Abstract
The effect of calcium (Ca) on cadmium (Cd) accumulation in plants was investigated using Gamblea innovans Sieb. & Zucc., a deciduous tree species that is an accumulator plant for Cd and zinc (Zn). Saplings of G. innovans were grown for 3 months and fed with solutions containing only Ca (+Ca), both Ca and Cd (Ca+Cd), or only Cd (+Cd). The Ca concentration in roots was higher in both treatments containing Cd alone (+Cd) and Ca+Cd compared to roots treated with Ca alone (+Ca). In addition, the Cd concentration in roots was higher in the Ca+Cd treatment than the Cd treatment. This showed that the presence of Ca2+ in the rhizosphere relates with Cd uptake into roots. The result that the transport of Cd from roots through stem to leaves was suppressed by Ca treatment indicates that the presence of Ca regulates Cd transport from the roots. A clear correlation between Cd and Zn concentrations in leaves suggests a possibility that the Cd treatment accelerates the transport of Zn into leaves via the same protein transporter in this species.
Introduction
Cadmium (Cd) is a non-essential and highly toxic element for both plants and animals. The problem of Cd contamination of soils is a serious concern, especially for agriculture. Phytoremediation is considered a low-cost and sustainable remediation technique to address this problem (Baker et al. Citation1994; Cunningham et al. Citation1995), although it is time-consuming. Previously, from the field sampling and chemical analysis of tree leaves in an old silver mining site, where the soils contained high concentrations of heavy metals (0.1 M hydrochloric acid (HCl) extractable Cd: 20 mg/kg), we determined that Gamblea innovans (Sieb. & Zucc.), taxonomically revised from Evodiopanax innovans (Sieb. & Zucc.) Nakai (Shang et al. Citation2000) is an accumulator plant for Cd and zinc (Zn) (Takenaka et al. Citation2009). This is a deciduous tree species that has a wide distribution in secondary forest in Japan. Since this plant has a larger biomass than herbaceous plants and accumulates Cd and Zn in above-ground organs, it has potential as a practical candidate for phytoremediation in Japan.
In a review of Cd toxicity in plants, DalCorso et al. (Citation2008) explained that the concentration of other nutrient elements, such as calcium (Ca), Zn, and iron (Fe) in the soil influences Cd uptake. Cd also imbalances nutrient metabolism (uptake, transport and use) at the root level, by interfering with the uptake of Ca, magnesium (Mg), potassium (K) and phosphorus (P). The reduction of Ca uptake due to Cd treatment has previously been reported for tree species (Arduini et al. Citation1998; Kim et al. Citation2003). In addition, the amelioration of Cd toxicity in roots by Ca has been reported in rice, and was considered to be due to the blocking of Cd transport by Ca (Kim et al. Citation2002). These reports indicate that Cd uptake and transport in plants is closely related to Ca movement.
In heavy metal contaminated sites, such as abandoned mining sites, it is common practice to apply lime (CaCO3) as a soil supplement for acidic soils (Khan and Jones Citation2008). Therefore, it is important to understand the effect of Ca on Cd accumulation in plants not only from the viewpoint of investigating the mechanism involved, but also from the perspective of its practical impact on phytoremediation. In this study, we aimed to clarify the effects of Ca on Cd absorption and transport in G. innovans.
Materials and Methods
G. innovans saplings (height 30–70 cm) were purchased from a plant nursery (Kusakiya, Japan; the saplings originated from natural forests in Yamagata prefecture). The saplings were potted in plastic pots (Fujimoto Kagaku Kogyo, Japan; 3.8 L volume) with a 2:1 mixture of Akadama soil (Sowa, Japan) and humus (Okabe, Japan) at the end of April 2006. The nutrient condition of the soil cultivation medium was considered to resemble that of forest soil. The saplings were grown in a greenhouse at Nagoya University. Three treatment solutions were prepared: 0.2 mM calcium chloride (CaCl2) solution (+Ca), 0.1 mM cadmium chloride (CdCl2) with 0.2 mM CaCl2 solution (Ca+Cd), and 0.1 mM CdCl2 (+Cd) solution. After 2 months growth without treatment, 100–200 mL of treatment solution was added to each pot twice a week for 3 months. No nutrient solution was added to the saplings during the treatments. Eight saplings were used for each treatment. The mean ambient temperature in the greenhouse from April 2006 to October 2006 was 31.6°C/24.6°C (day/night) and relative humidity was 44.9%. After treatment for 3 months, saplings and soil samples from the pots were collected. Plant samples were divided into leaves, stems and roots, and were rinsed with distilled water and dried at 80°C for 48 h, and then weighed. The concentrations of Cd, Ca and other elements in the plant samples were determined by inductively coupled plasma atomic emission spectrometry (ICP-AES; IRIS ICARP, Jarrell Ash Nippon Corp., Japan) after wet digestion with HNO3. Soil samples were air-dried and sieved thorough a 2-mm sieve and homogenized. Soil pH was measured using a pH meter (WM-22EP, TOA Electronics, Japan) after mixing with distilled water (1:2.5). For the determination of metal concentration in soil, soil samples were mixed with 0.1 M HCl (1:5) and shaken for 1 hour, and the extracts were filtered and then analyzed by ICP-AES.
The data were analyzed by a one-way analysis of variance (ANOVA), and the significant difference between treatments determined by Scheffe's method.
Results and Discussion
After 3 months’ treatment, there was no significant difference in dry weights of biomass (shoot, root, total) among the treatments and no visible symptoms of Cd toxicity with Cd treatments. Therefore, it is shown that the concentration of 0.1 mM Cd in rhizosphere is a tolerable level for G. innovans. The concentrations of Ca and Cd in roots, stems and leaves after 3 months’ treatment are shown in . We detected Cd in stems and leaves even in the saplings with the Ca treatment. In our field observations of the Cd concentration in leaves of G. innovans grown in secondary forests in Aichi prefecture, where the 0.1 M HCl extractable Cd concentrations in soils were low (0.1 ∼ 0.2 mg/kg), we found Cd in leaves at the concentration level of 1 ∼ 2 mg/kg (Takenaka et al. in press). This means that G. innovans has a high ability to accumulate Cd and most wild G. innovans might accumulate Cd in the plant tissues. Thus, G. innovans saplings used in this experiment should accumulate Cd during growth in the nursery or their original growing place.
Figure 1. Concentrations of calcium (Ca) and cadmium (Cd) in roots ((A) and (B)), stems ((C) and (D)), and leaves ((E) and (F)), following three treatment regimes. Bars are mean ± standard error for tissue from eight saplings. Significant differences at the P = 0.01 level are indicated by the different letters. DW, dry weight.
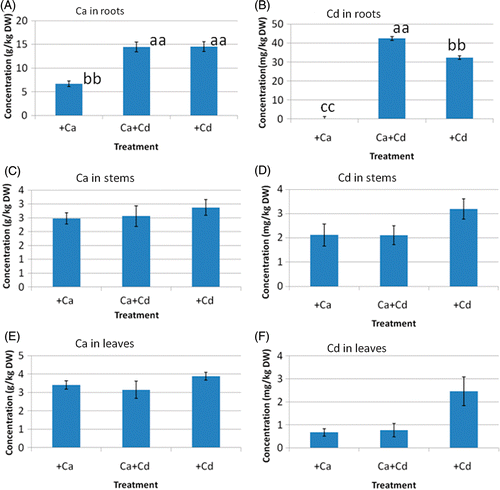
The Ca concentration in roots of G. innovans saplings treated with Cd was significantly higher than those treated with Ca, and was accompanied by high levels of Cd. Similar results have been reported for Brassica juncea (Jiang et al. Citation2004) and barley (Hordeum vulgare) (Brune and Dietz Citation1995), suggesting that the increase in Ca concentration in roots under Cd stress is a possible mechanism for reducing the toxic effects of Cd. Our results show that the Cd concentration in roots was greater after growth in the Ca+Cd treatment than in the Cd only treatment. DalCorso et al. (Citation2008) reviewed how plants cope with Cd toxicity, explaining that the uptake of Cd ions occurs via the same transmembrane carriers used for the uptake of other divalent cations including Ca2+, Fe2+ and Mg2+. In addition, the membrane potential of root epidermal cells provides a strong driving force for the uptake of cations. Therefore, it could be considered that the presence of excess Ca2+ in the rhizosphere during Ca+Cd treatment may have resulted in high membrane potential, and Cd uptake was increased compared with treatment using Cd alone. However, several studies have reported reduced Cd uptake in roots treated with Ca (Godbold Citation1991; Hardimann and Jacoby Citation1984). Godbold (Citation1991) showed that Ca supply reduced the Cd concentration of the apoplast but not the symplast in the roots of Norway spruce (Picea abies (L.) Karst.). In the case of Norway spruce, the decrease in the concentration of Cd in the apoplast by Ca was considered to be due to competition of the Ca with Cd for binding sites in the cell walls. Considering the results of this study, we concluded that the detoxification mechanism in roots of G. innovans should not be the same as that of Norway spruce, although the rhizospheric molar ratio of Cd to Ca in our experiment was different from that found by Godbold (Citation1991). The detoxification mechanism might include an isolation of Cd in the vacuoles as stable forms like as complexes with organic acids.
The transport of Cd through the stem to the leaves from the roots was suppressed by Ca addition, although the Cd concentrations in roots were high in both Cd treatments (). If detoxification for Cd occurred in the vacuoles of root cells as mentioned above, Cd transport from roots should be restricted. We suggest the possibility that the presence of excess Ca2+ in the rhizosphere might correlate with the control of Cd transport in roots. However, the behavior of Cd in G. innovans is supposed to be related not only to Ca but also to other elements, and the mechanism for such control should be clarified by further research.
Previously, we reported that G. innovans accumulates not only Cd but also Zn (Takenaka et al. Citation2009). In , the relationships between Cd and Zn in roots () and leaves () following each treatment are shown. In roots, no relationship between the concentrations of Cd and Zn was found. On the other hand, in leaves, a clear correlation was observed between Cd and Zn (R 2 = 0.8). This result indicates that Cd treatment accelerates the transport of Zn into leaves. The result of Zn contents in the soils after 3 months’ cultivation, in which the Zn contents of the Ca+Cd treatment (4.67 ± 0.10 mg/kg) and the Cd treatment (4.68 ± 0.07 mg/kg) were significantly decreased from that of the Ca treatment (5.66 ± 0.12 mg/kg), suggests that Cd treatment might increase Zn uptake.
Figure 2. Relationship between cadmium (Cd) and zinc (Zn) concentrations in (A) roots and (B) leaves following treatment with calcium (Ca) (triangles), Ca+Cd (squares) or Cd (diamonds). DW, dry weight.
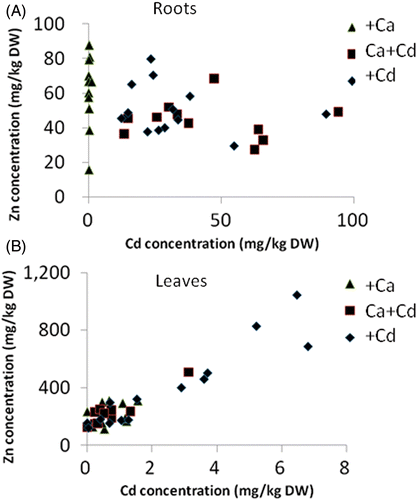
Several review papers have summarized the various factors involved in heavy metal transport in plants, such as mugineic acid, nicotianamine, organic acids, histidine, and several proteins (Lasat et al. Citation2000; Williams et al. Citation2000; Hall and Williams Citation2003; Grotz and Guerinot Citation2006; Haydon and Cobbett Citation2007). Many of these transporters for essential elements such as Zn can also transport Cd, a nonessential element. The clear relationship observed between Cd and Zn in leaves of G. innovans could indicate that Cd and Zn are transported via the same protein transporter in this species. On the other hand, Chen et al. (Citation2009) reported that iron (Fe), copper (Cu) and Zn accumulation were induced by Cd addition in Lotus japonicus, and speculated that phytochelatin synthases activated by Cd might be the key reason for Fe, Cu and Zn accumulation under Cd treatment. This speculation suggests that the synergistic relationship between Cd and Zn was not the result of the transport mechanism but of the detoxification mechanism. More detailed analysis of the chemical forms of Cd and Zn in G. innovans is necessary to determine the precise mechanism involved.
G. innovans is one of a number of potential tree species for use in phytoremediation of heavy metal contaminated soils. In addition, understanding the mechanisms of uptake and transport of heavy metals in this species is of interest from a plant physiological viewpoint. However, the lack of a stable supply of G. innovans remains a limitation, as it produces few fruits and is not very amenable to tissue culture. The establishment of a tissue cultivation method is therefore an essential step to aid further research on G. innovans.
Acknowledgments
Support of the work by the Steel Industry Foundation for the Advancement of Environmental Protection Technology (Water quality 07 · 08-292) is gratefully acknowledged.
Additional information
Notes on contributors
Rie Tomioka
These authors contributed equally to this work.Notes
These authors contributed equally to this work.
References
- Arduini , I , Godbold , DL , Onnis , A and Stefani , A . 1998 . Heavy metals influence mineral nutrition of tree seedlings . Chemosphere , 36 : 739 – 744 .
- Baker , AJM , McGrath , SP , Sidoli , CMD and Reeves , RD . 1994 . The possibility of in situ heavy metal decontamination of polluted soils using crops of metal-accumulating plants . Resour. Conserv. Recy. , 11 : 41 – 49 .
- Brune , A and Dietz , KJ . 1995 . A comparative analysis of element composition of roots and leaves of barley seedlings grown in the presence of toxic cadmium, molybdenum, nickel, and zinc concentrations . J. Plant Nutr. , 18 : 853 – 868 .
- Chen , Z , Watanabe , T , Shinano , T , Ezawa , T , Wasaki , J , Kimura , K , Osaki , M and Zhu , YG . 2009 . Element interconnections in Lotus japonicus: A systematic study of the effects of element additions on different natural variants . Soil Sci. Plant Nutr. , 55 : 91 – 101 .
- Cunningham , SD , Berti , WR and Huang , JW . 1995 . Phytoremediation of contaminated soils . Trends Biotechnol. , 13 : 393 – 397 .
- DalCorso , G , Farinati , S , Maistri , S and Furini , A . 2008 . How plants cope with cadmium: Staking all on metabolism and gene expression . J. Integr. Plant Biol. , 50 : 1268 – 1280 .
- Godbold , DL . 1991 . Cadmium uptake in Norway spruce (Picea abies (L.) Karst.) seedlings . Tree Physiol. , 9 : 349 – 357 .
- Grotz , N and Guerinot , ML . 2006 . Molecular aspects of Cu, Fe and Zn homeostasis in plants . Bba-Mol. Cell. Res. , 1763 : 595 – 608 .
- Hall , JL and Williams , LE . 2003 . Transition metal transporters in plants . J. Exp. Bot. , 54 : 2601 – 2613 .
- Hardiman , RT and Jacoby , B . 1984 . Absorption and translocation of Cd in bush beans (Phaseolus vulgaris) . Physiol. Plantarum. , 61 : 670 – 674 .
- Haydon , MJ and Cobbett , CS . 2007 . Transporters of ligands for essential metal ions in plants . New Phytol. , 174 : 499 – 506 .
- Jiang , XJ , Luo , YM and Zhao , QG . 2004 . Effect of cadmium on nutrient uptake and translocation by Indian Mustard . Environ. Geochem. Hlth. , 26 : 319 – 324 .
- Khan , MJ and Jones , DL . 2008 . Chemical and organic immobilization treatments for reducing phytoavailability of heavy metals in copper-mine tailings . J. Plant Nutr. Soil Sc. , 171 : 908 – 916 .
- Kim , CG , Bell , JNB and Power , SA . 2003 . Effect of soil cadmium on Pinus sylvestris L. seedlings . Plant Soil , 257 : 443 – 449 .
- Kim , YY , Yang , YY and Lee , Y . 2002 . Pb and Cd uptake in rice roots . Physiol. Plantarum. , 116 : 368 – 372 .
- Lasat , MM , Pence , NS , Garvin , DF , Ebbs , SD and Kochian , LV . 2000 . Molecular physiology of zinc transport in the Zn hyperaccumulator Thlaspi caerulescens . J. Exp. Bot. , 51 : 71 – 79 .
- Shang , CB , Lowry , IIPP and Frodin , DG . 2000 . A taxonomic revision and re-definition of the genus Gamblea (Araliaceae) . Adansonia ser. 3 , 22 : 45 – 55 .
- Takenaka , C , Hayakawa , N , Kobayashi , M and Kanaya , S . (2011): Accumulation and transport of heavy metals in Gamblea innovans. Jap. J. For. Environ. 53, 9–15
- Takenaka , C , Kobayashi , M and Kanaya , S . 2009 . Accumulation of cadmium and zinc in Evodiopanax innovans . Environ. Geochem. Hlth. , 31 : 609 – 615 .
- Williams , LE , Pittman , JK and Hall , JL . 2000 . Emerging mechanisms for heavy metal transport in plants . BBA-Biomembranes. , 1465 : 104 – 126 .