Abstract
The gut bacterial community structure for Pheretima hilgendorfi and P. heteropoda (Family Megascolecidae), and Allolobophora japonica (Family Lumbricidae) collected from agricultural grasslands in Japan was analyzed by denaturing gradient gel electrophoresis of polymerase chain reaction-amplified 16S rRNA gene fragments (PCR-DGGE) and compared with those in the surrounding soils. Denaturing gradient gel electrophoresis (DGGE) profiles indicated that each earthworm species had their own specific bacterial communities, and multidimentional scaling analysis grouped the DGGE profiles into three groups: gut samples from P. hilgendorfi and P. heteropoda, gut samples from A. japonica and samples from the surrounding soils. Nine dominant bands were identified by their direct sequencing and cloning. Major three bands from P. hilgendorfi and P. heteropoda were closely related to Bacillus species belonging to the phylum Firmicutes. Major four and two bands from A. japonica were closely related to the phyla Proteobacteria and Bacteroidetes, respectively.
Introduction
The importance of earthworm activity for improving soil quality was first recognized and described by Charles Darwin at the end of the nineteenth century (Darwin Citation1881). Today, it is widely accepted that earthworms, like other representatives of the macrofauna such as millipedes, modify the physical, chemical and biological properties of soil. These beneficial effects on soil properties are believed to relate to the close relationship that exists between earthworms and the microorganisms found in their gut and surrounding soil (Brown Citation1995). Flack and Hartenstein (Citation1984) reported that microorganisms (especially bacteria), with a combination of carbohydrate and grit, appeared necessary for earthworm growth. In another study, the existence of a specific gut microbial community provides essential fatty acids required for earthworm growth (Sampedro et al. Citation2006). Transition through the earthworm gut may also affect bacterial numbers (Monroy et al. Citation2008) and their activities (Aira et al. Citation2006) in several earthworm species, especially members of Lumbricidae. Pedersen and Hendriksen (Citation1993) reported that inoculated bacteria exhibited different survival patterns along the intestinal tract of Lumbricidae. Differences exist in the host effects on various bacterial strains in the earthworm intestine and may vary between separate earthworm species. In addition, earthworms have characteristic gut microbiota depending on the environments they live in: forest soil (Márialigeti Citation1979), forest and pasture soil (Wüst et al. Citation2009) and no-tilled agricultural soil (Furlong et al. Citation2002).
Lumbricidae species are dominant earthworms in Europe. However, within soils of Japan, the majority of earthworms are Pheretima species belonging to Megascolecidae, and only a few Lumbricidae species such as Allolobophora japonica are collected in the same area (Yamaguchi Citation1967; Aoki Citation1973). While many studies have been conducted on the gut microbiota of Lumbricidae in Europe, few have been reported for Megascolecidae (Risal and Ozawa Citation2002). Matsumoto (Citation1987) reported that the bacterial number in casts of Pheretima hupeiensis, Pheretima sp., and A. caliginosa collected in Japan were higher than that in soil under the feeding experiment. As for Eisenia foetida (Lumbricidae) collected from Japanese farmyard manure, they showed indigenous microbial community, comparing to the manure (Toyota and Kimura Citation2000). However, none of the studies have reported the gut microbiota of Japanese earthworm from field soil. In this study, we aimed to characterize the gut bacterial community structure from earthworms collected from Japanese filed soil by using denaturing gradient gel electrophoresis of polymerase chain reaction-amplified 16S rRNA gene fragments (PCR-DGGE). Their seasonal and yearly variation in different sections of the gut was comparatively analyzed.
Materials and methods
Sampling and gut preparation for earthworms
The Megascolecidae species, Pheretima hilgendorfi (MICHAELSEN) and P. heteropoda (GOTO ET HATAI) and Lumbricidae species, Allolobophora japonica (MICHAELSEN), were collected by a hand sorting method in June 2007, November 2007, and June 2008 from cattle grazed grassland at Kyushu University, Oita, Japan. The grassland was sectioned into 30 sites and randomly sampled for earthworm collection. Details of the earthworm species sampled are shown in (see also and ). Samples of surrounding soils were collected from 0–5, 5–10, and 10–20 cm depth. Species identification was conducted on the basis of characteristic morphological features (Ohfuchi and Yamaguchi Citation1965; Nakamura Citation1999). Most of three earthworms used in this study were adult, but two A. japonica was subadult. As it has been reported that activity of Japanese earthworms has a variety of seasonal changes (Nakamura Citation1972), P. heteropoda and A. japonica were found throughout the year and P. hilgendorfi was found only in June. Earthworms were immediately transported to the laboratory with their surrounding soils. Gut samples (gut contents + gut wall) were prepared as follows: All earthworms were washed with sterile deionized water three times and immediately frozen at −20°C until further use. Earthworms were dissected and the gut behind clitellum was equally divided into three parts (fore-gut, mid-gut, and hind-gut) in their length. The used gut section approximately correspond to the mid-gut sections A and B, and hind-gut as depicted by Horn et al. (Citation2003).
Table 1. Samples of earthworm guts used for this study
DNA extraction
Total DNA was extracted from earthworm gut samples and surrounding soil using the Fast DNA Spin Kit for soil (Qbiogene, Carlsbad, CA, USA). For further purification, a GENECLEAN® Turbo Kit (Qbiogene) was used. DNA concentration was estimated by visual comparison with authentic fragment DNA (λ/HindIII digest) after electrophoresis on agarose gel. Purified DNA was stored at −20°C until use.
Polymerase chain reaction amplification
A fragment of the16S rRNA gene (c. 194 bp) was PCR amplified from purified DNA diluted as template using the forward primer, 357f-GC (5′- CGC CCG CCG CGC GCG GCG GGC GGG GCG GGG GCA CGG GGG GCC TAC GGG AGG CAG CAG-3′) and the reverse primer, 518r (5′- ATT ACC GCG GCT GCT GG-3′) (Muyzer et al. Citation1993). Both primers are considered specific to most bacteria. The PCR reactions were performed in 25 or 50 µl (final volume) mixtures containing 20 µM of each primer and Premix Taq [Ex Taq Version] (TaKaRa, Otsu, Japan). Reactions were incubated in a Dice thermal cycler (TaKaRa, Otsu, Japan) using the following protocol: an initial denaturation step for 3 min at 94°C; then 30 cycles of 1 min at 52°C (annealing), 1 min at 72°C (elongation), and 1 min at 94°C (denaturation); followed by 1 min at 52°C and 10 min at 72°C. DNA amplification was verified using electrophoresis of the PCR products in 1.5% agarose gels.
Denaturing gradient gel electrophoresis (DGGE)
Denaturing gradient gel electrophoresis was performed with the DCode™ Universal Mutation Detection System (Bio-Rad, Hercules, CA, USA), according to the manufacturer's instructions. Polymerase chain reaction products (c. 100 ng) were loaded onto 1 mm polyacrylamide gels (8% wt/vol) containing a 30–60% linear gradient concentration of denaturant; 100% denaturant was defined as 7 M urea and 40% (vol/vol) formamide. Gel electrophoresis was conducted in 1 × TAE buffer (40 mM Tris, 20 mM acetic acid, 1 mM Na-EDTA; pH 8.0) at 200 V and 60°C for 5 h. Gels were stained for 30 min with 1 : 10,000 (vol/vol) SYBR® Green I (TaKaRa, Otsu, Japan) and photographed under ultraviolet (UV) transillumination.
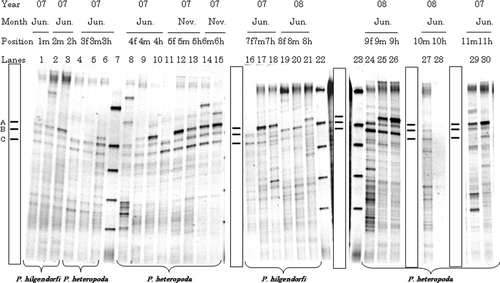
Figure 1. Typical denaturing gradient gel electrophoresis profiles for polymerase chain reaction-amplified fragments of 16S rRNA genes for gut samples from Pheretima hilgendorfi and P. heteropoda collected on 2007 (June or November) and 2008 (June): f, fore-gut; m, mid-gut; h, hind-gut. Numbers with f, m, or h on each lane correspond to individual number. Markers were electrophoresed in Lanes 7, 22, and 23. Bands typically found in most lanes (A–C) are illustrated in the box.
Statistical analysis of DGGE profiles
Gel images of the DGGE profiles were converted, normalized and digitized using Quantity One 3.0 software (Bio-Rad). Denaturing gradient gel electrophoresis banding patterns were analyzed in metric multidimensional scaling (MDS) as described by Iwamoto et al. (Citation2000). The distance matrix between gel Lane A and gel Lane B (D
AB) was calculated by using the following equation.where Ai
is the importance probability of each band on gel Lane A (no band is treated as zero); Bi
is the importance probability of each band on gel Lane B (no band is treated as zero); P is the (total number of bands on Lane A) + (total number of bands on Lane B) – (total number of bands common to Lane A and Lane B). The distance matrix was further evaluated by metric MDS analysis in SAS 9.2 (SAS Institute Japan Ltd., Tokyo, Japan). Multidimensional scaling is a mathematical technique that generates a spatial configuration map where the distance between data points reflects the relationship between individual variables in the underlying data set. Analysis was conducted using the computer facilities at the Research Institute for Information Technology, Kyushu University. To aid the conversion and normalization of gels, a DGGE marker (NIPPON GENE CO., LTD, Tokyo, Japan) consisting of five bands was added to the outside lane of each gel (Röling et al.
Citation2001).
In spite that PCR amplifications were conducted by bacterial 16S rRNA gene-specific primer sets, one major band (Band a; ) was found to be most related to the earthworm 18S rRNA gene by direct sequencing after excising the band. Similar results were reported by Singleton et al. (Citation2003) and thus, this band was excluded from analysis.
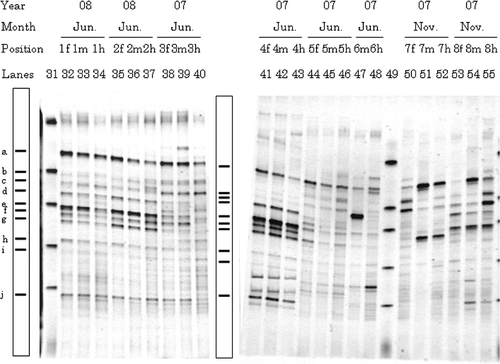
Figure 2. Typical denaturing gradient gel electrophoresis profiles for polymerase chain reaction-amplified fragments of 16S rRNA genes for gut samples from Allolobophora japonica. Explanations for each lane shown in the figure are the same as those in . Markers were electrophoresed in Lanes 31 and 49. Bands typically found in most lanes (a–j) are illustrated in the box.
Cloning and sequencing of 16S rRNA gene
Due to the limited success of direct sequencing from an electorphoresed gel, cloning of amplified 16S rRNA genes was conducted as supported by Burr et al. (Citation2006). Almost complete 16S rRNA gene sequences were amplified as follows: PCR reactions were performed in 50 µl (final volume) mixtures containing Premix Taq [Ex Taq Version] (TaKaRa) and of the primers 8F and 1492R (Burr et al. Citation2006). Amplified products were separated by electrophoresis on a 1% agarose gel and purified using a DNA recovery unit (SUPREC-EZ, TaKaRa). Products were ligated with the pTAC-1 vector using a DynaExpress TA PCR Cloning Kit (BioDynamics Laboratory Inc., Tokyo, Japan) and transformed into Escherichia coli JM109 competent cells (TOYOBO, Osaka, Japan) as specified by the manufacturer. Transformed cells were plated on selective Luria-Bertani medium plates containing 100 µg/mL ampicillin, 20 µL of 50 mg/mL 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal), and 100 µL of 0.1 M isopropyl-β-D-thiogalactopyranoside (IPTG) and incubated overnight at 37°C. Inserts were amplified using the pTAC-1 specific primers M13 BD FW and M13 BD Rev and further amplified using primers 357f-GC and 518r to compare the band position in DGGE gels. Plasmid DNA was purified from individual clones using QIAprep Spin Miniprep Kit (QIAGEN, GmbH, Hilden, Germany). Sequencing of the inserts was conducted using the primers M13 BD Fw and M13 BD Rev and 683r (CGCATTTCACYGCTACAC) and 1091r (CGCTCGTTGCGGGACTTA) (Sakai et al. Citation2000). Sequencing was performed by Dragon Genomics Center (TaKaRa).
The dominance of clones was assessed by comparing individual migration patterns in DGGE. Sequence homology was compared with 16S rRNA gene sequences available in the DDBJ/EMBL/GenBank DNA database using the FASTA algorithm (http://www.ddbj.nig.ac.jp/Welcom-j.html). The presence of chimeric sequences in the clones was checked using CHIMERA _CHECK ver.2.7 from the Ribosomal Database Project and all reference sequences were obtained from the Ribosomal Database Project II (http://rdp.cme.msu.edu/). Sequences were aligned using CLUSTAL W ver.1.83 (http://clustalw.ddbj.nig.ac.jp/top-j.html/) and phylogenetic analyses were conducted using MEGA ver.4 (Tamura et al. Citation2007). A distance-matrix method, using the Kimura two-parameter and neighbor-joining algorithms was used and bootstrap values were calculated from 1000 replications.
Nucleotide sequence accession numbers
The nucleotide sequence data of Clo2, Clo10, Clo13, Clo15, Clo29, Clo48, Clo52, Clo61 and Clo96 were deposited in the DDBJ database under accession numbers AB571082 to AB571090, respectively.
Results
Denaturing gradient gel electrophoresis fingerprinting
Denaturing gradient gel electrophoresis profiles for the fore-, mid-, and hind-gut from 11 Pheretima hilgendorfi and P. heteropoda sampled at different days are shown in . The DGGE profiles were similar to each other and were characterized by three strong bands (Bands A–C) at low denaturing concentration (c.40–44% denaturant). Bands A–C was common in both the P. hilgendorfi and P. heteropoda gut samples, and the effects of sampling year, sampling month, and earthworm species on the intensity of Bands A–C were small. The intensities of Bands B and C varied little for the fore-, mid-, and hind-gut, while the intensity of Band A gradually increased from fore-gut to hind-gut (example Lanes 4–6, 16–18).
Denaturing gradient gel electrophoresis profiles for fore-, mid-, and hind-gut samples from eight Allolobophora japonica are shown in . Strong dominant bands were found at the middle and lower region of the gel (c.44–55% denaturant), all of which were minor in the gut of P. hilgendorfi and P. heteropoda (). The effects of the sampling year or the sampling month on the profiles were not clear due to individual earthworm variation. The profiles for earthworms collected on June 2007 (n = 2) (Position 3 m and 4 m, Lanes 39 and 42) and June 2008 (n = 2) (Position 1 m and 2 m, Lanes 33 and 36) were similar but they were distinguishable from those of earthworms collected on June 2007 (n = 2) (Position 5m and 6m, Lanes 45 and 47). Profiles for earthworms collected on November 2007 (Position 7 m and 8 m, Lanes 51 and 54) were very different from each other. When comparing gut regions, the intensities of Bands b and f varied in the profiles of one earthworm collected on June 2007 (Lanes 38–40) and two earthworms on November 2007 (Lanes 50–55).
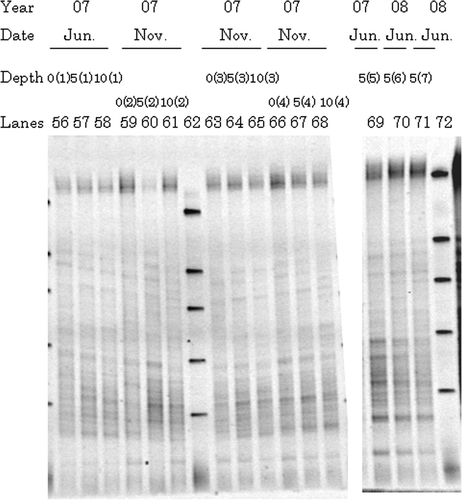
Denaturing gradient gel electrophoresis profiles of representative surrounding soils are shown in . Those collected from different sampling sites were similar to each other, irrespective of sampling year, sampling month and vertical depth. In contrast with gut samples, many minor bands were observed with an absence of any prominent major bands as typically observed for bulk soil patterns (Smalla et al. Citation2007). A majority of the dominant bands identified in earthworm gut samples also existed in the profiles for the surrounding soils.
Figure 3. Typical denaturing gradient gel electrophoresis profiles for polymerase chain reaction-amplified fragments of 16S rRNA genes from representative surrounding soils at 2007 and 2008: 0, 0–5 cm depth; 5, 5–10 cm depth; 10, 10–20 cm depth. Numbers in parenthesis behind 0, 5, or 10 correspond to sampling site. Markers were electrophoresed in Lanes 62 and 72.
Multidimensional scaling analysis of DGGE banding patterns
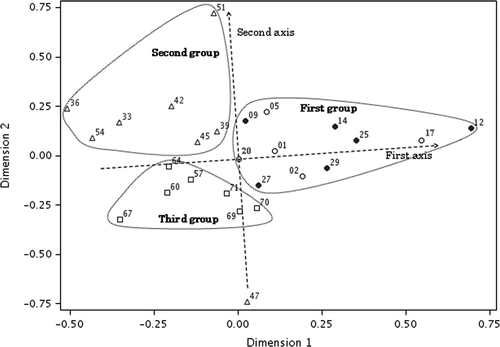
To further analyze the correlations between samples, DGGE banding patterns from mid-gut (five P. hilgendorfi, six P. heteropoda, and eight A. japonica) and surrounding soil samples of 5 to 10 cm depth (n = 7) were statistically compared using metric MDS analysis (). The configuration indicated the existence of three groups represented by those points inside the dotted line. The first group represented mid-gut samples from P. hilgendorfi and P. heteropoda; the second group comprised mid-gut samples from a majority of A. japonica and the third group represented samples from surrounding soils. One A. japonica gut (Lane 47) differed from all other samples in the second group. While the first axis separated most of the mid-gut and surrounding soil samples, three Pheretima spp. gut samples (Lanes 2, 27 and 29) were found to be similar to surrounding soils. The second axis divided the mid-guts according to earthworm families and the P. hilgendorfi and P. heteropoda mid-gut samples were grouped together. The relationships between sampling date and sampling year were not clear.
Figure 4. Two-dimensional plot of multidimensional scaling stimulus scores for denaturing gradient gel electrophoresis banding patterns from mid-guts and surrounding soils. Open circle, Pheretima hilgendorfi gut; closed circle, P. heteropoda gut; open triangle, Allolobophora japonica gut; open square, surrounding soil. Numbers correspond to those of the lanes shown in Figs .
Phylogenetic position of dominant DGGE bands
Clone libraries were constructed from DNA extracts of representative earthworm gut samples. Individual clones were screened by comparing their migration patterns to the DGGE profile of gut samples electrophoresed. Clones that migrated to the same position with the major bands of gut samples on DGGE were selected and near-complete 16S rRNA gene sequences were analyzed.
The phylogenetic relationships of three dominant bands in both P. hilgendorfi and P. heteropoda DGGE profiles (Bands A–C; ) were further verified. Denaturing gradient gel electrophoresis profiles for clones Clo61, Clo96, and Clo52 corresponded to Bands A, B, and C () respectively. Despite differences in migration behavior, these clones all showed the greatest degree of similarity with Bacillus longiquaesitum from soil: Clo61 and Clo96 to strain LMG 23783 (accession number AM747042) with 99.6 and 99.7% homology and Clo52 to strain LMG 23781T (accession number AM747040) with 97.6% homology.
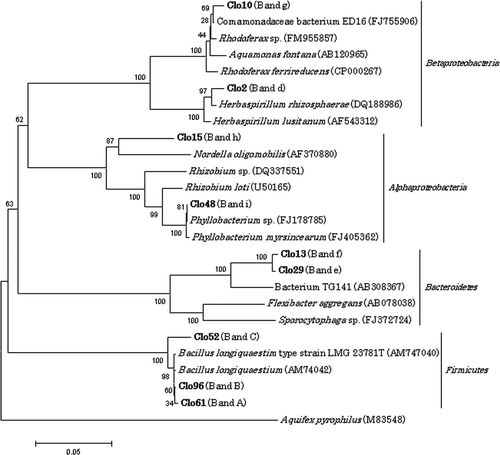
For A. japonica, the dominant bands were closely related to species in the phyla Proteobacteria and Bacteroidetes. Clone Clo2 migrated to the same position as Band d () and exhibited 98.4% similarity with Herbaspirillum rhizosphaerae, and clone Clo10 corresponding to Band g () with a high similarity to Comamonadaceae bacterium ED16 (98.9%) in the Betaproteobacteria. Clo15 (Band h) and Clo48 (Band i) () showed the greatest degree of similarity to the Rhizobium sp. (90.9%), and Phyllobacterium sp. (99.9%), respectively, both of which are members of the Alphaproteobacteia. Clo29 and Clo13 corresponded to Bands e and f () and were most homologous to the bacterium TG141 (93.4%, the isolate from lake sediment in Japan) in the phylum Bacteroidetes. Phylogenetic relationships among these clones are shown in .
Figure 5. Phylogenetic relationships of 16S rRNA gene sequences for clones which showed the same migration as dominant bands in direct polymerase chain reaction-denaturing gradient gel electrophoresis. Sequences of clones obtained in the present study are shown in boldface. Bootstrap values based on 1000 replications are shown as a percentage at branching points. Bar indicates 0.05 substitutions per nucleotide position.
Discussion
In this study, we report the comparative analysis of the bacterial community structure for three different earthworm species using PCR-DGGE. Our results identified distinct characteristics of bacterial community structure for earthworm gut samples and surrounding soils. Specific dominant bacterial species were also characterized for each of the earthworm species collected in this study.
First, the DGGE profiles for gut samples from earthworms collected from grassland soils were distinguishable from those of surrounding soils, and this was further supported by the MDS analysis. However, most bands found in gut samples also existed in soil samples. These results support claims by Furlong et al. (Citation2002) that the fresh cast clone library of 16S rRNA gene from Lumbricus rubellus (family Lumbricidae) was similar to the soil clone library, although their abundances were different. Similarly, some intestinal bacteria of several Lumricidae species (Horn et al. Citation2006) originated from surrounding soils. Although DGGE patterns in our study were specific for earthworm gut samples, each species did not appear to have indigenous bacterial species. A large portion of the microbial population in the soil is therefore likely to pass through the gastrointestinal tract of the earthworm unchanged, while representatives of some phyla increase in abundance. In addition, it was indicated that the gut microbiota of earthworms was mostly constructed until fore-gut, but certain bacteria increased during gut passage.
The bacterial community structure of the Pheretima hilgendorfi and P. heteropoda gut appeared to be distinguishable from those of the Allolobophora japonica due to differences in DGGE profile bands and MDS analysis results between the three earthworm species studied. Since DGGE only detects dominant members of the analyzed community (Muyzer et al. Citation1993), the interpretation from our results mainly paid attention to the variation of dominant bands. Activity of Japanese earthworms has also been reported to have a variety of seasonal changes (Nakamura Citation1972). This is in contrast to our DGGE profile results for gut samples, which exhibited a very uniform pattern for different seasons in both Pheretima species. However, gut samples in A. japonica varied among June 2007 samples and between June 2007 and 2008 samples. Differences in major members could therefore not be explained by the seasonality of earthworm activity. Rather, once established, the microbiota in the P. hilgendorfi and P. heteropoda gut seemed to be maintained for these two years () in this no-tilled grassland. Nakamura (Citation2000) grouped the Japanese earthworm species into four ecological categories based on the observation of Lumbricidae by Bouché (Citation1977), and suggested that P. hilgendorfi and P. heteropoda belong to different ecological categories, respectively. These ecological differences in habitat and food may also correspond to the earthworms ingesting different materials (bacteria, organic matters, soil particles, etc.). It is however noticeable that P. hilgendorfi and P. heteropoda possess similar gut microbiota, despite each being classified into different ecological categories. Different changes in bacterial numbers during gut transit of earthworms were partially explained by ecological categories, but variations by earthworm species were observed at the same time (Aira et al. Citation2009). Recently, Wüst et al. (Citation2009) suggested that earthworm species belonging to the Megascolecidae (Octochaetus multiporus) and Lumbricidae (Octolasion cyaneum, L. rubellus, Aporrectodea rosea) do not have equal capacities to emit nitrous oxide (N2O) and possess their own nosZ genes, indicating distinct family level differences in their intestinal denitrifying microbiota. Thus, we suggest that in such stable environments, the bacterial community structure of earthworm gut may be primarily associated with differences observed between higher taxonomical categories (Family level), such as variation in body structure.
Next, we discuss the dominant bacterial species. Dominant bands from both P. hilgendorfi (soil-type, are active in mineral soil layers) and P. heteropoda (surface soil-type, move soil surface) guts were most related to Bacillus longiquaesitum and their intensity increased from fore-gut to hind-gut. Among studies on the gut microbiota of Pheretima species, Khambata and Bhat (Citation1957) reported 129 bacterial isolates using nutrient agar plates, 111 of which were spore-forming Bacilli. Fischer et al. (Citation1997) reported the germination of inoculated Bacillus megaterium spores during gut passage of Lumbricus terrestris and thus, gut structure and condition of Pheretima may also be favorable to Bacillus species.
In contrast, many dominant bands for the A. japonica (surface soil-type) gut samples were most closely related to the phylum Proteobacteria. These proteobacteria were also found to dominate the gut contents of Lumbricus terrestris (Fischer et al. Citation1995; Schönholzer et al. Citation2002) and L. rubellus (Knapp et al. Citation2009). FISH analysis detected the α-, β-and γ-subgroups of the proteobacteria and the latter two classes increased prominently between the fore- and hind-gut of L. terrestris (Fischer et al. Citation1995). Passage through the digestive tract of L. terrestris however significantly reduced populations of bacteria belonging to the α-, β- and γ-subgroups of proteobacteria (Schönholzer et al. Citation2002).
Molecular fingerprinting techniques, including PCR-DGGE analysis, have become popular for assessing diversity, structural composition and dynamics of microbial communities (Nocker et al. Citation2007). Although PCR-DGGE allows the rapid assessment of the whole microbial community structure and identification of the dominant species, however, the analytical technique has some limitations: recovering DNA sequences information from excised gel bands ultimately requires cloning. Only relatively small fragment size of PCR products can be separated (up to c. 500 bp), and so on. In this study, we found characteristic features of the microbiota of Japanese earthworms, but due to the limited success of direct sequencing from an electorphoresed gel, cloning of amplified 16S rRNA genes was conducted. Thus, only information on dominant bacterial species was obtained. To understand the entire gut bacterial community structure of earthworms it is necessary to conduct a detailed clone library analysis or recent pyrosequencing approach.
Collectively, our results have revealed distinct differences between the gut bacterial community structures of P. hilgendorfi and P. heteropoda (Megasocolecidae) and A. japonica (Lumbricidae) earthworms from the same sampling site during different seasons and a similarity between P. hilgendorfi and P. heteropoda. The main bacterial groups observed were the Bacillus species belonging to Firmicutes in both P. hilgendorfi and P. heteropoda and Proteobacteria and Bacteroidetes for A. japonica. Such specific bacterial communities have likely originated from ingested soil bacteria and the bacterial community structure may be influenced by the alimental canal structure rather than by their ecological category and seasonality. Further sample collection and experimental feeding of the earthworm species used in this study would help to provide additional supporting evidence for our observations.
Acknowledgments
The authors are grateful to Dr. Takafumi Gotoh and the staff of Kuju Agricultural Research for their assistance in collecting the earthworms and to Emeritus Prof. Dr. Yoshio Nakamura for his assistance in identifying the earthworm species.
References
- Aira, M , Monroy, F , and Domínguez, J , 2006. Changes in microbial biomass and microbial activity of pig slurry after the transit through the gut of the earthworm Eudrilus eugeniae (Kinberg, 1867) , Biol. Fertil. Soils 42 (2006), pp. 371–376.
- Aira, M , Monroy, F , and Domínguez, J , 2009. Changes in bacterial numbers and microbial activity of pig slurry during gut transit of epigeic and anecic earthworms , J. Hazard. Mater. 162 (2009), pp. 1404–1407.
- Aoki, J , 1973. Soil Zoology . Tokyo (in Japanese): Hokuryukan; 1973.
- Bouché, MB , 1977. "Stratégies Lombriciennes". In: Lohm, U , and Person, T , eds. Soil Organisms as Components of Ecosystems, pp 122–132, Ecological Bulletins 25 . Stockholm: Swedish Natural Science Research Council; 1977.
- Brown, GG , 1995. How do earthworms affect microfloral and faunal community diversity? , Plant Soil 170 (1995), pp. 209–231.
- Burr, MD , Clark, SJ , Sper, CR , and Camper, AK , 2006. Denaturing gradient gel electrophoresis can rapidly display the bacterial diversity contained in 16S rDNA clone libraries , Microb. Ecol. 51 (2006), pp. 479–486.
- Darwin, CR , 1881. The Formation of Vegetable Mould through the Action of Worms, with Observations on their Habits . London: Murray; 1881.
- Fischer, K , Hahn, DR , Amann, I , Daniel, O , and Zeyer, J , 1995. In situ analysis of the bacterial community in the gut of the earthworm Lumbricus terrestris L. by whole-cell hybridization , Can. J. Microbiol. 41 (1995), pp. 666–673.
- Fischer, K , Hahn, D , Hönerlage, W , and Zeyer, J , 1997. Effect of passage through the gut of the earthworm Lumbricus terrestris L. on Bacillus megaterium studied by whole cell hybridization , Soil Biol. Biochem. 29 (1997), pp. 1149–1152.
- Flack, FM , and Hartenstein, R , 1984. Growth of the earthworm Eisenia foetida on microorganisms and cellulose , Soil Biol. Biochem. 16 (1984), pp. 491–495.
- Furlong, MA , Singleton, DR , Coleman, DC , and Whitman, WB , 2002. Molecular and culture-based analyses of prokaryotic communities from an agricultural soil and the burrows and casts of the earthworm Lumbricus rubellus , Appl. Environ. Microbiol. 68 (2002), pp. 1265–1279.
- Horn, MA , Drake, HL , and Schramm, A , 2006. Nitrous oxide reductase genes (nosZ) of denitrifying microbial populations in soil and the earthworm gut are phylogenetically similar , Appl. Environ. Microbiol. 72 (2006), pp. 1019–1026.
- Horn, MA , Schramm, A , and Drake, HL , 2003. The earthworm gut: an ideal habitat for ingested N2O-producing microorganisms , Appl. Environ. Microbiol. 69 (2003), pp. 1662–1669.
- Iwamoto, T , Tani, K , Nakamura, K , Suzuki, Y , Kitagawa, M , Eguchi, M , and Nasu, M , 2000. Monitoring impact of in situ biostimulation treatment on groundwater bacterial community by DGGE , FEMS Microbiol. Ecol. 32 (2000), pp. 129–141.
- Khambata, SR , and Bhat, JV , 1957. A contribution to the study of the intestinal microflora of Indian earthworms , Arch. Mikrobiol. 28 (1957), pp. 69–80.
- Knapp, BA , Podmirseg, SM , Seeber, J , Meyer, E , and Insam, H , 2009. Diet-related composition of the gut microbiota of Lumbricus rubellus as revealed by a molecular fingerprinting technique and cloning , Soil Biol. Biochem. 41 (2009), pp. 2299–2307.
- Matsumoto, S , 1987. Micro-organisms, sugars and amino acids in earthworm casts , Jpn. J. Soil Sci. Plant Nutr. 58 (1987), pp. 86–88, (in Japanese).
- Monroy, F , Aira, M , and Domínguez, J , 2008. Changes in density of nematodes, protozoa and total coliforms after transit through the gut of four epigeic earthworms (Oligochaeta) , Appl. Soil Ecol. 39 (2008), pp. 127–132.
- Muyzer, G , De Waal, EC , and Uitterlinden, AG , 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA , Appl. Environ. Microbiol. 59 (1993), pp. 695–700.
- Márialigeti, K , 1979. On the community-structure of the gut-microbiota of Eisenia lucens (Annelida, Oligochaeta) , Pedobiologia 19 (1979), pp. 213–220.
- Nakamura, Y , 1972. Studies on soil animals in grassland 1. Seasonal variations of the population density and biomass of macro soil animals in forage swords , J. Jpn. Grassl. Sci. 17 (1972), pp. 217–222, (in Japanese with English summary).
- Nakamura, Y , 1999. "Annelida". In: Aoki, J , ed. Pictorial Keys to Soil Animals of Japan, pp 101–110 . Tokyo: Tokai University Publishing; 1999, (in Japanese).
- Nakamura, Y , 2000. Earthworms and potworms as keystone functional animals in pedospheres , Farming Jpn. 34 (2000), pp. 10–15.
- Nocker, A , Burr, M , and Camper, AK , 2007. Genotypic microbial community profiling: a critical technical review , Microb. Ecol. 54 (2007), pp. 276–289.
- Ohfuchi, S , and Yamaguchi, H , 1965. "OLIGOCHAETA". In: Okada, K , Uchida, S , and Uchida, T , eds. New Illustrated Encyclopedia of the Fauna of Japan . Tokyo: Hokuryu-Kan Publishing Co.; 1965. pp. 533–563, (in Japanese).
- Pedersen, JC , and Hendriksen, NB , 1993. Effect of passage through the intestinal-tract of detritivore earthworms (Lumbricus spp.) on the number of selected gram-negative and total bacteria , Biol. Fertil. Soils 16 (1993), pp. 227–232.
- Risal, CP , and Ozawa, T , 2002. Isolation and characterization of diazotrophs from the intestinal tract of an earthworm (Pheretima vittata) , Soil Sci. Plant Nutr. 48 (2002), pp. 101–103.
- Röling, WFM , Van Breukelen, BM , Braster, M , Lin, B , and Van Verseveld, HW , 2001. Relationships between microbial community structure and hydrochemistry in a landfill leachate-polluted aquifer , Appl. Environ. Microbiol. 67 (2001), pp. 4619–4629.
- Sakai, K , Kudoh, E , Wakayama, M , and Moriguchi, M , 2000. Analysis of the microbial community in an activated sludge enriched with an inorganic nitrite medium , Microbes Environ. 15 (2000), pp. 103–112.
- Sampedro, L , Jeannotte, R , and Whalen, JK , 2006. Trophic transfer of fatty acids from gut microbiota to the earthworm Lumbricus terrestris L , Soil Biol. Biochem. 38 (2006), pp. 2188–2198.
- Schönholzer, F , Hahn, D , Zarda, B , and Zeyer, J , 2002. Automated image analysis and in situ hybridization as tools to study bacterial populations in food resources, gut and cast of Lumbricus terrestris L , J. Microbiol. Methods 48 (2002), pp. 53–68.
- Singleton, DR , Hendrix, PF , Coleman, DC , and Whitman, WB , 2003. Identification of uncultured bacteria tightly associated with the intestine of the earthworm Lumbricus rubellus (Lumbricidae; Oligochaeta) , Soil Biol. Biochem. 35 (2003), pp. 1547–1555.
- Smalla, K , Oros-Sichler, M , Milling, A , et al., 2007. Bacterial diversity of soils assessed by DGGE, T-RFLP and SSCP fingerprints of PCR-amplified 16S rRNA gene fragments: do the different methods provide similar results? , J. Microbiol. Methods 69 (2007), pp. 470–479.
- Tamura, K , Dudley, J , Nei, M , and Kumar, S , 2007. MEGA4: Molecular Evolutionay Genetics Analysis (MEGA) software version 4.0 , Mol. Biol. Evol. 24 (2007), pp. 1596–1599.
- Toyota, K , and Kimura, M , 2000. Microbial community indigenous to the earthworm Eisenia foetida , Biol. Fertil. Soils 31 (2000), pp. 187–190.
- Wüst, PK , Horn, MA , Henderson, G , Jassen, PH , Rehm, BHA , and Drake, HL , 2009. Gut-associated denitrification and in vivo emission of nitrous oxide by the earthworm families Megascolecidae and Lumbricidae in New Zealand , Appl. Environ. Microbiol. 75 (2009), pp. 3430–3436.
- Yamaguchi, H , 1967. "Oligochaeta". In: Uchida, T , ed. Systematic Zoology 6, pp 130–193 . Tokyo: Nakayama-Shoten Co.; 1967, (in Japanese).