, Araújo AC, Kruijt B, Nobre AD, Trumbore SE 2004: Respiration from a tropical forest ecosystem: Partitioning of sources and low carbon use efficiency. Ecol. Appl., 14, Supplement: LBA Experiment, 72–88
- Cisneros-Dozal , LM , Trumbore , SE and Hanson , PJ . 2006 . Partitioning sources of soil-respired CO2 and their seasonal variation using a unique radiocarbon tracer . Global Change Biol. , 12 : 194 – 204 .
- Cuevas , E . 1995 . “ Biology of the belowground system of tropical dry forests ” . In Seasonally Dry Tropical Forests , Edited by: Mooney , HA , Bullock , SH and Medina , E . 362 – 383 . Cambridge : Cambridge University Press .
- Díaz-Pinés , E , Rubio , A , Schindlbacher , A , PfeVer , M and Jandl , R . 2010 . Root trenching: a useful tool to estimate autotrophic soil respiration? A case study in an Austrian mountain forest . Eur. J. For. Res. , 129 : 101 – 109 .
- Doff sotta , E , Meir , P , Malhi , Y , Donato nobre , A , Hodnett , M and Grace , J . 2004 . Soil CO2 efflux in a tropical forest in the central Amazon . Global Change Biol. , 10 : 601 – 617 .
- Epron , D , Bosc , A , Bonal , D and Freycon , V . 2006 . Spatial variation of soil respiration across a topographic gradient in a tropical rain forest in French Guiana . J. Trop. Ecol. , 22 : 565 – 574 .
- Fang , Y , Gundersen , P , Zhang , W , Zhou , G , Christiansen , JR , Mo , J , Dong , S and Zhang , T . 2009 . Soil–atmosphere exchange of N2O, CO2 and CH4 along a slope of an evergreen broad-leaved forest in southern China . Plant Soil , 319 : 37 – 48 .
- Fisher , JB , Malhi , Y Bonal , D . 2009 . The land-atmosphere water flux in the tropics . Global Change Biol. , 15 : 2694 – 2714 .
- Gu , L , Post , WM and King , AW . 2004 . Fast labile carbon turnover obscures sensitivity of heterotrophic respiration from soil to temperature: A model analysis . Global Biogeochem. Cycles , 18 : GB1022 doi:10.1029/2003GB002119
- Hamilton , JG , DeLucia , EH , George , K , Naidu , SL , Finzi , AC and Schlesinger , WH . 2002 . Forest carbon balance under elevated CO2 . Oecologia , 131 : 250 – 260 .
- Hanpattanakit , P , Panuthai , S and Chidthaisong , A . 2009 . Temperature and moisture controls of soil respiration in a dry dipterocarp forest, Ratchaburi Province . Kasetsart J. (Nat. Sci.) , 43 : 650 – 661 .
- Hanson , PJ , Edwards , NT , Garten , CT and Andrews , JA . 2000 . Separating root and soil microbial contributions to soil respiration: A review of methods and observations . Biogeochemistry , 48 : 115 – 146 .
- Hashimoto , S . 2005 . Temperature sensitivity of soil CO2 production in a tropical hill evergreen forest in northern Thailand . J. Forest Res. , 10 : 497 – 503 .
- Hashimoto , S , Tanaka , N , Suzuki , M , Inoue , A , Takizawa , H , Kosaka , I , Tanaka , K , Tantasirin , C and Tangtham , N . 2004 . Soil respiration and soil CO2 concentration in a tropical forest, Thailand . J. Forest Res. , 9 : 75 – 79 .
- Hirobe , N , Tokuchi , N and Iwatsubo , G . 1999 . Spatial variability of soil nitrogen transformation patterns along a forest slope in a Cryptomeria japonica D. Don plantation . Eur. J. Soil Biol. , 34 : 123 – 131 .
- Hu , R , Hatano , R , Kusa , K and Sawamoto , T . 2004 . Soil respiration and net ecosystem production in an onion field in central Hokkaido, Japan . Soil Sci. Plant Nutr. , 50 : 27 – 33 .
- Huete , AR , Restrepo-Coupe , N , Ratana , P , Didan , K , Saleska , SR , Ichii , K , Panuthai , S and Gamo , M . 2008 . Multiple site tower flux and remote sensing comparisons of tropical forest dynamics in Monsoon Asia . Agric. For. Meteorol. , 148 : 748 – 760 .
- Ishida , A , Diloksumpun , S , Ladpala , P , Staporn , D , Panuthai , S , Gamo , M , Yazaki , K , Ishizuka , M and Puangchit , L . 2006 . Contrasting seasonal leaf habits of canopy trees between tropical dry-deciduous and evergreen forests in Thailand . Tree Physiol. , 26 : 643 – 656 .
- Ishizuka , S , Sakata , T Sawata , S . 2006 . High potential for increase in CO2 flux from forest soil surface due to global warming in cooler areas of Japan . Ann For. Sci. , 63 : 537 – 546 .
- Kang , S , Doh , S , Lee , D , Lee , D , Jin , VL and Kimboll , JS . 2003 . Topographic and climatic controls on soil respiration in six temperate mixed-hardwood forest slopes, Korea . Global Change Biol. , 9 : 1427 – 1437 .
- Katagiri , S . 1988 . Estimation of proportion of root respiration in total soil respiration in deciduous broadleaved stands . J. Jpn. For. Soc. , 70 : 151 – 158 .
- Knorr , W , Prentice , IC , House , JI and Holland , EA . 2005 . Long-term sensitivity of soil carbon: Turnover to warming . Nature , 433 : 298 – 301 .
- Kosugi , Y , Mitani , T , Itoh , M , Noguchi , S , Tani , M , Matsuo , N , Takanashi , S , Ohkubo , S and Nik , AR . 2007 . Spatial and temporal variation in soil respiration in a Southeast Asian tropical rainforest . Agric. For. Meteorol. , 147 : 35 – 47 .
- Kuzyakov , Y and Larionova , AA . 2005 . Root and rhizomicrobial respiration: A review of approaches to estimate respiration by autotrophic and heterotrophic organisms in soil . J. Plant Nutr. Soil Sci. , 168 : 503 – 520 .
- Lal , R . 2004 . Soil carbon sequestration to mitigate climate change . Geoderma , 123 : 1 – 22 .
- Lee , M , Nakane , K , Nakatsubo , T and Koizumi , H . 2003 . Seasonal changes in the contribution of root respiration to total soil respiration in a cool-temperate deciduous forest . Plant Soil , 255 : 311 – 318 .
- Marod , D , Kutintara , U , Tanaka , H and Nakashizuka , T . 2002 . The effects of drought and fire on seed and seedling dynamics in a tropical seasonal forest in Thailand . Plant Ecology , 161 : 41 – 57 .
- Marod , D , Kutintara , U , Yarwudhi , C , Tanaka , H and Nakashizuka , T . 1999 . Structural dynamics of a natural mixed deciduous forest in western Thailand . J. Veg. Sci. , 10 : 777 – 786 .
- McGroddy , M and Silver , WL . 2004 . Variations in belowground carbon storage and soil CO2 flux rates along a wet tropical climate gradient . Biotropica , 32 : 614 – 624 .
- Meir , P , Metcalfe , DB , Costa , ACL and Fisher , RA . 2008 . The fate of assimilated carbon during drought: Impacts on respiration in Amazon rainforests . Phil. Trans. R. Soc. B , 363 : 1849 – 1855 .
- Mitani , T , Kosugi , Y , Osaka , K , Okubo , S , Takanashi , S and Tani , M . 2006 . Spatial and temporal variability of soil respiration rate at a small watershed revegetated with Japanese Cypress . J. Jpn. For. Soc. , 88 : 496 – 507 .
- Nakane , K , Tsubota , H and Yamamoto , M . 1984 . Cycling of soil carbon in a Japanese red pine forest I. Before a clear-felling . Bot. Mag. (Tokyo) , 97 : 39 – 60 .
- Noguchi , K , Konopka , B , Satomura , T , Kaneko , S and Takahashi , M . 2007 . Biomass and production of fine roots in Japanese forests . J. Forest Res. , 12 : 83 – 95 .
- Nounmusig W, Wongwises P, Zhang M, Sukawat D, Chidthaisong A 2006: Effects of ENSO on precipitation over Northeast Thailand during rainy season 1997–1999. Proceedings of the 2nd Joint International Conference on “Sustainable Energy and Environment (SEE 2006)”, Technology and Policy Innovations. D-010(O), 21–23 November, Bangkok, Thailand
- Raich , JW and Schlesinger , WH . 1992 . The global carbon dioxide flux in soil respiration and its relationship to vegetation and climate . Tellus B , 44 : 81 – 99 .
- Raich , JW and Tufekciogul , D . 2000 . Vegetation and soil respiration: Correlations and controls . Biogeochemistry , 48 : 71 – 90 .
- Rodeghiero , M and Cescatti , A . 2006 . Indirect partitioning of soil respiration in a series of evergreen forest ecosystems . Plant Soil , 284 : 7 – 22 .
- Rundel , PW and Boonpragob , K . 1995 . “ Dry forest ecosystems of Thailand ” . In Seasonally Dry Tropical Forests , Edited by: Bullock , SH , Mooney , HA and Medina , E . 93 – 123 . New York : Cambridge University Press .
- Sakata , T , Ishizuka , S and Takahashi , M . 2007 . Separation of soil respiration into CO2 emission sources using 13C natural abundance in a deciduous broad-leaved forest in Japan . Soil Sci Plant Nutr. , 53 : 328 – 336 .
- Santantonio , D and Hermann , RK . 1985 . Standing crop, production, and turnover of fine roots on dry, moderate, and wet sites of mature Douglas-fir in western Oregon . Ann. For. Sci. , 42 : 113 – 142 .
- Schaefer , DA , Feng , W and Zou , X . 2009 . Plant carbon inputs and environmental factors strongly affect soil respiration in a subtropical forest of southwestern China . Soil Bio. Biochem. , 41 : 1000 – 1007 .
- Shimada , S , Toda , H , Haibara , K and Koike , T . 1998 . Characteristics of gas diffusion coefficient and CO2 flux at different slope site and different depth in forest soil . Jpn. J. For. Environ. , 40 : 1 – 8 . (in Japanese with English summary)
- Shinjo , H , Kato , A , Fujii , K , Mori , K , Funakawa , S and Kosaki , T . 2006 . Carbon dioxide emission derived from soil organic matter decomposition and root respiration in Japanese forests under different ecological conditions . Soil Sci. Plant Nutr. , 52 : 233 – 242 .
- Skopp , J , Jawson , MD and Doran , JW . 1990 . Steady-state aerobic microbial activity as a function of soil water content . Soil Sci. Soc. Am. J. , 54 : 1619 – 1625 .
- Soil Survey Staff 2006: Keys to Soil Taxonomy (10th edition). USDA-Natural Resources Conservation Service, Washington, DC
- Somrithipol S 1997: Decomposition of bamboo and Rang (Shorea siamensis Miq.) leaf litters in mixed deciduous forest and their decomposition fungi. http://agris.fao.org/agris-search/search/display.do?f=2000/TH/TH00010.xml;TH2000001591 (November, 2010)
- Subke , JA , Inglima , I and Cotrufo , MF . 2006 . Trends and methodological impacts in soil CO2 efflux partitioning: A metaanalytical review . Global Change Biol. , 12 : 921 – 943 .
- Suksawang S 1995: Site overview: Thong Pha Phum study site. Proceedings of the international workshop on the changes of tropical forest ecosystems by El Nino and others, JSTA, NRCT, and JISTEC, pp. 33–37, 7–10 February 2005, Kanchanaburi, Thailand
- Sulzman , EW , Brant , JB , Bowden , RD and Lajtha , K . 2005 . Contribution of aboveground litter, belowground litter, and rhizosphere respiration to total soil CO2 efflux in an old growth coniferous forest . Biogeochemistry , 73 : 231 – 256 .
- Swanson , FJ , Kratz , TK , Caine , N and Woodmansee , RG . 1988 . Landform effects on ecosystem patterns and processes . BioScience , 38 : 92 – 98 .
- Takahashi , M , Furusawa , H , Limtong , P , Sunanthapongsuk , V , Marod , D and Panuthai , S . 2007 . Soil nutrient status after bamboo flowering and death in a seasonal tropical forest in western Thailand . Ecol. Res. , 22 : 160 – 164 .
- Takahashi , M , Hirai , K , Limtong , P , Leaungvutivirog , C , Suksawang , S , Panuthai , S , Anusontpornperm , S and Marod , D . 2009 . Soil respiration in different ages of teak plantations in Thailand . JARQ , 43 : 337 – 343 .
- Tateno , R , Hishi , T and Takeda , H . 2004 . Above- and belowground biomass and net primary production in a cool-temperate deciduous forest in relation to topographical changes in soil nitrogen . For. Ecol. Manage. , 193 : 297 – 306 .
- Tsutsumi , T , Nishitani , Y and Sakai , M . 1985 . On the effects of soil fertility on the rate of soil respiration in a forest . Jpn. J. Ecol. , 35 : 207 – 214 .
- Tsui , C-C , Chen , Z-S and Hsieh , C-F . 2004 . Relationship between soil properties and slope position in a lowland rain forest of south Taiwan . Geoderma , 123 : 131 – 142 .
- Tulaphitak , T , Pairlntra , C and Kyuma , K . 1985 . Changes in soil fertility and tilth under shifting cultivation: III. Soil respiration and soil tilth . Soil Sci. Plant Nutr. , 31 : 251 – 261 .
- Vogel , JG , Valentine , DW and Ruess , RW . 2005 . Soil and root respiration in mature Alaskan black spruce forests that vary in soil organic matter decomposition rates . Can. J. For. Res. , 35 : 161 – 174 .
- Wang , XG , Zhu , B , Wang , YQ and Zheng , XH . 2006 . Field measures of the contribution of root respiration to soil respiration in an alder and cypress mixed plantation by two methods: Trenching method and root biomass regression method . Eur. J. For. Res. , 127 : 285 – 291 .
- Wen , XF , Yu , GR , Sun , XM , Li , QK , Liu , YF , Zhang , LM , Ren , CY , Fu , YL and Li , ZQ . 2006 . Soil moisture effect on the temperature dependence of ecosystem respiration in a subtropical Pinus plantation of southeastern China . Agric. For. Meteorol. , 137 : 166 – 175 .
- Yang , YS , Chen , GS , Guo , JF , Xie , JS and Wang , XG . 2007 . Soil respiration and carbon balance in a subtropical native forest and two managed plantations . Plant Ecol. , 193 : 71 – 84 .
- Yi , Z , Fu , S , Zhou , G , Mo , J , Zhang , D , Ding , M , Wang , X and Zhou , L . 2007 . Partitioning soil respiration of subtropical forests with different successional stages in south China . For. Ecol. Manage. , 243 : 178 – 186 .