Abstract
Mycorrhiza-free and mycorrhizal seedlings of onion were transplanted in the field along rows of previously established plants of ginger in order to evaluate the possibility of enhancing arbuscular mycorrhizal (AM) colonization of ginger via a companion crop. Arbuscular mycorrhizal fungal colonization of roots determined 138 days after transplanting of onion showed that roots of all plants were colonized by the fungi. However, the level of AM fungal colonization of onion roots was significantly higher if onion seedlings were colonized by the fungi prior to transplanting than if they were not. Dry matter yield of onion was more than tripled by AM fungal inoculation. The level of AM fungal colonization of ginger roots was significantly enhanced in the presence of a mycorrhizal companion onion, not in the presence of a non-mycorrhizal one. However, the yield of ginger was unaffected by the presence of onion irrespective of mycorrhizal status. Our data raise the possibility that the mycorrhizal status of an established plant may be enhanced through the introduction of a compatible companion crop well colonized by AM fungi.
Introduction
Although the mycotrophic nature of ginger (Zingiber officinale Roscoe) has been known since the early 1980s (Taber and Trappe 1982), the impact of arbuscular mycorrhiza fungi (AMF) on the nutrition and growth of ginger is not well established. The fact that ginger is conventionally propagated by means of the underground storage organ or rhizome may have contributed to this dearth of information.
Arbuscular mycorrhizal (AM) fungal propagules are normally applied to soil at the time of planting with the intent of maximizing their rapid interception by the roots of the host plants. In plants such as ginger (which are normally propagated from rhizomes and cuttings), this is not possible because of the relatively long time required for root formation. An alternative approach would be to establish these plants in the field first and to subsequently supply AM fungal inocula to them through companion crops. Although there is no specific information in this regard in the published literature, interconnection of roots of different plants through AM fungal hyphae has been reported, though the focus has been on the transfer of nutrients between the plants (Francis et al. Citation1986; Cruz et al. Citation2000; Giovannetti et al. Citation2004).
The aim of the current investigation was to determine the feasibility of transferring AM fungal propagules from mycorrhizal onion seedlings to an established ginger crop.
Materials and Methods
The medium used for raising thick green onion (Allium cepa L. cv Hikama) seedlings was a pasteurized peat-soil mixture (75% peat and 25% soil) by weight (Peters and Habte Citation2001). The soil used for this purpose was a subsurface sample of a moderately weathered soil (Leilehua series, oxidic, isothermic, Typic Kandihumult). The peat-soil mixture was limed to raise its pH from 4.5 to 6.2. Pasteurization was achieved by heating the moist (moisture content = 200%) material in an electric soil sterilizer (Progrow Soil Sterilizer, Pro Grow Supply Corporation, Buttler, PA) at 90°C for 72 h. The mycorrhiza-free medium was either not inoculated or inoculated with a crude inoculum of Glomus aggregatum Schenck and Smith emend Koske at the rate of 200 g of crude inoculum kg−1 of medium. The total number of infective propagules introduced into each kg of medium was estimated to be 4800. The medium was dispensed in 60 cm by 30 cm by 5 cm plastic trays. The medium not inoculated with the fungus received the equivalent amount of mycorrhiza-free inoculum carrier and 50 mL of a filtrate obtained by passing through a filter paper (Whatman #1) a 1% suspension of the crude inoculum in de-ionized water. The filtrate was applied along with nutrient solution. Seedlings were raised in trays containing the inoculated or non-inoculated medium. Watering of plants was achieved by means of sprinkler irrigation. During the first 8 weeks of growth of the seedlings on trays, the growth medium was supplemented with Hoagland's solution (see Habte and Osorio Citation2001) with the phosphorus (P) concentration adjusted to 8 mg L−1. The solution was supplied at weekly intervals at the rate of 500 mL of solution per tray. During the remainder of the growth of the seedlings in the greenhouse, macronutrients were supplied in the form of the controlled-release fertilizer Osmocote™ (19-6-12) which was evenly spread on the surface of the medium at the rate of 12 g kg−1 of medium.
Micronutrients were supplemented as Hoagland's solution minus macronutrients at the rate specified above every 14 days. Irrigation water was withheld 6 h before nutrient application and 24 h after nutrient application. Seedlings were allowed to grow in the greenhouse under natural light for 96 days. Ginger was planted in one of the plots assigned to the Hawaii branch of the Mokichi Okada Association, for the purpose of demonstrating nature farming, on the University of Hawaii Research Farm, Waimanalo, HI (latitude 21°20′ and longitude 158°20′). The site is located 20 m above sea level with a mean annual temperature of 24.6°C and a mean annual rainfall of 1380 mm. The soil of the experimental site belonged to the Waialua series (very fine, mixed, superactive, isohyperthermic, Pachic Haplustoll). At the beginning of the study, the pH of the soil (1:2 soil to water; w:v) was 6.9, and it had a solution P concentration of 0.04 mg L−1. The experiment was performed on an area of 616 m2. Marigold (Tagetes erecta cv Crackerjack) was grown on the land for 102 days prior to the planting of ginger. The marigold crop was mowed down and the soil was sub-soiled to a depth of 75 cm and tilled two weeks before the planting of ginger. Ginger rhizomes (100–200 g each) were planted in 10-cm depressions 30 cm apart in 15 m-long rows at a row spacing of 140 cm. Onion seedlings were transplanted along ginger rows at a distance of 15 cm from the ginger plants. Treatments were arranged in the field in a completely randomized design with 8 replicates per treatment. The level of AM fungal colonization on roots of onion seedlings at the time of transplanting was 72%. Onion and ginger plants were harvested 138 days after the onion was transplanted. Ginger rhizomes, onion bulbs, tops, and roots were harvested from the center two-meter portion of each row reserved for data collection. Measurements taken at harvest were: AM fungal colonization of roots; P contents of leaf tips (onion) and of leaf disks (ginger); dry mass of onion bulbs and tops, and dry mass of ginger rhizomes. To determine the extent to which roots were colonized by AMF, roots were excised and carefully washed free of soil with water under pressure. Root samples (0.5 g on fresh weight basis) were immersed into a 10% KOH solution for 24 h at 22°C in order to clear the roots. At the end of the incubation period, roots were rinsed with 4 changes of tap water. They were then acidified by soaking them in 10% hydrochloric acid (HCl) for 5 min in order to facilitate the retention of the staining material by AM fungal structures. After removing the excess acid, roots were stained by soaking them in a 0.15% acid fuchsin (dissolved in acidified glycerine). After 24 h of incubation at 22°C, the stain was removed and the roots were then soaked in acidified glycerine and incubated at 22°C for 24 hours in order to de-stain them. The spent de-staining solution was then replaced with a fresh de-staining solution at which time the roots were ready for observation. The stained roots were spread in petri plates marked with gridlines and observed under a stereoscopic microscope, and the extent to which the roots were colonized was estimated by employing the grid line intersect method (Giovannetti and Mosse Citation1980).
P contents of leaf tips (onion) and P contents of leaf disks (ginger) were determined after drying the samples at 70°C for 3 h and then transferring them into 18 × 150 mm Pyrex test tubes. The contents of the test tubes were ashed in a muffle furnace at 500°C for 3 h. The P content of the ash was determined spectrophotometrically after color was developed by the molybdenum blue technique (Murphy and Riley Citation1962). Additional methodological details are given in Habte and Turk (Citation1991).
Dry mass of onion bulbs and tops and dry mass of ginger rhizomes were determined after drying the materials at 70°C for 96 h.
Data were subjected to one-way ANOVA (General Linear Model) using the statistical software Statistix for Windows, Version 9 (Analytical Software, Tallahassee, Florida). The least significant difference (LSD) (p < 0.05) was used to separate treatment means when the F statistic was significant. Visual indication of data dispersion on bar graphs was achieved by means of standard error of the mean (SEM).
Results and Discussion
Arbuscular mycorrhizal (AM) fungal colonization of ginger and onion roots determined 138 days after transplanting of onion showed that roots of all plants were colonized by the fungi (). Intercropping ginger with onion stimulated AM fungal colonization of ginger roots significantly (p < 0.05) if onion seedlings were mycorrhizal at the time of transplanting (). The level of AM fungal colonization of onion roots was significantly higher (p < 0.05) if onion seedlings were mycorrhizal prior to transplanting than if they were not (). However, the yield of ginger was unaffected by the presence of onion irrespective of the latter's mycorrhizal status (). It is possible that the P demand of ginger was met by the prevailing soil solution P concentration (0.04 mg L−1). The recommended tissue P concentration for ginger is 0.24–0.33% which is low compared to that of onion (Uchida Citation1997). Unfortunately, the critical soil solution P concentration for ginger is not known. The relatively large planting materials used and the associated tissue P reserve (680–1360 mg P planting material−1) could also have contributed to the lack of growth response to AMF colonization (Habte and Byappanahalli Citation1994). Even when micropropagated materials are used, the growth response of ginger to AM fungal colonization has been reported to vary. da Silva et al. (Citation2008) noted that inoculation of micropropagated ginger stimulated oil production in ginger without significantly increasing rhizome yield. On the other hand, dos Santos et al. (Citation2010) evaluated the effect of P fertilization and AMF inoculation of soil on micropropagated ginger and noted significant mycorrhizal inoculation effect. They found that the effect of AM fungal inoculation on growth was comparable to fertilizing the test soil with 25 mg of P kg−1. It is evident from the results of da Silva et al. (Citation2008) that lack of growth response to AM fungal inoculation does not necessarily mean the absence of benefit. Arbuscular mycorrhizal fungi can have measurable effects on plant and soil health without necessarily enhancing plant yield (see Habte Citation2006).
Figure 1. Influence of pre-transplant mycorrhizal status of onion seedlings on arbuscular mycorrhizal (AM) fungal colonization of ginger roots (a) and on the post-transplant AM fungal colonization (colon.) of onion (b). Vertical bars represent standard error of the mean (ranges for ginger are 30 and 24; ranges for onion are 15 and 41; n = 8). For each species, the first set of range values is associated with mycorrhiza-free onion seedlings while the second one is associated with mycorrhizal ones. AMF, arbuscular mycorrhiza fungi.
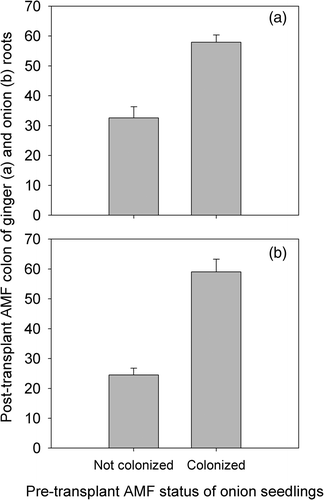
Figure 2. Influence of pre-transplant arbuscular mycorrhizal (AM) fungal colonization of onion seedlings on dry mass of ginger rhizomes plant−1 (a) and on the post-transplant dry mass of onion plant−1 (b). Vertical bars represent standard error of the mean (ranges for ginger are 93.7 and 129.1; ranges for onion are 16.1 and 38.1; n = 8); for each species, the first set of range values is associated with mycorrhiza-free onion seedlings while the second one is associated with mycorrhizal ones. AMF, arbuscular mycorrhiza fungi.
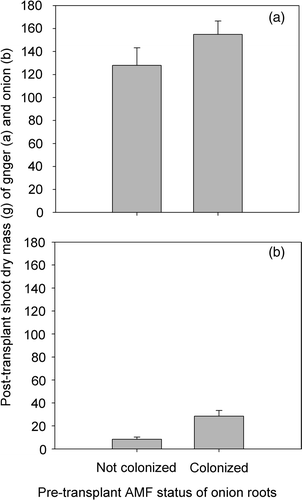
Dry matter yield of onion was more than tripled by mycorrhizal inoculation (). Onion is considered to be highly dependent on the mycorrhizal condition for its P uptake and growth (Miyasaka and Habte Citation2001). The significant response (p < 0.05) it exhibited to mycorrhization in the current study despite the relatively high soil solution P concentration suggests that onion's dependence on the mycorrhizal condition is even higher under field conditions. This may be explained by the presence under field conditions of other organisms that compete with onion for the same available soil solution P pool.
In the current study, treatments did not influence leaf P contents of ginger or onion determined at harvest (data not shown). The influence of AM fungal colonization on tissue P status is often associated with the earlier, most vigorous phase of the activity of AMF (Miyasaka and Habte Citation2001), the levels generally declining after reaching peak values and coinciding with levels attained under mycorrhiza-free conditions. The fact that both ginger and onion have erect growth habits has contributed to their compatibility, and hence to the lack of competitive interaction for above ground resources, notably light. Results could be different if non-compatible plants are intercropped for the purpose of transferring mycorrhizal propagules from an introduced companion crop to an established one.
Our hypothesis that it may be feasible to enhance the AM fungal colonization level of roots of established ginger through a mycorrhizal companion onion crop is supported by our data. The fact that AM colonization of ginger roots was enhanced only when it was inter-cropped with mycorrhizal onion and not with mycorrhiza-free ones argues for the possibility of a wider application of this approach.
Acknowledgments
This research was supported, in part, by the College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa (USDA-CSREES-ARS Hatch Funding Program).
References
- Cruz , AF , Ishii , T and Kadoya , K . 2000 . Distribution of vesicular-arbuscular mycorrhizal hyphae in the rhizosphere of trifoliage orange and bahia grass seedlings under intercropping system . J. Japan. Soc. Hort. Sci. , 164 : 175 – 181 .
- da Silva , MF , Pescador , R , Rebelo , RA and Sturmer , SL . 2008 . The effect of arbuscular mycorrhizal fungal isolates on the development and oleoresin production of micropropagated Zingiber officinale . Braz. J. Plant Physiol. , 20 : 119 – 130 .
- dos Santos , R , Girardi , CG , Pescador , R and Sturmer , SL . 2010 . Effects of arbuscular mycorrhizal fungi and phosphorus fertilization on post vitro growth of micro-propagated Zingiber officinale Roscoe . R. Bras. Ci. Solo , 34 : 765 – 771 .
- Francis , R , Finlay , RD and Read , DJ . 1986 . Vesicular-arbuscular mycorrhiza in natural vegetation systems IV. Transfer of nutrients in inter-and intra-specific combinations of host plants . New Phytol. , 102 : 103 – 111 .
- Giovannetti , M and Mosse , B . 1980 . An evaluation of techniques for measuring vesicular-arbuscular mycorrhyzal infection in roots . New Phytol. , 84 : 489 – 500 .
- Giovannetti , M , Sabrana , C , Avio , L and Strani , P . 2004 . Patterns of below ground plant interconnections established by means of arbuscular mycorrhizal networks . New Phytol. , 164 : 175 – 181 .
- Habte , M . 2006 . “ The roles of arbuscular mycorrhizas in plant and soil health ” . In Biological Approaches to Sustainable Soil Systems , Edited by: Uphoff , N , Ball , AS Palm , C . 129 – 147 . Boca Raton, , FL : CRC .
- Habte , M and Byappanahalli , MN . 1994 . Dependency of cassava (Manihot esculenta Crantz) on vesicular-arbuscular mycorrhizal fungi . Mycorrhiza , 4 : 241 – 245 .
- Habte , M and Osorio , NW . 2001 . Arbuscular Mycorrhizas: Producing and Applying Arbuscular Mycorrhizal Inoculum. College of Tropical Agriculture and Human Resources (CTAHR) , Honolulu, , HI : University of Hawaii at Mano .
- Habte , M and Turk , D . 1991 . Response of two species of Cassia and Gliricidia sepium to vesicular-arbuscular mycorrhizal infection . Commun. Soil Sci. Plant Anal. , 22 : 1860 – 1872 .
- Miyasaka , SC and Habte , M . 2001 . Plant mechanisms and mycorrhizal symbioses to increase phosphorus uptake efficiency . Commun. Soil Sci. Plant Anal. , 32 : 1101 – 1147 .
- Murphy , J and Riley , JP . 1962 . A modified single solution of phosphate in natural waters . Anal. Chim. Acta. , 27 : 31 – 35 .
- Peters , SM and Habte , M . 2001 . Optimizing solution concentration in a peat-based medium for producing mycorrhizal seedlings in containers . Arid Land Res. Manage. , 15 : 359 – 370 .
- Taber , RA and Trappe , JM . 1982: Vesicular- arbuscular mycorrhiza in rhizomes, scale-like leaves, roots, and xylem of ginger. Mycologia, 74, 156–161
- Uchida , R . 1997 . “ Recommended tissue nutrient levels: vegetables, fruits, ornamental plants, and ornamental flowering plants ” . In Plant Nutrient Management in Hawaii's Soils: Approaches for Tropical and Subtropical Agriculture , Edited by: Silva , JA and Uchida , R . 57 – 64 . Honolulu, , HI : College of Tropical Agriculture and Human Resources (CTAHR), University of Hawaii at Manoa .