Abstract
A five-step sequential extraction procedure was developed for the partitioning of soil aluminum (Al) into five fractions: exchangeable, weakly organic bound, strongly organic bound, inorganic non-crystalline and crystalline. The results obtained by the sequential extraction method for Al were compared with those estimated from single extractions using identical operating conditions applied in each individual sequential fraction. The Al content in the extracts was measured by inductively coupled plasma optical emission spectrometry. The results indicated that the first four steps [potassium chloride (KCl), copper chloride (CuCl2), sodium pyrophosphate (Na4P2O7), acid ammonium oxalate ((NH4)2C2O4)] in the sequential procedure could be as effective as single extraction methods at estimating exchangeable Al, weakly organic bound Al, strongly organic bound Al, and non-crystalline Al. However, the crystalline Al content by sequential procedure was not in agreement with single extraction procedures. Further, the sequential extractions resulted in more consistent estimates of the aluminum/silicon (Al/Si) molar ratio and allophane and crystalline Al contents than single extractions method. Results of X-ray diffraction on the soil samples confirm the presence of appreciable amounts of at least three types of crystalline minerals, including gibbsite, vermiculite or halloysite. Our result implies that the sequential method can be more reliable in estimating the various Al forms in Andisols.
Introduction
Aluminum (Al) is one of the major elements in the earth's crust, comprising about 7% of its mass. When soils become acidic as a result of natural processes or human activities, Al is solubilized into the toxic trivalent cation, Al3+. Micromolar concentrations of Al3+ can inhibit root growth within minutes or hours of many agriculturally important plant species (Kochian Citation1995), resulting in poor growth and productivity. The toxic effect of certain Al species on different plants has been well documented (Lukaszewski and Blevins Citation1996; Takabatake and Shimmen Citation1997). However, some plant species, for example tea plants (Camellia sinensis L.) which naturally grow in soils containing elevated levels of Al, have the ability to accumulate unusually high concentrations of Al in mature leaves without any impact on their growth and development (Konishi et al. 1985; Matsumoto et al. Citation1976). Although the Al accumulation potential is dependent on the ability of plants to absorb and translocate the metal from root to shoot, Al bioavailability in rhizosphere soil is considered to be a critical factor. Since a large proportion of Al in soils is bound to organic and inorganic soil constituents, it is generally unavailable for root uptake.
A number of chemical extraction methods have been developed to identify various aluminum fractions in the soils and characterize their bioavailability. For instance, single extraction methods, including extractions with potassium chloride (KCl), copper chloride (CuCl2), sodium pyrophosphate (Na4P2O7), acid ammonium oxalate ((NH4)2C2O4), and cold sodium hydroxide (NaOH), are widely used in the quantification of Al fractions in acid soils. Aluminum extracted by KCl is considered to represent salt-exchangeable Al, while Al extracted with CuCl2 is used to estimate the potentially reactive non-exchangeable Al (AlCu) (Juo and Kamprath Citation1979). Likewise, extraction with Na–pyrophosphate at pH 10 (Alp) has been proposed to estimate the Al in organic complexes. Thus the difference between AlCu and Alp is considered to represent the Al that forms stable complexes with organic matter (Urrutia et al. Citation1988). Acid ammonium oxalate is commonly used to dissolve total non-crystalline Al (Alo), including short-range order Al hydroxides and oxihydroxides, and Al bounded to organic matter (Higashi and Ikeda Citation1974; Kodama and Schnitzer Citation1971; McKeague and Day Citation1966; McKeague et al. Citation1971; Theng et al. Citation1982; Wada Citation1977). Similarly Al extracted with 0.5 M NaOH (Aln) is used to estimate the total free Al pool in allophane, imogolite and gibbsite (Darke and Walbridge Citation1994; Eduardo Garcia-Rodeja et al. Citation2004; Wada Citation1980). Although the single extraction methods are efficient for the estimation of particular fractions of Al, various uncertainties are also associated with the use of these extractants. For instance, although CuCl2 removes Al from organic matter via ligand exchange reactions, it can extract some inorganic Al from interlayer clay minerals (Hargrove and Thomas Citation1981; Juo and Kamprath Citation1979). Likewise Na4P2O7 may extract some interlayer Al and relatively labile forms of surface-precipitated Al (Page and Kimpe Citation1989; Paterson et al. Citation1993; Soon Citation1993). Similarly, Kaiser and Zech (Citation1996) have shown that pyrophosphate extracted amorphous Al hydroxides as well as crystalline gibbsite. It has been established that acid oxalate also extracted a portion of the hydroxy-Al interlayer material from 2:1 layer silicates (Farmer et al. Citation1988; Iyengar et al. Citation1981; Paterson et al. Citation1993). Eduardo Garcia-Rodeja et al. (Citation2004) also reported that the amount of Al extracted by acid oxalate was significantly greater than that by cold NaOH.
The objective of this work was to compare, using some Japanese soil samples, Al fractions estimated from a sequential extraction procedure with those obtained by single extraction methods employing similar operating conditions to the corresponding sequential individual steps. The advantages and drawbacks of the two procedures were considered and discussed in the light of available literature.
Materials and methods
The soils
The soil samples used in this study were collected from 10 different regions of Japan. The samples were taken from the surface layer (0–20 cm).
General characterization of soil samples
The soils were air-dried, ground, and passed through a 1-mm screen to remove rocks, roots, and other large particles. Soil pH was measured in water (pH-H2O) and 1 M KCl (pH-KCl). Fluoride reactivity was measured as the pH in 1 M sodium fluoride (NaF) after 2 min of equilibration (Fieldes and Perrot Citation1966). Total aluminum oxide (Al2O3) and silicon dioxide (SiO2) contents of the soils were measured by X-ray fluorescence analysis (Primini, Rigaku, Japan). Total organic carbon content was determined using a vario MAX CN elemental analyzer (Elementar Analysen systeme GmbH, Hanau, Germany). Phosphate adsorption was determined by the standard method according to (Nair et al. Citation1984). Clay minerals of the soils were determined by X-ray diffraction (Wada Citation1966).
Aluminum extraction procedures
Single extraction procedures
Extractions consisted in shaking soil samples with solutions given below (), thus producing extracts containing one or several specified forms of Al. Following commonly used interpretations, the Al pools were classified as follows:
| Alk | = | exchangeable Al. |
| Alp-AlCu | = | Al bounded to organic matter, in order of decreasing stability. |
| Alo-Alp | = | Al in allophane and imogolite. |
| Aln-Alo | = | crystalline Al. |
Table 1. Experimental conditions of the single extraction procedure
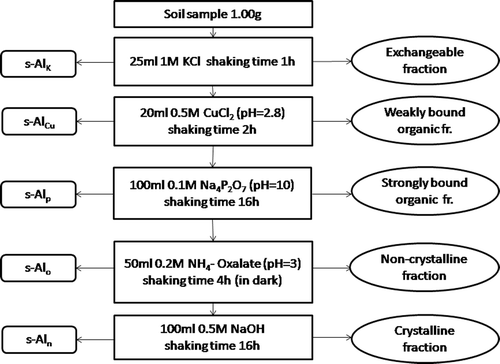
Sequential extraction procedure
The reagents and operating conditions for the sequential extraction procedure are presented in . The procedure was based on the single extraction methods but the shaking time and soil/extractant ratio in the first two steps were modified. The extraction was carried out in 50 mL polyethylene tubes, which were also used for centrifugation to minimize the possible loss of solid in the centrifuge-washing steps. After each extraction step the supernatant liquid was separated from the solid phase by centrifugation at 2500 rpm for 15 min. It was then decanted into polyethylene vessels and stored at 4°C before analysis.
Figure 1. Schematic diagram of the sequential extraction procedure employed. Abbreviations: CuCl2, copper chloride; KCl, potassium chloride; Na4P2O7, sodium pyrophosphate; NaOH, sodium hydroxide; NH4-Oxalate, ammonium oxalate. s-Alk, exchangeable aluminum (Al) sequentially extracted; s-AlCu, Al weakly bounded to organic matter sequentially extracted; s-Alp, Al strongly bounded to organic matter sequentially extracted; s-Alo, Al in allophane or immoglite sequentially extracted; s-Aln, crystalline Al sequentially extracted. fr., fraction.
Aluminum in the extracts and silicon (Si) extracted with (NH4)2C2O4 were measured in duplicates using inductively coupled plasma spectroscopy (SPS3100, SII Nano Technology Inc., Japan).
Soil classification
The soils studied were classified into “allophanic” (A) and “non-allophanic” (N) by using the following criteria:
| Non-allophanic | = | Alp/Alo ≥ 0.5%; Alp/Alo < 0.5%, Sio% < 0.5% and clay% ≥ 8%. |
| Allophanic | = | Alp/Alo < 0.5%, and Sio% ≥ 0.5%; Alp/Alo < 0.5%, Sio% < 0.5% and clay% < 8%, as defined by Masahiko Saigusa et al. (Citation1993). |
Allophane calculation
Allophane contents were calculated as follows (Dahlgren Citation1994):
The factor f depends on the Si/Al molar ratio and distinguishes Si-rich allophane (Al/Si, 1:1) and Al-rich allophane or imogolite (Al/Si, 2:1). Factor f can be determined as follows:
For an Al/Si ratio of 1:1, the factor is 5, for one of 2:1 the factor is 7.
Results and discussion
General characteristics of the soils
shows some of the general parameters analyzed in the soils. The pH in water ranged between 4.4 and 6.9. Among the soils studied, A1 and A2 showed the highest aluminum oxide (Al2O3) content (33%). Similarly silicon dioxide (SiO2) content in soil N2 was higher than those observed in other soils. The highest carbon content (12.8%) was recorded in the N3 soil.
Table 2. Location, pH, carbon content, phosphate retention and some general properties of the studied soils
Table 3. Aluminum (Al) concentration (in g kg−1) of each fraction extracted by single extraction methods, Alp/Alo ratio and classification of the soils studied
Aluminum fractionation by single extraction methods
Extractable Al contents in soils applying single extraction procedure are shown in . Extractable Al by three single extraction methods from KCl to pyrophosphate is in the order: Alk < AlCu < Alp. This was in good agreement with the results from Eduardo Garcia-Rodeja et al. (Citation2004) and Fransco Matus et al. (Citation2008). Extactable Al by pyrophosphate varied between 3.2 g kg−1 and 18.0 g kg−1, and was the highest extractable Al form after Aln and Alo; extractable Al in ammonium oxalate ranged from 12.8 g kg−1 to 64.8 g kg−1 and exhibited the greatest mean value; extractable Al in NaOH ranged from 7.5 g kg−1 to 49.9 g kg−1; excluding soil N3, Aln values of the other soils were less than Alo values, causing values of [Aln–Alo] negative. It indicates that there are no crystalline minerals in these soils.
The aim was to separate samples according to properties that are mainly related to the crystalline minerals, non-crystalline alumino-silicates, and Al–organic matter complexes (Masahiko Saigusa et al. 1993). According to Shoji et al. (Citation1993) and Dahlgren et al. (Citation2004), in Japan, allophanic andisols are typically younger than 10,000 years and have a high content of allophanic constituents, which contrasts with non-allophanic Andisols that are typically older than 10,000 years and have a low content of allophanic constituents. All the allophanic soils (A1–A6) had little pyrophosphate-extractable Al (<1%) but high oxalate-extractable Al (> 1%), causing Alp/Alo values <0.5%. Soil A2, is Akadama soil and developed from the loamy layer of the Kanto District. Although the soils A1 and A2 had the highest Al2O3 content (33%), they showed the lowest Alp/Alo ratio, indicating that these two soils are richer in allophane or imoglite than the other allophanic soils studied.
The non-allophanic soil, N1, was collected from the cultivated field, soil N2 from a no-till soil, soil N3 from a forest, and soil N4 from a vegetable plot. Carbon contents in non-allophanic soil, N1, N2 and N3 (>10%) were much higher than in allophanic soils. Since these soils are rich in pyrophosphate-extractable Al, they showed higher Alp/Alo ratio than allophanic soils. Further the results revealed that the non-allophanic soils are rich in Al–organic matter complex.
Aluminum fractionation by sequential extraction methods
Extractable Al contents in soils applying sequential extraction procedure are shown in . It is evident that the five-step sequential procedure (Sum-s-Al) extracted more Al (13.9–71.4 g kg−1) than the single oxalate method (Alo, 12.8–64.8 g kg−1). Further, the fourth step (s-Alo) of sequential procedure extracted greatest amount of Al in allophanic soils. It indicates that Al in allophanic soils mainly exist as non-crystalline Al. However in the case of non-allophanic soils, the highest amounts of Al found in the third step (s-Alp) indicating the greatest proportion of Al in non-allophanic soils was associated with organic bound fraction. In contrast to estimation using the single extraction method (Aln–Alo), the positive s-Aln values obtained with the sequential extraction, indicated the presence of crystalline-bound Al in both allophanic and non-allophanic soils.
Table 4. Aluminum (Al) concentration (in g kg−1) of each fraction extracted by the five-step sequential procedure and the sum quantity of extractable Al (Sum-s-Al)
Relationships amongst extractable aluminum between single extraction methods and sequential procedure
A simple correlation analysis was performed to assess relationships between the Al concentrations in the different Al fractions obtained by the single and sequential extraction procedures. The correlation coefficients for these relationships are listed in . It is evident that although the shaking time and soil/extractant ratio were modified in the first two steps, the amounts of Al released in the first four fractions extracted by the sequential procedure were correlated with the quantities extracted in the first four steps by the single extraction procedures. These results indicated that the first four steps in the sequential procedure could be as effective as the single extraction methods at estimating exchangeable Al, weakly organic bound Al, strongly organic bound Al, and non-crystalline Al. On the contrary, Al content extracted by Step 5, s-Aln, in the sequential procedure was not correlated with the Al content in the Aln–Alo in the single procedure. This suggests the possibility that cold NaOH was not an efficient extractor in the crystalline Al form, especially without a pretreatment using (NH4)2C2O4 to remove amorphous Al and expose the crystalline Al form in the single extraction procedure.
Table 5. Relationships of extractable aluminum (Al) between single extraction methods and sequential method
Aluminum in allophane
According to Parfitt and Henmi (1982), a molar ratio (Alo–Alp)/Sio close to 2 indicates the presence of allophane. The molar ratios of (Alo–Alp)/Sio obtained from single extraction methods ranged from 1.6 to 5.1 in the allophanic soils and from 0.1 to 1.3 in non-allophanic soils (). However, with the sequential procedure, this ratio was close to 2 for all the allophanic soils tested, and ranged from 2.9 to 7.9 for non-allophanic soils (). These results clearly show evidence that the sequential procedure can distinguish between non-allophanic soils and allophanic soils based on the molar ratio (Alo–Alp)/Sio. The molar ratio (Alo–Alp)/Sio of soil A6 obtained from the single oxalate procedure had a very high value (5.1), because the single oxalate procedure could also extract Al from crystalline minerals (Farmer et al. Citation1988; Iyengar et al. Citation1981; Paterson et al. Citation1993) and probably also because of the lower content of carbon () in this soil. This suggests that using single oxalate and pyrophosphate extractions may not be suitable for estimating the allophane contents in Andisols when the soil carbon content is low.
Table 6. Comparison of molar ratio aluminum/silicon (Al/Si) of allophane and allophane content calculated by single extraction methods and sequential procedure
Allophane contents in soils calculated by applying both the sequential and single extractions are shown in . The allophane contents obtained from single extraction methods ranged from 1.9% to 23% in allophanic soils, and from 1.4% to 2.4% in non-allophanic soils. The allophane content obtained from the sequential procedure were 3.1% to 23.7% for the allophanic soils, and 0.3% to 1.4% for non-allophanic soils. In general, allophane and imogolite are formed preferentially at pH >4.9, because at pH values <4.9 complexation of organic matter with Al leaves no available Al to react with silica to form allophane or imogolite (Mizota and Van Reeuwijk Citation1989; Shoji and Fujiwara Citation1984). In the present case, because the pH of N2 and N3 soils are lower than 4.9 (), there is negligible possibility of the presence of allophane. Interestingly our results also show the sequential extraction to be more effective than the single extraction in evidencing the low allophone content in soils N2 and N3. Hence, it is possible to conclude that the sequential procedure provides more discriminatory results than the single extraction methods.
Crystalline aluminum
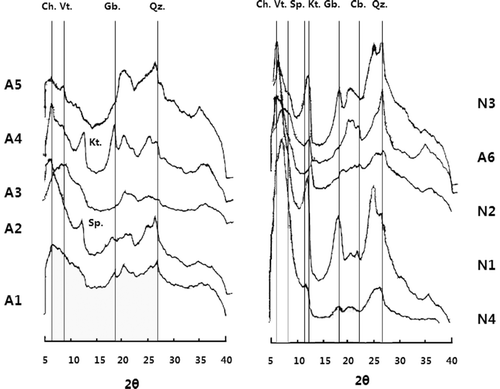
Negative Aln–Alo values (crystalline Al contents) found in the studied soil samples using the single procedure indicated that more Alo was extracted than was Aln. Farmer et al. (Citation1988) and Paterson et al. (Citation1993) also reported that acid oxalate can extract a part of the hydroxy-Al from silicates interlayer. Similarly, Eduardo Garcia-Rodeja et al. (Citation2004) also reported that (NH4)2C2O4 was more efficient than 0.5 M NaOH in extracting Al from organic carbon-rich horizons (> 10% carbon). In contrast to the single extraction procedure, the sequential procedure revealed the existence of crystalline clays or minerals (crystalline Al-ranged from 2.0 to 17.6 g kg−1) in the soil samples. Thus in order to confirm the existence of Al in crystalline minerals, all the soil samples were subjected to X-ray diffraction. Scans of magnesium ion (Mg2+) saturated oriented specimens of the clay fraction are presented in . The results of X-ray diffraction on the soil samples confirm the presence of appreciable amounts of more than three types of crystalline minerals, including gibbsite, vermiculite or halloysite. Our result implies that the sequential method to be more reliable in estimating the crystalline Al contents in the soil.
Conclusion
The proposed five-step sequential procedure can be as effective as the single extraction methods at estimating exchangeable Al, weakly organic bound Al, strongly organic bound Al fractions. However, the single extraction methods showed a high risk of overestimating allophane contents of non-allophanic soils. Further, the sequential extractions were more discriminatory with respect to Al/Si molar ratio and allophane and crystalline Al contents than single extraction methods. Moreover, the sequential procedure seems to be more reliable in evaluating the crystalline minerals in the soil. Hence, the use of sequential extractions should allow one to evaluate the extractable Al in soil samples.
Acknowledgments
The authors are grateful to Professor Masami Nanzyo (Graduate School of Agricultrual Science, Tohoku University) for collecting soil samples from Kawatabi Miyagi and to Ms Noami for her assistance in sample preparation.
References
- Bascomb, CL , 1968. Distribution of pyrophosphate-extractable iron and organic carbon in soils of various groups , J. Soil Sci. 19 (1968), pp. 251–268.
- Blackmore, LC , Searle, PL , and Daly, BK , 1981. Methods for Chemical Analysis of Soils . Wellington: New Zealand Soil Bureau Scientific Report 10 A. Department of Scientific and Industrial Research (DSIR); 1981.
- Dahlgren, RA , 1994. "Quantification of allophane and imogolite". In: Amonette, JE , and Zelazny, L , eds. Quantitative Methods in Soil Mineralogy . Madison, WI: SSSA; 1994. pp. 430–451.
- Dahlgren, RA , Saigusa, M , and Ugolini, FC , 2004. The nature, properties and management of volcanic soils , Adv. Agron. 82 (2004), pp. 113–182.
- Darke, AK , and Walbridge, MR , 1994. Estimating non-crystalline and crystalline aluminum and iron by selective dissolution in a riparian forest soil , Commun. Soil Sci. Plant Anal. 25 (1994), pp. 2089–2101.
- Eduardo, Garcia-Rodeja , Nóvoa Juan, C , Xabier, Pontevedra , Antonio, Martínez-Cortizas , and Peter, Buurman , 2004. Aluminum fractionation of European volcanic soils by selective dissolution techniques , Catena 56 (2004), pp. 155–183.
- Farmer, VC , Smith, BFL , Wilson, MJ , Loveland, PJ , and Payton, RW , 1988. Readily-extractable hydroxyaluminum interlayers in clay- and silt-sized vermiculite , Clay Miner. 23 (1988), pp. 271–277.
- Fieldes, M , and Perrot, KW , 1966. The nature of allophane in soils: 3 , Rapid field and laboratory test for allophane. New Zealand J. Sci. 9 (1966), pp. 629–639.
- Hargrove, WL , and Thomas, GW , 1981. Extraction of aluminum from aluminum-organic matter complexes , Soil Sci. Soc. Am. J. 45 (1981), pp. 151–153.
- Hashimoto, IM , and Jackson, ML , 1960. Rapid dissolution of allophone and kaolinite and halloysite after dehydration , Clays Clay Miner. 7 (1960), pp. 102–113.
- Higashi, T , and Ikeda, H , 1974. Dissolution of allophane by acid oxalate solution , Clay Sci. 4 (1974), pp. 205–211.
- Iyengar, SS , Zelazny, LW , and Martens, DC , 1981. Effect of photolitic oxalate treatment on soils hydroxy-interlayered vermiculites , Clays Clay Miner. 29 (1981), pp. 429–434.
- Juo, AS , and Kamprath, EJ , 1979. Copper chloride as an extractant for estimating the potentially reactive aluminum pool in acid soils , Soil Sci. Soc. Am. J. 43 (1979), pp. 35–38.
- Kaiser, K , and Zech, W , 1996. Defects in estimation of aluminum in humus complexes of podzolic soils by pyrophosphate extraction , Soil Sci. 161 (1996), pp. 452–458.
- Kochian, LV , 1995. Cellular mechanisms of aluminum toxicity and resistance in plants , Annu. Rev. Plant Physiol. Plant Mol. Biol. 46 (1995), pp. 237–260.
- Kodama, H , and Schnitzer, M , 1971. Evidence for interlamellar adsorption of organic matter by clay in a podzol soil , Can. J. Soil Sci. 51 (1971), pp. 509–512.
- Konishi, S , Miyamoto, S , and Taki, T , 1985. Stimulatory effects of aluminium on tea plants grown under low and high phosphorus supply , Soil Sci. Plant Nutr. 31 (1985), pp. 361–368.
- Lin, C , and Coleman, MT , 1960. The measurement of exchangeable aluminum in soils , Soil Sci. Soc. Am. Pro. 24 (1960), pp. 444–446.
- Lukaszewski, KM , and Blevins, DG , 1996. Root growth inhibition in boron-deficient plants , Plant Physiol. 112 (3) (1996), pp. 1135–1140.
- Matsumoto, H , Hirasawa, E , Morimura, S , and Takahashi, E , 1976. Localization of aluminum in tea leaves , Plant Cell Physiol. 17 (1976), pp. 627–631.
- Matus, F , Garrido, E , Sepulveda, N , Carcamo, I , Panichini, M , and Zagal, E , 2008. Relationship between extractable Al and organic C in volcanic soils of Chile , Geoderma 148 (2008), pp. 180–188.
- McKeague, JA , Brydon, JE , and Miles, NM , 1971. Differentiation of forms of extractable iron and aluminum in soils , Soil Sci. Soc. Am. Pro. 35 (1971), pp. 33–38.
- McKeague, JA , and Day, JH , 1966. Dithionite and oxalate extractable Fe and Al as aids in differentiating various classes of soils , Can. J. Soil Sci. 46 (1966), pp. 13–22.
- Mizota, C , and van Reeuwijk, LP , 1989. Clay Mineralogy and Chemistry of Soils Formed under Volcanic Materials in Diverse Climatic Regions . Wageningen: Soil Monograph 2. ISRIC; 1989.
- Nair, PS , Logan, TJ , Sharpley, AN , Sommers, LE , Tabatabai, MA , and Yuan, TL , 1984. Interlaboratory comparison of a standardized phosphorus adsorption procedure , J. Environ. Qual. 13 (1984), pp. 591–595.
- Page, CR , and Kimpe, CR , 1989. Dissolution des composes ferrugineux et alumineux des horizons B podzoliques de sols du Quebec par le dithionite-citrate, l’oxalate, lepyrophosphate et le tetraborate , Can. J. Soil Sci. 69 (1989), pp. 451–459.
- Parfitt, RL , and Henmi, T , 1982. Comparison of an oxalate extraction method and an infrared spectroscopic method for determining allophane in soil clays , Soil Sci. Plant Nutr. 28 (1982), pp. 183–190.
- Paterson, E , Clark, L , and Birnie, C , 1993. Sequential selective dissolution of iron, aluminum, and silicon from soils , Commun. Soil Sci. Plant Anal. 24 (1993), pp. 2015–2023.
- Saigusa, M , Mastuyama, N , and Abe, T , 1993. Distribution of allophanic andisols and nonallophanic andisols in Tohoku district , Jpn. J. Soil Sci. Plant Nutr. 64 (1993), p. 424, (in Japanese).
- Shoji, S , and Fujiwara, Y , 1984. Active aluminum and iron in the humus horizons of Andisols from northeastern Japan: their forms, properties and significance in clay weathering , Soil Sci. 137 (1984), pp. 216–226.
- Shoji, S , Nanzyo, M , and Dahlgren, RA , 1993. Volcanic Ash Soils . Amsterdam: p. 288. Elsevier Science Publishers; 1993.
- Soon, YK , 1993. Fractionation of extractable aluminum in acid soils: a review and a proposed procedure , Commun. Soil Sci. Plant Anal. 24 (1993), pp. 1683–1708.
- Takabatake, R , and Shimmen, T , 1997. Inhibition of electrogenesis by aluminum in characean cells , Plant Cell Physiol. 38 (11) (1997), pp. 1264–1271.
- Theng, BKG , Russell, M , Churchman, GJ , and Parfitt, RL , 1982. Surface properties of allophane, imogolite and halloysite , Clays Clay Miner. 30 (1982), pp. 143–149.
- Urrutia, MM , Garcia-Rodeja, E , and Macias, F , 1988. Effectividad delas soluciones no tamponadas (KCl, CuCl2 y LaCl3) en la extraccion de aluminio en suelos ricos en materia organica . Sevilla: II. Congreso Nacional de la Ciencia del Suelo; 1988.
- Wada, K , 1966. Identification and quantitative analysis of clay minerals , Jpn. J. Soil Sci. Plant Nutr. 37 (1966), pp. 7–17, (in Japanese).
- Wada, K , 1977. "Allophane and imogolite". In: JB, Dixon , and SB, Weed , eds. Minerals in Soil Environments . Madison, WI: SSSA; 1977. pp. 603–638.
- Wada, K , 1980. "Mineralogical characteristics of Andisols". In: BKG, Theng , ed. Soile Charge . Lower Hutt: New Zealand Society of Soil Science; 1980. pp. 87–107.