Abstract
The phytic acid (myo-inositol hexakisphosphate or InsP6) content of seed crops is important to their nutritional quality. Since it represents 75 ± 10% of the total seed phosphorus (P), phytic acid is also important regarding the management of P in agricultural production. A low-phytate F5 line, No. T-2-250-4-20, was selected from the progeny of a cross between the low-phytate soybean line CX1834 and the Japanese commercial cultivar Tanbakuro. This line and its parents were grown in a field nursery, and the growth characteristics, phytate accumulation, and processing suitability for tofu were evaluated. At full maturity, the weight of seeds per plant of line T-2-250-4-20 was 5.2- and 1.3-fold higher than that of CX1834 and Tanbakuro, respectively. The amount of phytate-phosphorus as a percentage of the total P content in seeds was 23% in line T-2-250-4-20-34, 30% in CX1834, and 69% in Tanbakuro. No significant difference was observed among the three cultivars/lines in their seed magnesium (Mg), potassium (K), crude protein, and sugar. However, the calcium (Ca), crude fat and ash contents in seeds of line T-2-250-4-20-34 and Tanbakuro was lowered compared to that of CX1834. The breaking stress of tofu was estimated employing a rheometer with a decreasing concentration of the coagulant magnesium chloride (MgCl2), starting at 15.7 mmol L−1. In tofu made from Tanbakuro, the concentration of MgCl2 required to achieve the maximum breaking stress was 12.6 mmol L−1; however, it was 9.5 mmol L−1 for tofu made from T-2-250-4-20-34 and CX1834. The tofu made from Tanbakuro was soft and broke at 6.3 mmol L−1 MgCl2, but, in line T-2-250-4-20-34, harder tofu was made with lower MgCl2 concentrations. No difference was observed among the cultivars/lines in the SDS-PAGE patterns of protein in soymilk. These results indicate that we have developed a low-phytate soybean with adequate productivity, and confirmed that tofu made from the low-phytate T-2-250-4-20-34 soybean becomes coagulated and harder at a lower MgCl2 concentration than that from high-phytate soybean cultivars.
Introduction
Phytic acid (myo-inositol hexakisphosphate or InsP6) is the storage form of phosphorus (P) in seeds and exists as phytate, typically representing 75 ± 10% of the total seed P (Lott et al. Citation2000; Raboy Citation2009). Phytate is poorly digested by monogastric animals. Therefore, large amounts of P are present in poultry feces. As a result, P is lost to the environment in cereal-consuming regions, where it may contribute to excessive levels in soil and water (Brinch-Pedersen et al. Citation2002). The anti-nutritional effects of phytate are primarily related to its strong chelating activity due to its six reactive P esters (Loewus and Murthy Citation2000; Urbano et al. Citation2000). Multivalent cations, such as magnesium (Mg), calcium (Ca), zinc (Zn), iron (Fe), and manganese (Mn), are particularly susceptible and form insoluble and indigestible complexes (Persson et al. Citation1998). To address these problems, various crops, for example, rice (Oryza sativa L.; Larson et al. Citation2000), maize (Zea mays L.; Raboy et al. Citation2000), barley (Hordeum vulgare L.; Larson et al. Citation1998; Rasmussen and Hatzack Citation1998), and wheat (Triticum aestivum L.; Guttieri et al. Citation2004), have been developed with lower contents of phytate. For the soybean, a low-phytate mutant has been produced by mutation breeding through chemical mutagenesis (Wilcox et al. Citation2000).
Tofu is one of the most nutritional and traditional foods made from soybean. Tofu manufacturing represents 51.4% of the food-use demand for soybean in Japan; however, domestically-produced soybean supplies only 25% of this demand (MAFF Citation2010). While tofu made from domestically-produced soybean is praised for its taste and provides consumers with a sense of safety (MAFF Citation2010), the phytate content of this domestically-produced soybean ranges widely due to the cultivated area and environmental conditions. Phytate acts as a buffering agent against the coagulation reaction of soymilk at a low concentration of coagulant (e.g., magnesium chloride, MgCl2) generally used for tofu processing (Toda et al. Citation2006). For this reason, a soybean variety is demanded which has a low phytate content and can be efficiently processed for tofu production.
In order to address these problems, we developed a low-phytate soybean by crossing a Japanese commercial cultivar and the CX1834 low-phytate line. In this study, we investigate the growth characteristics, productivity, and seed quality of this new low-phytate line compared to the parental stock.
Materials and Methods
Plant materials
The low-phytate soybean line CX1834, isolated from the mutant originally referred to as M153 (Wilcox et al. Citation2000), was obtained from USDA-ARS, Small Grains and Potato Germplasm Research Unit, Aberdeen, Idaho, USA. This low-phytate line was used as a pollen parent in crosses to develop several decades of Japanese commercial cultivars. They were crossed in August 2004 under greenhouse conditions, and crossing with Tanbakuro was successful. Five progeny populations were developed from the F1 seeds. The F1 generation and parents were grown in the field of the Graduate School of Biosphere Science, Hiroshima University, Higashi-Hiroshima, Japan, during the summer of 2005, and low-phytate lines were selected by measuring phytate-phosphorus and inorganic and total P contents in seeds after harvesting. From 2006 to 2008, the low-phytate soybean line was selected by measuring the phytic acid-phosphorus, inorganic P, and total P concentrations, yield per plant, weight of 100 seeds, numbers of seeds and seed coat color. Thirty-seven plants of the F5 line (Line No. T-2-250-4-20) and the two parents were sown in the field in June 2009 at a plant density of 2.2 plants m−2 (50 cm intra-row and 90 cm inter-row spacing), and the number of nodes on the main stem, total number of nodes, length of the main stem, weight of seeds per plant, and the 100-seed weight of each plant were measured at full maturity. The T-2-250-4-20-34 plant which showed a higher yield per plant and 100-seed weight was selected from 37 plants of the F5 line, and the seeds of T-2-250-4-20-34 were used for the analysis of seed quality and tofu properties.
Analysis of seed quality
Seeds were dried in an oven at 80°C for over 48 h and milled using a pulverizing mill. The total phosphorus (TP) content of seeds was determined using colorimetric methods (Chen et al. Citation1956), and Mg, Ca, and K contents were measured by atomic absorption spectrometry and a flame photometer after wet-ashing in sulfuric acid. Inorganic phosphorus (Pi) and phytate-P were determined as previously described (Raboy and Dickinson Citation1987). Crude protein was determined employing the standard micro-Kjeldahl method, and it was converted to the protein content using the conversion factor 5.71. Sugar was measured with the anthron method (Spiro Citation1966), crude ash by the dry ashing method, and crude fat was analyzed employing the Soxhlet method (Thiex et al. Citation2003).
Soymilk preparation and tofu making
Soybeans were washed and soaked in water at 25°C for 15 h. Hydrated seeds were drained and ground into homogenates with water. After the anti-foam agent Kureton (Kao Co., Tokyo, Japan) was added (0.6% of the dry seed weight), they were heated over a high flame until boiling, and, after boiling, heated over a low flame for seven minutes. Water was added equivalent to seven times the weight of dry seeds. Boiled soymilk was separated from the homogenate by squeezing with a hydraulic press machine and filtering with thin nylon mesh. The soymilk was cooled and kept in a refrigerator until used to make tofu. MgCl2 (3.1–15.7 mmol L−1 of soymilk) was added as a coagulant. After stirring with a glass rod, the soymilk was put into 25-ml syringes. The coagulated soymilk was incubated at 80°C for 1.0 h and cooled in flowing water.
Texture measurements
The texture profiles of the tofu samples were investigated using a rheometer (NRM-2002 J, Fudo Industry Co., Tokyo, Japan) with a cylindrical plunger 15 mm in diameter at a compression rate of 1 mm sec−1. Three cylindrical pieces of tofu (13 mm in height and 20 mm in diameter) were cut from the middle part of the tofu sample and used for texture analysis. The breaking stress of tofu was expressed based on three measurements at the same MgCl2 concentration.
Protein analysis of soymilk
Analysis of the subunit composition was conducted using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) according to the method of Yagasaki et al. (Citation1997). The protein concentration of soymilk was assayed employing the method of Lowry et al. (Citation1951) using bovine serum albumin as a standard protein. The gel was stained with Coomassie brilliant blue G-250 (CBB G-250). Densitometric analysis of the stained gel was carried out with ImageJ, a public domain open source software for analyzing images in Java.
Results and Discussion
Flowering began in line T-2-250-4-20 at the beginning of August, and bean development began mid-August. These growth stages were earlier than in Tanbakuro and later than in CX1834 (data not shown). The date when 95% of the beans reached sufficient maturity for harvest in line T-2-250-4-20 was slightly earlier than in Tanbakuro. From these growth characteristics, it is estimated that line T-2-250-4-20 is a late-maturity group soybean. The main stem length, number of nodes in the main stem, and total number of nodes in line T-2-250-4-20 were greater than in Tanbakuro and CX1834 (). The 100-seed weight of line T-2-250-4-20 was 1.8-fold greater than that of CX1834 and 15% lower than that of Tanbakuro, but the seed weight per plant of line T-2-250-4-20 was 1.35-fold higher than that of Tanbakuro (). These data indicate that the low-phytate line T-2-250-4-20 displays adequate vigor and productivity compared with the Japanese commercial cultivar Tanbakuro.
Table 1. Growth characteristics and yield components of line T-2-250-4-20, the low-phytate line CX1834, and the Japanese commercial cultivar Tanbakuro
The TP concentration in seeds of line T-2-250-4-20-34 was 6.59 mg g−1 dry weight (DW), and that of CX1834 and Tanbakuro was 7.26 and 7.72 mg g−1 DW, respectively (). The TP concentration in seeds of line T-2-250-4-20-34 was lower than in Tanbakuro (ANOVA, p < 0.05), but there was no significant difference among these cultivars/lines (p ≥ 0.05). The amount of phytate-P as a percentage of the TP in line T-2-250-4-20-34 (23.1%) and CX1834 (30.3%) was less than half of that in Tanbakuro (68.6%), and the amount of Pi as a percentage of the TP in line T-2-250-4-20-34 (75.0%) and CX1834 (66.1%) was about 20-fold higher than in Tanbakuro (3.5%).
Figure 1. Phosphorus fractions in seeds of line T-2-250-4-20-34, the low-phytate line CX1834, and the Japanese commercial cultivar Tanbakuro. Phy-P, phytate-phosphorus; Pi, inorganic phosphorus; Other-P, other phosphorus, calculated by subtracting Phy-P and Pi from the total phosphorus; DW, dry weight. Vertical bars represent standard errors of the mean of the total and Pi concentration.
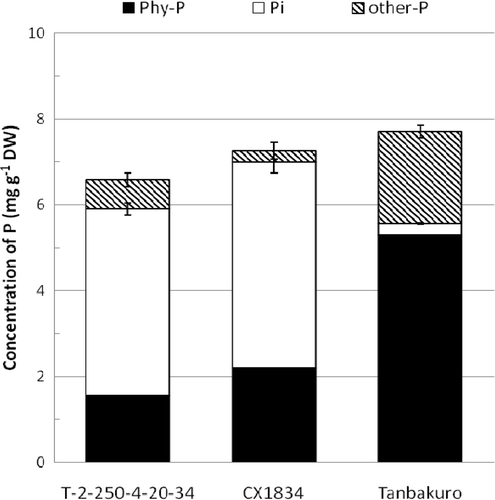
The contents of Mg, K, crude protein, and sugar in line T-2-250-4-20-34 were similar to Tanbakuro and CX1834 (). The Ca, crude fat and ash contents of T-2-250-4-20-34 and Tanbakuro was slightly lower than in CX1834. Numata (Citation1998) reported that the concentration of crude protein of 15 soybean varieties was 339 to 404 mg g−1, and he also indicated the positive correlation between the protein concentration and hardness of tofu. In this study the protein concentration was 308 to 316 mg g−1 in tested cultivars/lines, and it was lower than that found by Numata. For the lower protein concentration, in this study, the texture of the tofu made from tested cultivars/lines might have become soft.
Table 2. Calcium (Ca), magnesium (Mg), potassium (K), crude protein, crude fat, sugar, and crude ash contents (in mg−1 dry weight) in seeds of line T-2-250-4-20-34, the low-phytate line CX1834, and the Japanese commercial cultivar Tanbakuro
The seed water absorption ratio, pH of soymilk, solids, and phytate content, and tofu crude protein and phytate contents in tofu made from line T-2-250-4-20-34, CX1834, and Tanbakuro are shown in . The seed water absorption ratio and solids content in soymilk, and crude protein in tofu, made from line T-2-250-4-20-34 did not differ from those of the other two; however, the phytate content of soymilk made from lines T-2-250-4-20-34 and CX1834 was 70% lower than that made from Tanbakuro. Also, the pH of soymilk made from lines T-2-250-4-20-34 and CX1834 was lower than that made from Tanbakuro.
Table 3. Seed water absorption ratio, soymilk solid and phytate concentration, and crude protein and phytate concentration in tofu made from line T-2-250-4-20-34, the low-phytate line CX1834, and the Japanese commercial cultivar Tanbakuro
Phytate has been hypothesized to influence the coagulant requirement in tofu production (Ishiguro et al. Citation2008; Ono et al. Citation1993; Torikata et al. Citation1987). To examine the influence of phytate on the MgCl2 requirement, we measured the breaking stress of tofu made from T-2-250-4-20-34, CX1834, and Tanbakuro with various MgCl2 concentrations (). Tofu made from Tanbakuro showed a maximum breaking stress at 12.6 mmol L−1 MgCl2, while tofu made from T-2-250-4-20-34 and CX1834 reached its maximum breaking stress at 9.5 mmol L−1 MgCl2. Tofu made from Tanbakuro became softer and, as a result, was not hard enough to measure the breaking stress at 6.3 mmol L−1 MgCl2; however, the breaking stress of tofu made from T-2-250-4-20-34 was still high. Also, while it was impossible to measure the breaking stress at 3.1 mmol L−1 MgCl2, soymilk made from Tanbakuro did not coagulate, and that made from CX1834 coagulated slightly, but soymilk made from T-2-250-4-20-34 coagulated and formed tofu curd. These results indicate that high-phytate tofu tends to have a softer curd than low-phytate tofu at lower coagulant concentrations. Magnesium chloride, CaSO4 (calcium sulphate), and GDL (glucono-lactone) are coagulants used for tofu production. Magnesium chloride is used the most frequently, and an MgCl2 concentration of 12.3 mmol L−1 is generally employed for tofu processing in Japan (Toda et al. Citation2003; Toda et al. Citation2006). In this study the high-phytate cultivar Tanbakuro made the hardest tofu at 12.6 mmol L−1 MgCl2, whereas the low-phytate line T-2-250-4-20-34 made the hardest tofu at 9.5 mmol L−1 MgCl2. Thus, improvement of the coagulation properties at a lower concentration of MgCl2 solution was shown in T-2-25-4-20-34. These results indicate that improvement of the coagulation properties at a lower coagulant concentration might be due to a decrease in phytate, which affects the tofu texture by reacting with protein and coagulants such as magnesium salts.
Figure 2. Relationship between the MgCl2 concentration in soymilk and breaking stress of tofu made from line T-2-250-4-20-34, the low-phytate line CX1834, and the Japanese commercial cultivar Tanbakuro. Vertical bars indicate the standard error. MgCl2, magnesium chloride.
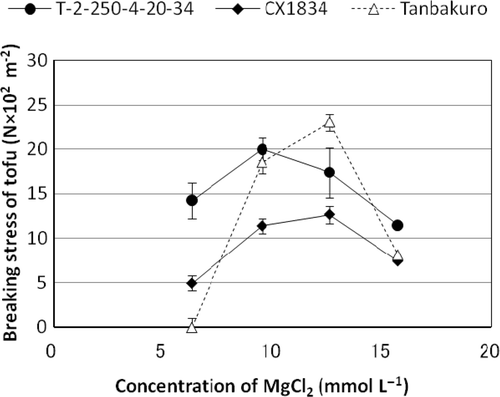
In this study, the pH of soymilk made from low-phytate lines was lower than that made from Tanbakuro (). Ono et al. (Citation1993) and Tezuka et al. (Citation1995) indicated that the coagulation of tofu was affected by a decrease in the pH of soymilk. Ishiguro et al. (Citation2006) also reported that the breaking strain of tofu decreased with increasing phytate concentration, and phytic acid prevented a decrease in the pH of soymilk during tofu processing.
In tofu production, soymilk is heated to cause protein dissociation and a coagulant is added to form a protein matrix, which gives tofu its firmness and hardness (Poysa et al. 2006). The quantity and quality of protein in the soymilk affects tofu processing. Glycinin (11S globulin) and β-conglycinin (7S globulin) are the most important proteins. Glycinin is generally in hexameric form, and each monomer unit consists of one acid and one basic polypeptide linked together by one disulfide bond. β-conglycinin is a trimeric glycoprotein with a molecular mass of 180 kDa, consisting of three subunits (Tezuka et al. 2004; Poysa et al. 2006). Tofu gel made from 11S protein was found to be significantly harder than that from 7S globulin, and 11S gel also showed a greater cohesiveness and elasticity than 7S globulin (Saio et al. Citation1971). Toda et al. (Citation2008) reported that the coagulant concentration for the maximum breaking stress of tofu decreased as the11S/7S ratio increased. Cai et al. (Citation1997) also reported that tofu from crude glycinin is harder than that from crude β-conglycinin, and the difference was attributed to higher numbers of disulfide bonds. SDS-PAGE patterns of protein in the soymilk of tested varieties are shown in ; no difference in SDS-PAGE patterns was observed among the three tested cultivars/lines. The 11S/7S ratio of tested cultivars/lines calculated by this SDS-PAGE band pattern was 3.36 in T-2-250-4-20-34, 2.75 in CX1834, and 2.99 in Tanbakuro. This result suggests that a higher breaking stress of tofu made from T-2-250-4-20-34 at a low concentration of coagulant (MgCl2) may not be related to a difference in the quality of protein in soymilk.
Figure 3. SDS-PAGE patterns of protein in soymilk made from line T-2-250-4-20-34 (lane A), the low-phytate line CX1834 (lane B), and the Japanese commercial cultivar Tanbakuro (lane C).
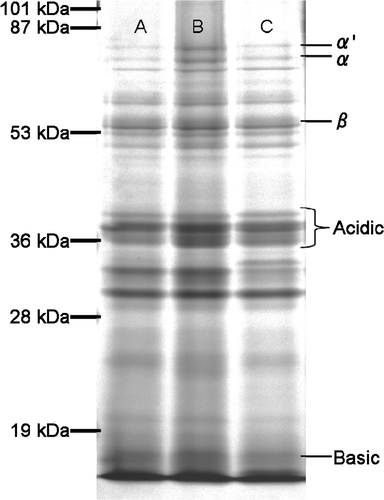
In this study, however, the tofu made from CX1834 was softer than that made from T-2-250-4-20-34. The differences in tofu hardness between CX1834 and T-2-250-4-20-34 with low concentrations of coagulant could not be clarified. However, Toda et al. (Citation2008) indicated that the concentration of oil globules in the coagulum may have influenced the formation of tofu curd. Further studies are needed to clarify coagulation activity in low-phytate soybean varieties.
Conclusions
Line T-2-250-4-20-34, developed by introducing the low-phytate trait into the cultivar Tanbakuro, displayed an adequate yield equal to that of Tanbakuro. The phytate content of soymilk and phytate concentration in tofu made from line T-2-250-4-20-34 were less than half of those from Tanbakuro. Line T-2-250-4-20-34 could be used to produce tofu at a lower concentration of coagulant compared to the high-phytate cultivar Tanbakuro. These results indicate that CX1834 can be used as a parental plant to introduce the low-phytate trait into Japanese cultivars, and line T-2-250-4-20-34 may be useful as a new soybean variety after the selection of pure lines employing the pedigree method, etc.
Acknowledgments
This research was supported by a Grant-in-Aid for Scientific Research (19380013) from the Japan Society for the Promotion of Science.
References
- Brinch-Pedersen , H , Sørensen , LD and Holm , PB . 2002 . Engineering crop plants: getting a handle on phosphate . Trends Plant Sci. , 7 : 118 – 125 .
- Cai , TD , Chang , KC , Shih , MC , Hou , HJ and Ji , M . 1997 . Comparison of bench and production scale methods for making soymilk and tofu from 13 soybean varieties . Food Res. Int. , 30 : 659 – 668 .
- Chen , PSJ , Toribara , TY and Warner , H . 1956 . Microdetermination of phosphorus . Anal. Chem. , 28 : 1756 – 1758 .
- Guttieri , M , Bowen , D , Dorsch , JA , Raboy , V and Souza , E . 2004 . Identification and characterization of low phytic acid wheat . Crop Sci. , 44 : 418 – 424 .
- Ishiguro , T , Ono , T and Nakasato , K . 2008 . The localization of phytate in tofu curd formation and effects of phytate on tofu texture . J. Food Sci. , 73 : C67 – 71 .
- Ishiguro , T , Ono , T , Wada , T , Tsukamoto , C and Kono , Y . 2006 . Changes in soybean phytate content as a result of field growing conditions and influence on tofu texture . Biosci. Biotechnol. Biochem. , 70 : 874 – 880 .
- Larson , SR , Rutger , JN , Young , KA and Raboy , V . 2000 . Isolation and genetic mapping of a non-lethal rice (Oryza sativa L.) low phytic acid 1 mutation . Crop Sci. , 40 : 1397 – 1405 .
- Larson , SR , Young , KA , Cook , A , Blake , TK and Raboy , V . 1998 . Linkage mapping of two mutations that reduce phytic acid content of barley grain . Theor. Appl. Genet. , 97 : 141 – 146 .
- Loewus , FA and Murthy , PPN . 2000 . myo-Inositol metabolism in plants . Plant Sci. , 150 : 1 – 19 .
- Lott , JNA , Ockenden , I , Raboy , V and Batten , GD . 2000 . Phytate and phosphorus in crop and fruits: a global estimate . Seed Sci. Res. , 10 : 11 – 33 .
- Lowry , OH , Rosebrough , NJ , Farr , AL and Randall , RJ . 1951 . Protein measurement with the folin phenol reagent . J. Biol. Chem. , 193 : 265 – 275 .
- MAFF 2010: Ministry of Agriculture, Forestry and Fisheries of Japan 2010: About the recent trend over the soybean. Available at http://www.maff.go.jp/j/seisan/ryutu/daizu/pdf/daizu_doukou.pdf (March 2010). MAFF, Tokyo (in Japanese)
- Numata , K . 1998 . Analysis of chemical composition of soybean grains, relating to packed tofu-making . Bull. Tokyo Metropolitan Technol. Res. Cent. , 7 : 21 – 27 . (in Japanese with English summary)
- Ono , T , Katho , S and Mothizuki , K . 1993 . Influences of calcium and pH on protein solubility in soybean milk . Biosci. Biotech. Biochem. , 57 : 24 – 28 .
- Persson , H , Türk , M , Nyman , M and Sandberg , AS . 1998 . Binding of Cu2 +, Zn2 +, and Cd2+ to Inositol Tri-, Tera-, Penta-, and Hexaphosphates . J. Agric. Food Chem. , 46 : 3194 – 3200 .
- Poysa , V , Woodrow , L and Yu , K . 2006 . Effect of soy protein subunits composition on tofu quality . Food Res. Int. , 39 : 309 – 317 .
- Raboy , V . 2009 . Approaches and challenges to engineering seed phytate and total phosphorus . Plant Sci. , 177 : 281 – 296 .
- Raboy , V and Dickinson , DB . 1987 . The timing and rate of phytate accumulation in developing soybean seeds . Plant Physiol. , 85 : 841 – 844 .
- Raboy , V , Gerbasi , PF , Young , KA , Stoneberg , SD , Pickett , SG , Bauman , AT , Murthy , PPN , Sheridan , WF and Ertl , DS . 2000 . Origin and seed phenotype of maize low phytic acid 1-1 and low phytic acid 2-1 . Plant Physiol. , 124 : 355 – 368 .
- Rasmussen , SK and Hatzack , F . 1998 . Identification of two low-phytate barley (Hordeum vulgare L.) grain mutants by TLC and genetic analysis . Hereditas , 129 : 107 – 112 .
- Saio , K , Kajikawa , M and Watanabe , T . 1971 . Food processing characteristics of soybean proteins. II. Effects of sulfhydryl group on physical properties of tofu-gel . Agric. Biol. Chem. , 35 : 890 – 898 .
- Spiro , RG . 1966 . “ Analysis of sugars found in glycoprotein ” . In Complex Carbohydrates vol. VIII, Methods in Enzymology , Edited by: Neufeld , ES and Ginsburg , V . 3 – 26 . New York : Academic Press .
- Tezuka , M , Ono , T and Ito , T . 1995 . Properties of soymilk prepared from soybeans of different varieties . Nippon Shokuhin Kagaku Kogaku Kaishi , 42 : 556 – 561 . (in Japanese with English summary)
- Tezuka , M , Yagasaki , K and Ono , T . 2004 . Changes in characters of soybean glycinin groups I, IIa, and IIb caused by heating . J. Agric. Food Chem. , 52 : 1693 – 1699 .
- Thiex , NJ , Anderson , S and Gildemeister , B . 2003 . Crude fat, diethyl ether extraction, in feed, cereal grain, and forage (Randall/Soxtec/submersion method): collaborative study . J. AOAC Int. , 86 : 888 – 898 .
- Toda , K , Ono , T , Kitamura , K , Hajika , M , Takahashi , K and Nakamura , Y . 2003 . Seed protein content and consistency of tofu prepared with different magnesium chloride concentrations in six Japanese soybean varieties . Breed. Sci. , 53 : 217 – 223 .
- Toda , K , Takahashi , K , Ono , T , Kitamura , K and Nakamura , Y . 2006 . Variation in the phytate content of soybeans and its effect on consistency of tofu made from soybean varieties with high protein content . J. Sci. Food Agric. , 86 : 212 – 219 .
- Toda , K , Yagasaki , K and Takahashi , K . 2008 . Relationship between protein composition and coagulation reactivity, particle formation, and incorporation of lipids in soymilk . Biosci. Biotechnol. Biochm. , 72 : 2824 – 2830 .
- Torikata , Y , Ishihara , J and Yano , T . 1987 . Protein coagulation through reversible and irreversible bindings of calcium . Agric. Biol. Chem. , 51 : 707 – 714 .
- Urbano , G , López-Jurado , M , Aranda , P , Vidal-Valverde , C , Tenorio , E and Porres , J . 2000 . The role of phytate in legumes: antinutrient or beneficial function? J . Physiol. Biochem. , 56 : 283 – 294 .
- Wilcox , JR , Premachandra , GS , Young , KA and Raboy , V . 2000 . Isolation of high seed inorganic P, low-phytate soybean mutants . Crop Sci. , 40 : 1601 – 1605 .
- Yagasaki , K , Takagi , T , Sakai , M and Kitamura , K . 1997 . Biochemical characterization of soybean protein consisting of different subunits of glycinin . J. Agric. Food Chem. , 45 : 656 – 660 .