Abstract
Non-allophanic Andosols often show aluminum (Al) toxicity to Al-sensitive plant roots. The toxicity was evaluated by the 1 M potassium chloride (KCl)-extractable Al (AlKCl) content. In contrast, natural allophanic Andosols rarely show any Al toxicity, whereas AlKCl can appear with strong acidification. It is still not clear whether the acidic allophanic soils cause the Al toxicity, or the acid injury in Andosols is totally explained by the Al toxicity. In this study, plant cultivation experiments were performed using non-allophanic and allophanic soils over a wide-range of pH values; two typical non-allophanic soils [pH(H2O) 4.4–4.7], their limed soils [pH(H2O) 5.9–6.1], two typical allophanic soils [pH(H2O) 5.7–7.0], three acidic allophanic soils [pH(H2O) 4.6–5.4] and a commercial Kanuma pumice. We cultivated the Al-sensitive plants [burdock (Arctium lappa L.) and barley (Hordeum vulgare)] and an Al-accumulative plant [buckwheat (Fagopyrum esculentum)] in these soil samples, then measured the root lengths of the burdock and barley and determined the Al concentrations in the buckwheat. The toxic (available) Al was evaluated from the results of these cultivations. The typical non-allophanic soils showed a strong inhibition of the roots of the burdock and barley. Although the inhibition was not observed in the typical allophanic soils for the Al-sensitive plants, the acidic allophanic soils did show the inhibition as observed in the non-allophanic soils. Close negative correlations were observed between the root lengths of the Al-sensitive plants and the Al concentrations in the buckwheat (P < 0.05); the Al concentrations in the buckwheat grown in the non-allophanic soils were much higher (2.6–4.3 mg g−1) than those in the typical allophanic soils (0.4–1.4 mg g−1), and these concentrations in the buckwheat in the acidic allophanic soils were comparable (2.7–4.0 mg g−1) to those in the non-allophanic soils. Thus, it was shown that the acid injury of the Andosols is definitely caused by the Al toxicity, regardless of the type of Andosols, i.e. non-allophanic or allophanic. Possible substances controlling the Al toxicity were discussed in relation to soil properties.
Introduction
Soils derived from volcaniclastic materials have generally developed into Andosols (IUSS Working Group WRB 2006) or Andisols (Soil Survey Staff Citation2006). Andosols are divided into two major groups on the basis of their colloidal compositions (Nanzyo et al. Citation1993). One group is referred to as allophanic Andosols (the sil-andic type, IUSS Working Group WRB 2006), whose colloidal fraction is dominated by allophanic materials. The other group is non-allophanic Andosols (the alu-andic type, IUSS Working Group WRB 2006), which are dominated by aluminum (Al)–humus complexes. Both types of Andosols display unique properties such as a thick dark A horizon, high reactivity with phosphate and fluoride ions and a low bulk density. However, there are significant differences in their soil acidity and Al toxicity to plants between the two groups (Nanzyo et al. Citation1993).
Non-allophanic Andosols often show an Al toxicity to Al-sensitive plant roots (Dahlgren et al. Citation2004; Nanzyo et al. Citation1993). The toxic Al has been evaluated by 1 M potassium chloride (KCl)-extractable Al (AlKCl) (Saigusa et al. Citation1980; Soil Survey Staff Citation1999) and considered to be primarily Al3+ adsorbed on permanent charge sites of the 2:1 type minerals (Dahlgren et al. Citation2004; Nanzyo et al. Citation1993; Saigusa et al. Citation1980). Non-allophanic Andosols are dominated not only by the 2:1 type minerals, but also by Al–humus complexes. It has been reported that the Al solubility or Al release/retention kinetics of humus-rich A horizons of non-allophanic Andosols was significantly influenced by the ion exchange reaction of the H+ and Al ions on the negatively charged sites of the organic matter (Dahlgren and Saigusa Citation1994; Takahashi et al. Citation1995). Some of the Al complexes with the humus were easily released using an acidic buffer solution (Takahashi et al. Citation1995). Therefore, several researchers showed that the Al solubility of non-allophanic Andosols is controlled by the Al–humus complexes (Dahlgren and Saigusa Citation1994; Takahashi et al. Citation1995). Furthermore, Takahashi et al. (Citation2007) showed that synthetic Al–humus complexes injured Al-sensitive plant roots. Thus, the Al solubility and toxicity in non-allophanic soils was remarkably influenced by the Al–humus complexes.
In contrast, the typical allophanic Andosols rarely show any Al toxicity to plant roots (Dahlgren et al. Citation2004; Nanzyo et al. Citation1993). Several researchers reported that the Al solubility of the allophane-rich Andosols is usually controlled by the dissolution equilibrium of imogolite and gibbsite (Dahlgren et al. Citation1990; Takahashi et al. Citation1995; Yagasaki et al. Citation2006). With strong acidification by human activities, however, the allophanic Andosols can then possess high amounts of AlKCl which may injure the plant roots (Matsuyama et al. Citation2005; Takahashi et al. Citation2008). The Al solubility of the acidified allophanic soils is close to that of the non-allophanic Andosols and is controlled by the Al–humus complexes (Takahashi et al. Citation2008; Yagasaki et al. Citation2006). However, it is still not clear whether the acidic allophanic soils actually cause Al toxicity to plants.
As mentioned earlier, AlKCl is often used as an indicator of the Al toxicity potential in soils. Thus, 2 cmolc kg−1 of AlKCl is considered to be the threshold for indicating the Al toxicity to Al-sensitive plant roots (Saigusa et al. Citation1980; Soil Survey Staff Citation1999). Unbuffered salt solutions, such as the KCl solution, are generally believed to remove exchangeable Al adsorbed on the permanently charged sites of the 2:1 type minerals (Jardine and Zelazny Citation1996; Saigusa et al. Citation1980; Shoji et al. Citation1980). However, there is a close relationship between the exchangeable Al (AlKCl) and the organically complexed Al (pyrophosphate-extractable Al) of the humus-rich horizons of the non-allophanic Andosols from an extensive area in eastern Japan (Takahashi et al. Citation2003), indicating that the organically complexed Al greatly contributes to AlKCl (Takahashi et al. Citation2003). Ross et al. (Citation2008) criticized that the measurement of extractable Al with a neutral salt from humus-rich soils cannot fit the definition of exchangeable. Furthermore, the precipitation of Al displaced by 1 M KCl during the extraction was found in allophanic and non-allophanic Andosols (Wada 1987a,b; Dahlgren and Walker 1994; Takahashi and Dahlgren Citation1998). These facts suggest the possibility that AlKCl does not always reflect accurately the Al toxicity potential in soils dominated by Al–humus complexes. Thus, AlKCl values of the soils rich in organically complexed Al may underestimate its Al-toxicity because of the precipitation of Al during the KCl extraction.
The purpose of this study was to determine whether soil acidification causes Al toxicity (or availability for Al-accumulative plants) in both non-allophanic and allophanic Andosols and to estimate the substances regulating the Al toxicity. To evaluate the toxic (available) Al in soils, we performed plant cultivation experiments instead of using chemical extraction. We prepared various Andosols having different properties, such as soil pH values, AlKCl values, the amount of Al–humus complexes and clay mineralogy. Using these soils, we cultivated burdock and barley as Al-sensitive plants and measured the degree of hindrance to plant root growth. Buckwheat was cultivated as an Al-accumulative plant and the Al concentrations in the shoots were determined. The toxic (available) Al was evaluated based on the results of cultivation experiments.
Materials and methods
Soil sampling and soil analysis
Seven A horizon samples were collected from eastern Japan. Sampling sites and classification according to the Soil Taxonomy (Soil Survey Staff Citation2006) of these soils are shown in . Kawatabi soils (Kawatabi 08 and Kawatabi 07 soils; both pedons are approximately 10 m away from each other) are A horizons of typical non-allophanic Andosols (Ito and Saigusa Citation1996; Takahashi Citation2008). The Kawatabi 08 (lime) and Kawatabi 07 (lime) soils are limed soil samples. The Utsunomiya, Yunodai, Tsutanuma, Morioka and Tsukuba soils are A or buried A horizons of the allophanic Andosols. Kanuma pumice that is commercially available consists mostly of allophanic materials.
Table 1. Location of sampling sites and classification of the soils according to Soil Taxonomy
The air-dried, fine-earth fractions (<2 mm) of the soil samples were used for the following analyses: The soil pH was measured in water or 1 M KCl (1:2.5 soil/solution) following a 1-h equilibration period. Total carbon was determined using the dry combustion method. The amount of AlKCl was determined by the method of Blakemore et al. (Citation1981). The concentrations of the pyrophosphate-extractable Al (Alp) were determined according to McKeague (Citation1967), and the concentrations of acid-oxalate-extractable Al (Alo), iron (Feo) and silicon (Sio) were determined as described by McKeague (Citation1976). The content of the allophanic materials was calculated using the formula Sio/(−0.067 × Al/Si + 0.27) (Parfitt and Wilson Citation1985). The content of ferrihydrite was calculated by multiplying the Feo by 1.7 (Parfitt and Childs Citation1988). The clay content of the soil samples was determined by the pipette method (Gee and Bauder Citation1986). The content of the crystalline minerals was calculated by subtracting the allophanic materials and the ferrihydrite content from the clay content. The mineralogy of the crystalline minerals in the clay fraction was analyzed by X-ray diffractometry (XRD) (MiniFlex, Rigaku Corp., Tokyo, Japan) using Cu Kα radiation generated with a 30 kV accelerating potential and a 15 mA tube current. Samples were step-scanned for 2 s at a 0.02° 2θ step. All samples were saturated with magnesium ion (Mg2+) (non-treated and solvated with glycerol) and potassium ion (K+) (then heated to 20, 300 and 550°C) for the XRD analysis.
Cultivation of aluminum-sensitive plants and evaluation of acid injury of soils
To investigate the acid injury (assuming mainly Al-toxicity) of the soil samples to the Al-sensitive plants, we cultivated burdock (Arctium lappa L.) and barley (Hordeum vulgare). Seventy grams of field-moist soil samples were placed in 100 mL beakers. The water content was adjusted and maintained at 70% of the maximum water-holding capacity. After a two-day germination period, three seedlings of burdock and four seedlings of barley per beaker were sown. We prepared one pot for each soil sample. The seedlings were cultured in a greenhouse kept at 25°C. After four days, the plants were sampled and the length of the roots was measured (total length of the fibrous root system for barley and length of the main root for burdock).
Cultivation of aluminum-accumulative plant and evaluation of available aluminum in soils
Buckwheat (Fagopyrum esculentum) is highly resistant to Al stress and is known to be an Al-accumulator (Shen et al. Citation2006). We determined the Al concentrations in the shoots of the buckwheat plant grown in the soil samples. Field-moist soil samples (corresponding to 750 g of dry soils) were placed in 2 L pots (15 cm diameter at the top) and mixed with 150 mg urea and 200 mg potassium dihydrogenphosphate per pot as basal fertilizer. Ten seeds of buckwheat were sown per pot. We prepared three pots for each soil sample. On day seven, the plants were thinned to five per pot. The plants were grown in a greenhouse kept at 25°C and watered with distilled water. After a growth period of 36 days, the plants were sampled. Plant shoot samples were dried (75°C) and ground to a fine powder. The ground samples were digested with concentrated nitric acid (HNO3) and 70% perchloric acid (HClO4). The Al concentrations in the solutions were determined by atomic absorption spectrometry (A2000, Hitachi High-Technologies Corp., Tokyo, Japan).
To monitor the Al concentrations in the soil solutions, non-destructive soil solution samplers [looped hollow fiber (LHF)] were installed in the soils as described by Yanai et al. (Citation1993) before the buckwheat was planted. The soil solutions were sampled at 7, 14, 21 and 28 days after the start of the cultivation. The monomeric Al concentrations in the soil solutions (Alsol) were determined by the ferron-o-phenanthroline method (American Society of Agronomy and Soil Science Society of America Citation1982).
Statistical analysis
Data were expressed as mean ± standard error (SE). To determine the statistical significance of the differences in the root length of the burdock and barley between the soil samples, the averages were compared using a one-way analysis of variance (ANOVA). Separation of the means was carried out using the least significant differences (LSD) at P = 0.05. Spearman's rank correlation coefficient (r s) was determined for relationships among plant root lengths, Al concentration of buckwheat, and AlKCl value.
Results
Chemical properties and clay mineralogy of soil samples
The chemical properties and clay mineralogy of the soil samples are shown in and , respectively. The XRD patterns of the Kawatabi 08, Utsunomiya, Tsutanuma and Morioka soil samples with Mg-saturation are shown in . The XRD patterns of the Kawatabi 08 sample saturated with Mg2+ (non-treated and solvated with glycerol) and K+ (20, 300 and 550°C) are shown in .
Figure 1. X-ray diffractometry patterns of the clay fractions. Samples were treated with magnesium-saturation.
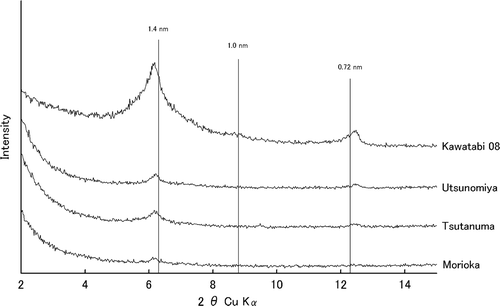
Figure 2. X-ray diffractometry patterns of the clay fractions of Kawatabi 08 soil sample. Samples were saturated with magnesium ion (Mg2+) (non-treated and solvated with glycerol) and potassium ion (K+) (20, 300, and 550°C).
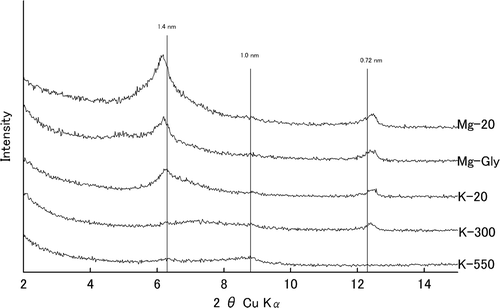
Table 2. Selected properties and extractable aluminum (Al) with pyrophosphate and acid-oxalate of soil samples
Table 3. Clay mineralogy of soil samples
The Kawatabi 08 and Kawatabi 07 soils showed the typical properties of non-allophanic soil materials, such as low pH values [pH(H2O) range 4.4–4.7], a high amount of AlKCl (4.6–6.0 cmolc kg−1), high Alp values (>16 g kg−1) and high Alp/Alo ratios (>0.8) (). These soils had high ratios of crystalline minerals to total clay minerals (77–78%) (, ). Their XRD patterns showed that most 2:1 type minerals were mostly chloritized (). The amount of AlKCl in the Kawatabi samples significantly decreased by liming ().
The Morioka and Tsukuba soils were slightly acidic or neutral [pH(H2O) range 5.7–7.0] with low Alp values (3.2–7.5 g kg−1) and low Alp/Alo ratios (<0.2) (), which were typical properties of the allophanic soils. Both soils were rich in allophanic materials (121–155 g kg−1) and the crystalline minerals/total clay minerals ratios in the soils were not high (26–42%) (). In contrast, the Utsunomiya soil showed low pH values [pH(H2O) 4.6] and a high amount of AlKCl (4.6 cmolc kg−1) similar to the Kawatabi soils. The Alp value of the Utsunomiya soil (11.8 g kg−1) was higher than that of the other allophanic soils, showing that these soil properties are similar to those of the non-allophanic soils. The Yunodai and Tsutanuma soils were moderately acidic [pH(H2O) range 5.3–5.4] and showed medium properties between those of the Utsunomiya soil and typical allophanic soils. The Tsutanuma soil possessed a slight amount of AlKCl (<0.1 cmolc kg−1), while the Yunodai soil possessed a slightly higher amount (0.7 cmolc kg−1). The crystalline minerals/total clay minerals ratios of the Utsunomiya, Yunodai and Tsutanuma soils (15–24%) were much lower than those of the Kawatabi non-allophanic soils. Based on the XRD analysis, the 2:1 type minerals of all the allophanic soils were also chloritized (data not shown).
Evaluation of acid injury in soil samples to aluminum-sensitive plants
The results of the root length of the burdock and barley are shown in . These results were considered to mainly reflect the Al toxicity, but other factors, such as the H+ and heavy metals, may also have an affect.
Table 4. Root lengths of burdock and barley
The typical non-allophanic soils (Kawatabi 08 and Kawatabi 07) showed strong suppression of the root lengths of burdock (13–16 mm). The suppression was ameliorated by liming [Kawatabi 08 (lime) and Kawatabi 07 (lime)] (44–46 mm). Although the inhibition was not observed in the typical allophanic soils (Morioka and Tsukuba) and Kanuma pumice (root lengths range 35–59 mm), strongly acidic allophanic soil (Utsunomiya) significantly damaged the roots of the burdock (9 mm) as observed in the non-allophanic soils. The root length in the moderately acidic allophanic soils (Yunodai and Tsutanuma; 23–29 mm) were also significantly shorter compared to those in the typical allophanic soils.
A similar tendency of injury was observed in the results for barely. The typical non-allophanic soils (Kawatabi 08 and Kawatabi 07) and strongly acidic allophanic soil (Utsunomiya) showed a strong inhibition (root lengths range 0–100 mm). The inhibition was ameliorated by liming [480–640 mm in Kawatabi 08 (lime) and Kawatabi 07 (lime)]. Although the injury was also not observed in the typical allophanic soils (Morioka and Tsukuba) and Kanuma pumice (490–540 mm), moderately acidic allophanic soils (Yunodai and Tsutanuma) damaged the roots of the barley (200–210 mm).
Evaluation of aluminum-availability in soil samples by aluminum-accumulative plant
The dry weights, Al concentrations in the shoots, and amounts of absorbed Al by the buckwheat plants are shown in . The Al concentrations in the buckwheat plant grown in Kawatabi 08 and Kawatabi 07 were very high (2.6–4.3 mg g−1), but were low in the limed soils (0.8–1.3 mg g−1). The concentrations were low in the typical allophanic soils (Morioka and Tsukuba) and Kanuma pumice (0.5–1.4 mg g−1), but high in the acidic allophanic soils (Utsunomiya, Yunodai and Tsutanuma) (2.7–4.0 mg g−1). Higher dry-matter productions were observed in the buckwheat with low internal concentrations of Al (). Therefore, differences in the amount of absorbed Al were smaller than those of the Al concentration among the soil samples (). However, both values reflect the differences in the Al availability.
Table 5. Dry weight, aluminum (Al) concentration and amounts of absorbed Al of buckwheat
The monomeric Al concentrations in the soil solutions during cultivation of the buckwheat are shown in . The concentrations were high in the Kawatabi 07 and Utsunomiya soils (3.5–4.1 mg L−1), but were lower than 0.5 mg L−1 in all the other soils.
Table 6. pH and aluminum (Al) concentration in soil solution during the cultivation of buckwheat
Discussion
Relationship between aluminum-toxicity to aluminum-sensitive plants and aluminum-availability to aluminum-accumulative plant
From these plant cultivation experiments, significant negative correlations were observed between the Al concentrations in the shoots of the buckwheat and the root lengths of the Al-sensitive plants (burdock: r s = −0.733, P < 0.05, barley: r s = −0.673, P < 0.05) (). Thus, the Al concentrations in the buckwheat reflect the strength of the Al toxicity of soils on the Al-sensitive plants.
Figure 3. The relationships between the aluminum (Al) concentration in the buckwheat and the root length of burdock or barley: (a) burdock and (b) barley. *P < 0.05.
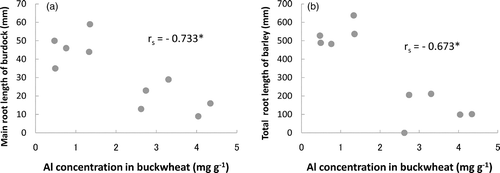
These relationships confirm that the injury to the Al-sensitive plant roots in the acidic allophanic Andosols is caused by the Al-toxicity because the shoot of the buckwheat accumulates a large amount of Al. This means that acid injury in the Andosols is mainly caused by the Al toxicity, regardless of the types of Andosols, i.e. non-allophanic or allophanic.
The monomeric aluminum concentrations in the soil solutions (Alsol) and 1M potassium chloride (KCl)-extractable aluminum (AlKCl) as indicators of aluminum toxicity (availability)
The Alsol was high in the Kawatabi 07 and Utsunomiya soils during the buckwheat cultivation (3.5–4.1 mg L−1) (). In these soils, the root growths of the Al-sensitive plants were extremely limited by the toxic Al, and the concentrations in the buckwheat were high. Despite the fact that the Alsol was low (<0.5 mg L−1) in all the other soils, the Kawatabi 08, Yunodai and Tsutanuma soils also showed toxicity (availability). This might be due to the large contribution of contact exchange (Jenny and Overstreet Citation1938) of Al ions between the roots and the soil surfaces. Thus, it is difficult to judge the degree of Al toxicity (availability) from only the monomeric Al concentrations in the soil solutions.
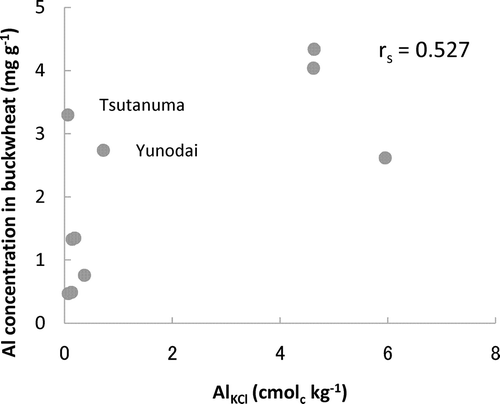
shows a fairly close correlation between the Al concentrations in the buckwheat and AlKCl (r s = 0.527, P > 0.05). A considerable relationship was also observed between the root length of the Al-sensitive plants and AlKCl (r s = −0.636, P > 0.05 for burdock and r s = 0.709, P < 0.05 for barley). However, some soil samples did not follow these correlations; the Yunodai and Tsutanuma soils having low AlKCl values showed a strong Al toxicity and high Al availability (), indicating that some of the Al, which was not extracted by the 1 M KCl, was also toxic (available). Thus, AlKCl does not adequately evaluate the Al toxicity (availability) potential of some soils.
Substance controlling the toxic aluminum in non-allophanic Andosols
The Kawatabi soils showed a strong Al toxicity and high Al availability ( and ). In the non-allophanic Andosols, the toxic Al has been considered to be primarily Al3+ adsorbed on the permanently charged sites of 2:1 type minerals (Dahlgren et al. Citation2004; Nanzyo et al. Citation1993; Saigusa et al. Citation1980). Indeed, the XRD analysis showed that the Kawatabi soils contained a large amount of 2:1 type minerals that were mostly chloritized (, ). The soils are also dominated by Al–humus complexes as shown by the high Alp contents (). Takahashi et al. (Citation1995) performed Al-release and Al-equilibrium experiments using A horizon samples of the non-allophanic Andosols including Mukaiyama soil (38°42′N, 140°33′E) that is near (approximately 4 km) and has almost the same properties as the Kawatabi soils. They showed that the Al solubility of the soils was mainly controlled by the Al–humus complexes, or the exchange reaction of Al ions and H+ on the charge sites of humus. According to Cronan et al. (Citation1986), the upper limit of applicability for the Al–humus model would be approximately pH 5.2. The pH(H2O) values () and pH values of soil solution () of the Kawatabi soils were less than 5.2. Thus, it is highly probable that the Al–humus complexes are one of the important regulators for toxic Al in the non-allophanic Andosols (Takahashi et al. Citation1995; Takahashi et al. Citation2003).
Possible substances controlling the toxic aluminum in acidic allophanic Andosols
Allophanic Andosols rarely show an Al toxicity to plant roots (Dahlgren et al. Citation2004; Nanzyo et al. Citation1993). Several researchers reported that the Al solubility of allophane-rich Andosols is usually controlled by the dissolution equilibrium of imogolite and gibbsite (Dahlgren et al. Citation1990; Takahashi et al. Citation1995; Yagasaki et al. Citation2006). With strong acidification, however, the allophanic Andosols can then possess high amounts of AlKCl (Matsuyama et al. Citation2005; Takahashi et al. Citation2008). The Al solubility of the acidified allophanic soils is close to that of the non-allophanic Andosols and is controlled by the Al–humus complexes (Takahashi et al. Citation2008; Yagasaki et al. Citation2006).
In this study, typical allophanic soils (Morioka and Tsukuba soils) and Kanuma pumice did not show an Al toxicity (availability), but the acidic allophanic soils (Utsunomiya, Yunodai and Tsutanuma soils) did show this ( and ). Some possibilities for the reasons these substances affected the Al toxicity (availability) in these acidic allophanic soils were considered, such as the Al3+ adsorbed on the permanent negative charge site of the 2:1 type minerals, dissolved allophanic materials, or the humus complexed Al.
All the allophanic soils contained a lower amount of the 2:1 type clay minerals compared to the Kawatabi soils (, ). Therefore, it is not considered that Al ions adsorbed on the permanent negative charges of the 2:1 minerals mainly affect the Al toxicity. The Utsunomiya and Yunodai soils, showing an Al toxicity (availability), possessed lower soil pH values, higher Alp values and higher Alp/Alo ratios. These properties are approaching those of the non-allophanic Andosols and acidified allophanic soils in which the Al–humus complexes control the Al solubility of the soils (Takahashi et al. Citation1995; Takahashi et al. Citation2008; Yagasaki et al. Citation2006). Therefore, it is likely that the Al–humus complexes significantly affect the Al toxicity (availability) in the acidic allophanic Andosols as well as non-allophanic Andosols. However, the possible effect of the dissolved allophanic materials on the Al-toxicity is not denied because a significant amount of the materials exist even in the acidic allophanic Andosols. This may be true for Tsutanuma soil, which has the rather low Alp value and Alp/Alo ratio ().
Acknowledgments
The authors are grateful to Professor H. Hirai (Utsunomiya University), Dr S. Hiradate (National Institute for Agro-Environmental Science), and Ms K. Yoshizumi (National Agricultural Research Center for Tohoku Region) for providing soil samples. This study was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 20580059).
References
- American Society of Agronomy and Soil Science Society of America 1982: Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties (2nd edition). American Society of Agronomy and Soil Science Society of America, Madison, WI.
- Blakemore, LC , Searle, PL , and Daly, BK , , 1981: Soil Bureau Laboratory Methods. A. Methods for Chemical Analysis of Soils. New Zealand Soil Bureau Scientific Report, 10A (revised). New Zealand Soil Bureau, Wellington.
- Cronan, CS , Walker, WJ , and Bloom, PR , 1986. Predicting aqueous aluminium concentrations in natural waters , Nature 324 (1986), pp. 140–143.
- Dahlgren, RA , McAvoy, DC , and Driscoll, CT , 1990. Acidification and recovery of a Spodosol Bs horizon from acidic deposition , Environ. Sci. Technol. 24(4) (1990), pp. 531–537.
- Dahlgren, RA , and Saigusa, M , 1994. Aluminum release rates from allophanic and nonallophanic Andosols , Soil Sci. Plant Nutr. 40 (1994), pp. 125–136.
- Dahlgren, RA , Saigusa, M , and Ugolini, FC , 2004. The nature, properties and management of volcanic soils , Adv. Agron. 82 (2004), pp. 113–182.
- Dahlgren, RA , and Walker, WJ , 1994. Solubility control of KCl extractable aluminum in soils with variable charge , Commun. Soil Sci. Plant Anal. 25 (1994), pp. 2201–2214.
- Gee, GW , and Bauder, JW , , 1986: Particle-size analysis. In: Methods of Soil Analysis, Part 1 (2nd edition), pp. 383–411. American Society of Agronomy and Soil Science Society of America, Madison, WI.
- International Union of Soil Sciences (IUSS) Working Group World Reference Base (WRB) 2006: World Reference Base for Soil Resources 2006. World Soil Resources Reports No. 103. Food and Agriculture Organization, Rome.
- Ito K, Takahashi T, Nanzyo M 2009: Aluminum toxicity of synthetic aluminum-humus complexes derived from non-allophanic and allophanic Andosols and its amelioration with allophanic materials. Soil Sci. Plant Nutr., 55, 35–41.
- Ito, T , and Saigusa, M , 1996. Characteristics of non-allophanic Andosols at Tohoku University Farm , Bull. Exp. Farm, Tohoku Univ. 12 (1996), pp. 91–103.
- Jardine, PM , and Zelazny, LW , , 1996: Surface reactions of aqueous aluminum species. In Sposito G (ed) The Environmental Chemistry of Aluminum (2nd edition), pp. 221–270. Lewis Publishers, Washington, DC.
- Jenny, H , and Overstreet, R , 1938. Contact effect between plant roots and soil colloids , Proc. Natl. Acad. Sci. USA 24 (1938), pp. 384–392.
- Matsuyama, N , Saigusa, M , Sakaiya, E , Tamakawa, K , Oyamada, Z , and Kudo, K , 2005. Acidification and soil productivity of allophanic Andosols affected by heavy application of fertilizers , Soil Sci. Plant Nutr. 51 (2005), pp. 117–123.
- McKeague, JA , 1967. An evaluation of 0.1 M pyrophosphate and pyrophosphate-dithionite in comparison with oxalate as extractants of the accumulation products in podzols and some other soils , Can. J. Soil Sci. 47 (1967), pp. 95–99.
- McKeague, JA , , 1976: Manual on Soil Sampling and Methods of Analysis. Soil Resource Institute, Agriculture Canada, Ottawa.
- Nanzyo, M , Dahlgren, RA , and Shoji, S , , 1993: Chemical characteristics of volcanic ash soils. In: Shoji S, Nanzyo M, Dahlgren RA (eds) Volcanic Ash Soils – Genesis, Properties and Utilization, pp. 145–187. Elsevier, Amsterdam.
- Parfitt, RL , and Childs, CW , 1988. Estimation of forms of Fe and Al: a review and analysis of contrasting soils by dissolution and Möessbauer methods , Aust. J. Soil Res. 26 (1988), pp. 121–144.
- Parfitt, RL , and Wilson, AD , , 1985: Estimation of allophane and halloysite in three sequences of volcanic soils, New Zealand. In: Caldas EF, Yaalon DH (eds) Volcanic Soils on Tephra and Basalt, Catena Suppl. 7, pp. 1–8. Catena-Verlag, Cremlingen-Destedt.
- Ross, DS , Matschonat, G , and Skyllberg, U , 2008. Cation exchange in forest soil: the need for a new perspective , Eur. J. Soil Sci. 59 (2008), pp. 1141–1159.
- Saigusa, M , Shoji, S , and Takahashi, T , 1980. Plant root growth in acid Andosols from northeastern Japan: 2. Exchange acidity Y1 as a realistic measure of aluminum toxicity potential , Soil Sci. 130 (1980), pp. 242–250.
- Saito, M , and Kato, T , 1994. Relationships between dry-matter production, nitrogen and phosphorus accumulation soybean grown under cool weather conditions , Soil Sci. Plant Nutr. 40 (1994), pp. 73–81.
- Shen, RF , Chen, RF , and Ma, JF , 2006. Buckwheat accumulates aluminum in leaves but not in seeds , Plant Soil 284 (2006), pp. 265–271.
- Shoji, S , Saigusa, M , and Takahashi, T , 1980. Plant root growth in acid Andosols from northeastern Japan: 1. Soil properties and root growth of burdock, barley, and orchard grass , Soil Sci. 130 (1980), pp. 124–131.
- Shoji, S , Takahashi, T , Saigusa, M , Yamada, I , and Ugolini, FC , 1988. Properties of Spodosols and Andisols showing climosequential and biosequential relations in southern Hakkoda, northeastern Japan , Soil Sci. 145 (1988), pp. 135–150.
- Soil Survey Staff 1999: Soil Taxonomy, Agriculture Handbook, vol. 436 (2nd edition). US Department of Agriculture-National Resource Conservation Service, Washington, DC.
- Soil Survey Staff 2006: Keys to Soil Taxonomy (10th edition). US Department of Agriculture-National Resource Conservation Service, Washington, DC.
- Takahashi, K , , 2008: Search of the crops developing the roots to forage phosphorus in non-allophanic Andosols and inspection of efficiency of the effects in frame tests (Master's thesis). Tohoku University (in Japanese).
- Takahashi, T , and Dahlgren, RA , 1998. Possible control of aluminum solubility by 1 M KCl treatment in some soils dominated by Al-humus complexes , Soil Sci. Plant Nutr. 44 (1998), pp. 43–51.
- Takahashi, T , Fukuoka, T , and Dahlgren, RA , 1995. Aluminum solubility and release rates from soil horizons dominated by aluminum–humus complexes , Soil Sci. Plant Nutr. 41 (1995), pp. 119–131.
- Takahashi, T , Mitamura, A , Ito, T , Ito, K , Nanzyo, M , and Saigusa, M , 2008. Aluminum solubility of strongly acidified allophonic Andosols from Kagoshima Prefecture, southern Japan , Soil Sci. Plant Nutr. 54 (2008), pp. 362–368.
- Takahashi, T , Nanzyo, M , and Hiradate, S , 2007. Aluminum status of synthetic Al–humic substance complexes and their influence on plant root growth , Soil Sci. Plant Nutr. 53 (2007), pp. 115–124.
- Takahashi, T , Sato, T , Sato, A , and Nanzyo, M , 2003. Relationship between 1M KCl-extractable aluminum and pyrophosphate-extractable aluminum in A horizons of non-allophanic Andosols , Soil Sci. Plant Nutr. 49 (2003), pp. 729–733.
- Wada, S-I , 1987a. A critical evaluation of 1 M KCl-extraction method for determining exchangeable Al ions in variable charge soils , Soil Sci. Plant Nutr. 33 (1987a), pp. 153–160.
- Wada, S-I , 1987a. Adsorption of Al(III) on allophane, imogolite, goethite, and noncrystalline silica and extractability of the adsorbed Al(III) in 1 M KCl solution , Soil Sci. Plant Nutr. 33 (1987a), pp. 487–491.
- Yagasaki, Y , Mulder, J , and Okazaki, M , 2006. The role of soil organic matter and short-range ordered aluminosilicates in controlling the activity of aluminum in soil solutions of volcanic ash soils , Geoderma 137 (2006), pp. 40–57.
- Yanai, J , Araki, S , and Kyuma, K , 1993. Use of a looped hollow fiber sampler as a device for nondestructive soil solution sampling from the heterogeneous root zone , Soil Sci. Plant Nutr. 39 (1993), pp. 737–743.
- Yonemura, S , Kawashima, S , and Tsuruta, H , 1999. Continuous measurements of CO and H2 deposition velocities onto an andisol: uptake control by soil moisture , Tellus. 51B (1999), pp. 688–700.