Abstract
Movement of nitrous oxide (N2O) produced in subsoil and shallow groundwater is important in determining the direct and indirect N2O emissions from agricultural soils. From the results of our previous study in a lysimeter-contained Gray lowland soil in the summer, we hypothesized that if a large amount of N2O is produced near shallow groundwater table in the summer, it will diffuse upward to the atmosphere. To examine this hypothesis, we conducted a one-year experiment in the same lysimeters for the cultivation of soybean–wheat double cropping (SW) or upland rice (UR). Dissolved N2O concentration in the drainage water in the UR plots exceeded 0.4 mg N L−1 in the summer, whereas that in the SW plots remained <0.1 mg N L−1. Analyses of the concentrations of nitrate and dissolved N2O in the drainage water and their nitrogen and oxygen isotopic compositions (δ15N and δ18O) during the summer revealed that denitrification was the main process for the N2O production near the groundwater table. There was a significant positive correlation between the dissolved N2O concentration and soil-surface N2O flux in the summer. Calculated upward diffusive N2O fluxes at three soil depths by Fick's law also supported our hypothesis. The δ15N values of N2O in the soil-surface flux were similar to those in the shallow groundwater in the UR plots during the summer. Such similarity was not found in the SW plots. We conclude that our hypothesis was confirmed by the above results. Comparison of the monitored data with other seasons indicates that low soil water content was a driving force for the upward N2O diffusion as well as the high dissolved N2O concentration.
Introduction
Nitrous oxide (N2O) is predicted to be 298 times as potent a greenhouse gas as carbon dioxide (CO2) on a 100-year time scale (Forster et al. Citation2007), and is indirectly involved in the catalytic destruction of stratospheric ozone. Agriculture is the largest anthropogenic N2O source, and accounted for 58% of the global anthropogenic N2O emissions in 2005 (Smith et al. Citation2007). Nitrogen (N) fertilization inevitably enhances the production and emission of N2O through its effects on microbial nitrification and denitrification processes in agricultural soils. However, it is well known that soil-surface N2O emissions have high spatial variability (Clemens et al. Citation1999; Yanai et al. Citation2003). Akiyama et al. (Citation2006) compiled long-term field monitoring data on soil-surface N2O emissions from Japanese agricultural fields (246 measurements from 36 sites), and reported that the mean emission from poorly drained upland fields was more than four times that of fields with well-drained soils. In such wet soils, N2O is produced mainly via denitrification (Davidson Citation1991).
The N2O production by denitrification is not just confined to topsoil; subsoil and shallow groundwater can also be hot spots. Understanding the mechanism and fate of N2O produced in subsoil is important for accurately assessing the direct and indirect N2O emissions (Clough et al. Citation2005). The vertical transfer of N2O from the groundwater table to the atmosphere depends on biophysical soil properties, such as the rates of N2O production and consumption and the rates of convective and diffusive transport (Deurer et al. Citation2008; Well et al. Citation2001). According to Mosier et al. (Citation1998), N2O emitted from groundwater to the atmosphere by upward diffusion is also considered as an indirect emission associated with N leaching.
Clough et al. (Citation1999) conducted a short-term soil column experiment, and reported that 0.4% of the 15N-labeled nitrate (), injected at a depth of 0.8 m in the presence of a carbon (C) substrate, diffused upward to the atmosphere as N2O. In a German aquifer, Weymann et al. (Citation2009) used a 15N tracer method and found that groundwater-derived N2O was diffused upward to the atmosphere though its amount was hardly significant. On the basis of the results of a six-year field experiment in Gray lowland soil, Kusa et al. (Citation2010) reported that 14% of soil-surface N2O emission was derived from N2O produced in the subsoil by a concentration gradient method. These studies have demonstrated that N2O produced in subsoil and shallow groundwater can contribute more or less to soil-surface N2O emission.
In our previous study conducted in lysimeters with a shallow groundwater table, soil-surface N2O flux and dissolved N2O concentration in drainage water increased synchronously during the summer (Minamikawa et al. Citation2010). As far as we know, such a relationship has not been observed in any previous studies. Given the accumulated knowledge of N2O dynamics in a soil profile, we proposed the following hypothesis to explain the observed relationship: if a large amount of N2O is produced near the groundwater table in the summer, it will diffuse upward and significantly contribute to soil-surface N2O emission.
The objective of the present study was to examine this hypothesis. We measured soil-surface N2O emissions, N2O concentrations in the soil profile, and dissolved N2O emissions in the lysimeter drainage water for one year. Diffusive N2O fluxes in the soil profile were calculated with Fick's law. We also measured nitrogen and oxygen isotopic compositions (δ15N and δ18O) of and N2O in the drainage water during the summer to discuss the production process of N2O dissolved in the drainage water. Then we compared the δ15N and δ18O values of N2O in the soil-surface flux with those in the shallow groundwater and the drainage water to discuss a link between dissolved N2O concentration and soil-surface N2O flux. In addition, we discussed whether our hypothesis could apply to the other seasons.
Materials and Methods
Study site and crop cultivation
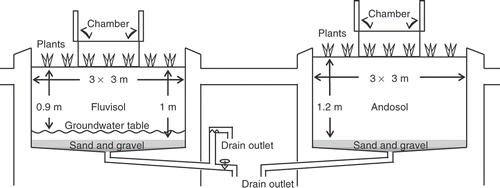
We carried out a one-year experiment at the lysimeter facility of the National Institute for Agro-Environmental Sciences (NIAES), Tsukuba, Ibaraki, Japan (36°01′N, 140°07′E) from March 2007 to April 2008. Each lysimeter contained Gray lowland soil (Gleyic Fluvisols) and had a cross-sectional area of 9 m2 (3 m × 3 m) and a 1 -m soil depth (). The bottom of each lysimeter was filled with a layer of sand and gravel. Total C and N contents in the topsoil (0 to 0.2 m depth) were 18.8 g C kg−1 and 1.6 g N kg−1, respectively, and those in the subsoil (0.2 to 1 m depth) were 13.7 g C kg−1 and 1.4 g N kg−1, respectively. Soil textures in the topsoil and subsoil were classified into Light Clay (36% clay, 34% silt, and 30% sand) and Silty Clay Loam (19% clay, 46% silt, and 35% sand), respectively. The topsoil's pH (H2O) was 5.7 and its bulk density was 1.01 g cm−3. The groundwater table had been set to a depth of 0.9 m from the soil surface since the winter of 2005 () to simulate soil water condition in actual lowland fields, but evapotranspiration could lower the groundwater level. Subsurface drainage water was discharged through the bottom drain outlet whenever enough precipitation occurred. We collected samples of drainage water and shallow groundwater from the deep and shallow (0.9-m depth) valves, respectively ().
Figure 1. Schematic diagram of a lysimeter. The dotted line represents a pre-installed soil gas sampler.
We examined two kinds of upland cropping systems, each with two replicates: double-cropping of soybean (Glycine max L. cv. Enrei) in the summer with wheat (Triticum aestivum L. cv. Norin 61) in the winter (SW), and single cropping of upland rice (Oryza sativa L. cv. Toyohatamochi) (UR). Single cropping of paddy rice (Oryza sativa L. cv. Nipponbare) had been conducted in all four plots for more than ten consecutive years before 2002. Thereafter, crop rotation between paddy rice and upland crops was conducted at intervals of two years: cultivation of upland crops in 2002 and 2003, followed by cultivation of paddy rice in 2004 and 2005, and cultivation of upland crops in 2006 and 2007.
Field management followed conventional practices for the region. Total urea application rates in the SW and UR plots were 12 and 6 g N m−2, respectively. Fused magnesium phosphate and potassium chloride were applied as basal dressing for soybean, wheat, and upland rice at 6, 10, and 10 g P2O5 or K2O m−2, respectively. At crop maturity, all aboveground biomass was harvested and oven-dried for three days at 80°C to determine dry mass. The wheat and rice crop residues were incorporated into the topsoil of each plot after harvesting, whereas soybean residues were removed. Other details of lysimeter facility and crop cultivation are described in Minamikawa et al. (Citation2010).
Gas and water measurements
All the measurements were conducted at one place or for one sample in each plot. We continuously monitored the soil-surface N2O flux six times a day in each lysimeter by the automated chamber system (Nishimura et al. Citation2005b). The chamber covered 0.81 m2 (0.9 m × 0.9 m), and its height was adjustable to 0.6 or 1.2 m according to crop height. We analyzed N2O concentration inside the chamber using a gas chromatograph (GC) equipped with an electron-capture detector (GC-14B, Shimadzu, Kyoto, Japan), and determined soil-surface N2O flux from a linear increase in the N2O concentration for 30 min. Total soil-surface N2O emission was calculated by integrating the fluxes over time. The volumetric soil water content at a depth of 0.1 m was monitored using a time-domain reflectometry (TDR) moisture sensor (CS616S, Campbell Scientific Instruments, Logan, UT, USA), and the water-filled pore space (WFPS) was calculated from the volumetric water content and soil porosity (0.61 ± 0.03 m3 m−3).
We collected soil gas samples at a depth of 0.15, 0.45, and 0.75 m in the soil profile once a week using pre-installed stainless-steel tube samplers (outer dimension 1.26 mm, inner dimension 0.90 mm), at a distance of 0.5 m from the lysimeter's side wall (). We analyzed the concentrations of N2O and CO2 using the automated gas analysis system which consisted of two GCs (Sudo Citation2006).
We collected the drainage water samples whenever drainage occurred after precipitation and also once a week without regard to precipitation. The concentrations of dissolved N2O and CO2 in the drainage water were measured by a headspace technique (Minamikawa et al. Citation2010) using the automated analysis system. We monitored the volume of drainage water with a tipping bucket pluviometer (UIZ-TB200, Uizin, Tokyo, Japan), and calculated the total emissions of dissolved N2O and CO2 by integrating daily emissions (i.e., dissolved gas concentration × daily drainage volume). Shallow groundwater was sampled for the stable isotope analysis during the summer (discussed later, in the section “Stable isotope analysis”).
Aliquots of the drainage water sample were passed through a 0.45-µm membrane filter. We analyzed the concentrations of and ammonium (
) using an ion chromatograph (DX-500, Dionex, Sunnyvale, CA, USA) and the concentration of dissolved organic carbon (DOC) with using a total organic carbon analyzer (TOC-V, Shimadzu, Kyoto, Japan). Total amounts of leached
and DOC were calculated in the same manner as were the amounts of dissolved gases.
Estimation of diffusive N2O flux in the soil profile
Vertical diffusive N2O fluxes at a depth of 0.075, 0.3, and 0.6 m were calculated from the measured concentration gradients between the two neighboring depths on the basis of Fick's law:
Stable isotope analysis
Water and gas samplings for the stable isotope analysis were conducted more than once a week from late June to September (from July to August for gas). The δ15N and δ18O of and N2O in the water and gas samples were measured using an isotope ratio mass spectrometer (Mat 252, Finnigan Mat, Bremen, Germany) equipped with a GC (5890 Series II, Hewlett-Packard, Waldbronn, Germany) (GC-IRMS). The measurement precision for the GC-IRMS was ±0.22‰ for δ15N and ±0.28‰ for δ18O. The standard for 15N was atmospheric dinitrogen (N2), and that for 18O was Vienna Standard Mean Ocean Water (VSMOW). The required amount of N2O for reliable measurement by the GC-IRMS was 15 nmol, and the required volume of N2O gas was dependent on the N2O concentration of each gas sample.
The δ15N and δ18O of in the drainage water (
drain) were analyzed by the denitrifier method (Sigman et al.
Citation2001; Casciotti et al.
Citation2002), in which
was converted quantitatively into N2O by denitrifying bacteria that lack N2O reductase activity (Pseudomonas chlororaphis f. sp. aureofaciens, ATCC 13985). We extracted and purified the N2O produced by the bacteria using a preparation line (PreCon, Thermoquest, Bremen, Germany), and analyzed it using the GC-IRMS. Details of the apparatus are given in Sigman et al. (Citation2001) and Casciotti et al. (Citation2002). Gas samples of dissolved N2O in water were prepared using the headspace method.
We also measured the δ15N and δ18O of N2O in the air inside a closed chamber (N2Ochamber) and of N2O in the soil air at a depth of 0.15 m (N2Osoil). An incomplete adjustment of the GC-IRMS caused some loss of data. We installed a static closed chamber (0.75 m ×0.2 m × 0.1 m height) between crop rows without including crops for two hours to collect N2O derived from the soil (N2Oflux-c). We also collected atmospheric ambient N2O (N2Oair) at a height of 2 m above the ground on site. To simplify calculations of gas movement within the soil profile, we assumed that only gas diffusion was responsible for gas movement, and that the diffusion was driven by the N2O concentration gradient. Therefore, although there could be both upward and downward N2O fluxes in the soil profile, we assumed that only the upward flux occurred. This means that the gross downward N2O flux was assumed to be zero and that the gross upward N2O flux equaled the net upward N2O flux. Hence, the amount of N2Ochamber consisted of the amount of N2Oair inside the chamber just after placement plus the amount of N2Oflux-c. Given these assumptions, the amount (molar basis) of N2O can be replaced by its concentration (µL L−1 basis), and thus, the concentration of N2Ochamber (=Cchamber) consists of the concentration of N2Oair (Cair) and the calculated concentration of N2Oflux-c (=Cchamber − Cair). Then, δ15N and δ18O of N2Oflux-c were calculated from the following isotope mass-balance equation:
As for N2Osoil, we used the same assumptions as those for N2Ochamber, except for the direction of gas fluxes. Instead, we assumed that the soil at the 0.15 -m depth functioned as a semi-closed system, in which N2O could enter and leave based on the concentration gradient. Hence, N2Oair was assumed to enter the system, but downward movement to depths >0.15 m was assumed to be zero due to the existence of a plow pan below 0.15 m. The N2O concentration in the system (Csoil) could increase as a result of the supply of N2O derived from the soil (N2Oflux-s), though some escaped outside the system. On this basis, we used the following equation to calculate δ15N and δ18O of N2Oflux-s:
We preliminarily checked the validity of the assumptions used for Eq. Equation3 by comparing the calculated δ15N and δ18O of N2Oflux-s with or without the entry of atmospheric N2O against those of N2Oflux-c. We did not report δ15N and δ18O values of N2Oflux-c and of N2Oflux-s when Cchamber and Csoil were below 0.33 µL L−1, as we considered this value to be the lower threshold value for reliable analysis. For example, given δ15N-N2Osoil = −10.0‰, δ18O-N2Osoil = 40.0‰, Csoil = 0.50 µL L−1, δ15N-N2Oair = 6.0‰, δ18O-N2Oair = 42.0‰, and Cair = 0.31 µL L−1, calculated δ15N-N2Oflux-s (−41.4‰) and δ18O-N2Oflux-s (36.7‰) have an uncertainty of ±0.72‰ and ±1.55‰, respectively, due to error propagation by instrument precision.
We compared the δ15N and δ18O values of N2Oflux-c and N2Oflux-s with those of N2O in the drainage water (N2Odrain) and N2O in the shallow groundwater (N2Osgw).
Statistical analysis
A paired t-test (p < 0.05) was performed to examine the effect of cropping system on the harvested aboveground biomass, drainage volume, leached and DOC, and N2O and CO2 emissions using statistical software (JMP 8.0, SAS Institute, Cary, NC, USA). Residual normality between dissolved N2O concentration and soil-surface N2O flux was preliminary analyzed with the Shapiro-Wilk's test for the spring (May 2007 and March–April 2008), the summer (June–August 2007), the autumn (September–November 2007), and the winter (December 2007–February 2008). Because the normality was rejected for all the seasons, the relationship between them was examined using the Spearman rank correlation coefficient. For the same reason, the relationship between the concentrations of DOC and dissolved N2O through the year was examined by plot using the Spearman rank correlation coefficient.
Results
Crop biomass and leaching of  and DOC
and DOC
Aboveground biomasses of soybean and wheat were similar to those of local crops, whereas that of upland rice was 75% lower than expected () probably because of its weak drought tolerance. The normal biomass in the SW plots and the low precipitation in August (23.5 mm versus the normal value of 121.8 mm) decreased the topsoil WFPS to < 30% (). Accordingly, the groundwater level in the SW plots lowered by >0.2 m from the initial depth during this period (data not shown), which made it impossible to collect samples of the shallow groundwater. Such water movement was also observed in the previous studies (Thorburn et al. Citation1995; Zhang et al. Citation1999). The total drainage volume in the SW plots was significantly lower than that in the UR plots, and each accounted for an average of 32% and 54% of the total precipitation (1280 mm yr−1), respectively ().
Figure 2. Seasonal courses of (a) daily precipitation and mean air temperature; (b) topsoil water-filled pore space (WFPS) and cumulative drainage volume; soil-surface nitrous oxide (N2O) flux in (c) the soybean–wheat double cropped (SW) plots and (d) the upland rice (UR) plots; (e–g) soil N2O profile, and the concentrations of (h) dissolved N2O; (i) nitrate (); (j) dissolved carbon dioxide (CO2), and (k) dissolved organic carbon (DOC) in the drainage water. Horizontal bars in (d) indicate the period of crop cultivation. Arrows indicates urea application and the rate (g N m−2). Basal dressing of urea in the UR plots (3 g N m−2) was conducted on 26 April 2007.
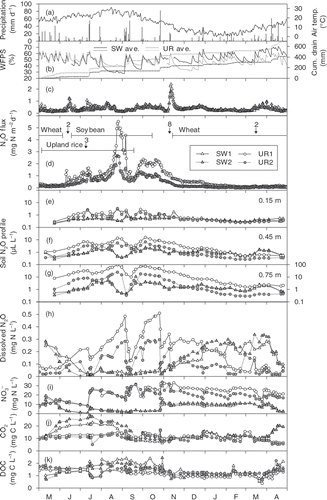
Table 1. Total crop biomass, drainage water volume, leached nitrate ( ) and dissolved organic carbon (DOC), and the emissions of nitrous oxide (N2O) and carbon dioxide (CO2)
) and dissolved organic carbon (DOC), and the emissions of nitrous oxide (N2O) and carbon dioxide (CO2)
The concentration ranged from 7.4 to 34.0 mg N L−1 in the UR plots, whereas it ranged from 0.5 to 19.0 mg N L−1 in the SW plots (). Heavy precipitation often supplied
to the resident groundwater. The total leached
-N in the UR plots was significantly greater than that in the SW plots (). The
concentration was generally <0.1 mg N L−1 through the year (data not shown). The DOC concentration in the SW plots exceeded 2 mg C L−1 from July to October (); however, the total leached DOC did not differ between the SW and UR plots ().
N2O dynamics
Soil-surface N2O flux in the SW plots had sharp increases just after fertilizer N applications, and a slight increase in the flux occurred from March to April (). Soil-surface N2O flux in the UR plots had a broad and great increase from August to October that was interrupted by a large precipitation event in early September ().
The N2O concentration in the soil profile increased with depth through the year (). From July to November, N2O concentrations at the three depths stayed high. In addition, N2O concentrations in the SW plots had slight increases from March to April. The N2O concentrations in the SW plots decreased temporarily from early August to early September, the period of lowered groundwater level. The CO2 concentration in the soil profile also increased with depth through the year, and the highest concentration at a depth of 0.75 m exceeded 20 mL L−1 in all the plots (data not shown).
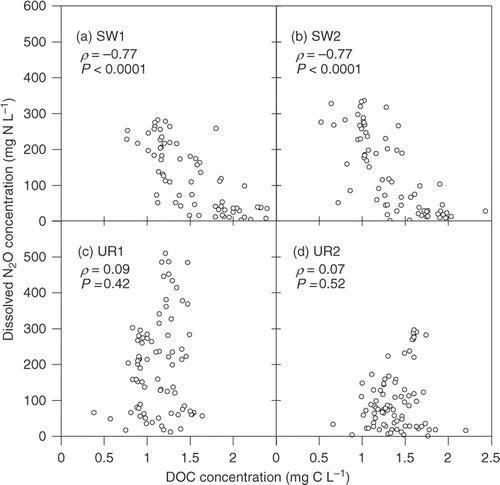
Dissolved N2O concentrations in the drainage water of the UR plots reached 0.5 mg N L−1 from July to October, which was interrupted by heavy precipitation () that subsequently diluted and displaced dissolved N2O in the resident groundwater. Dissolved N2O concentrations in the SW plots remained <0.1 mg N L−1 from July until heavy precipitation in late October. The highest concentrations of dissolved N2O (0.28 to 0.51 mg N L−1) were comparable to those observed in our previous study (0.47 to 0.89 mg N L−1; Minamikawa et al. Citation2010), and within the range of those observed in actual agricultural drainage water (0.03 to 9.98 mg N L−1; Heincke and Kaupenjohann Citation1999, and references therein). Dissolved CO2 concentration in the SW plots exceeded 20 mg C L−1 from May to August and in April (). In the SW plots, there were significant negative correlations between the concentrations of DOC and dissolved N2O ().
Figure 3. Relationships between dissolved organic carbon (DOC) concentration and dissolved nitrous oxide (N2O) concentration in the drainage water. Statistical significance was tested by the Spearman rank correlation coefficient. SW, soybean–wheat double cropping; UR, upland rice.
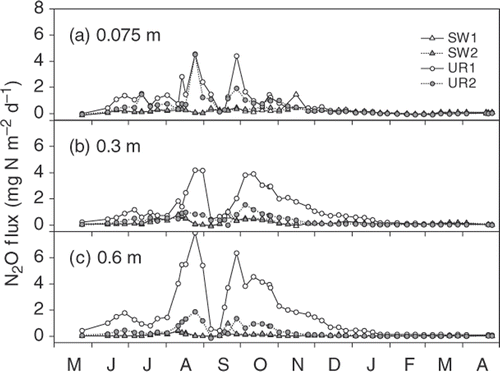
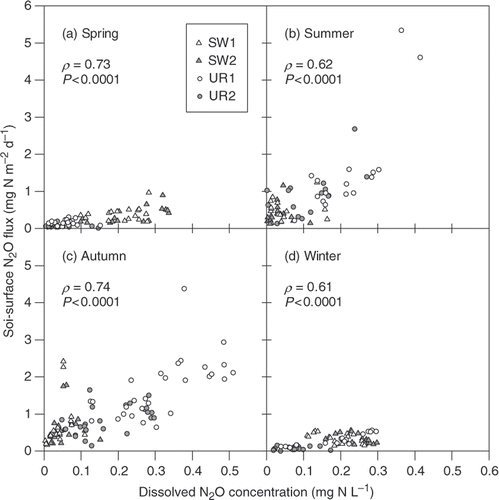
As shown in , there were significant positive correlations between dissolved N2O concentration and soil-surface N2O flux for all the seasons. However, the magnitude of soil-surface N2O flux against dissolved N2O concentration in the spring and winter was generally lower than that in the summer and autumn. Calculated diffusive N2O fluxes at the three depths in the UR plots () had similar seasonal course and magnitude to the soil-surface N2O flux (), whereas those in the SW plots were generally lower than the soil-surface flux ().
Figure 4. Relationships between dissolved nitrous oxide (N2O) concentration in the drainage water and soil-surface N2O flux for (a) the spring, (b) the summer, (c) the autumn, and (d) the winter. Statistical significance for all four plots was tested by the Spearman rank correlation coefficient. SW, soybean–wheat double cropping; UR, upland rice.
δ15N and δ18O values of  drain and N2Odrain
drain and N2Odrain
The concentrations in the drainage water steadily decreased from June to September, except for the heavy precipitation events (). The δ15N and δ18O of
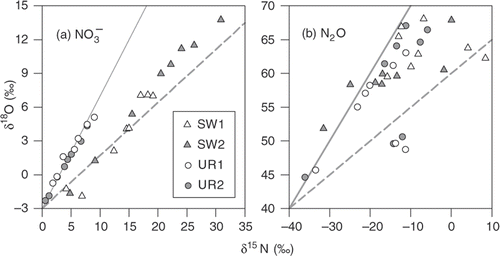
drain followed courses opposite to those for the concentration (). The slope for the relationship between δ15N and δ18O (Δδ18O/Δδ15N) of
drain between the two heavy precipitation events (i.e., from 19 July to 31 August) ranged between 0.61 and 0.66 in the SW plots and between 0.82 and 0.90 in the UR plots ().
Figure 6. Detailed time courses of the concentration, nitrogen (δ15N), and oxygen (δ18O) of (a–c) nitrate concentrations in the drainage water (
drain) and (d–f) nitrous oxide concentrations in the drainage water (N2Odrain) from June to September 2007. Arrows indicates the timing of urea application and the rate (g N m−2). SW, soybean–wheat double cropping; UR, upland rice.
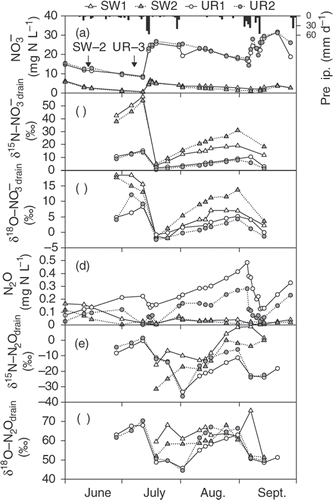
Figure 7. Relationships between nitrogen (δ15N) and oxygen (δ18O) for (a) nitrate concentrations in the drainage water (
drain) and (b) nitrous oxide concentrations in the drainage water (N2Odrain) from 19 June to 31 August 2007. Dashed and solid lines indicate a slope of 0.5 and 1.0, respectively. SW, soybean–wheat double cropping; UR, upland rice.
Dissolved N2O concentrations in the UR plots increased during this period, except for the heavy precipitation events, whereas those in the SW plots remained at their lowest level of the year (). There were significant (P < 0.01) and strong (r = −0.79 to −0.81) negative linear correlations between the concentrations of and dissolved N2O in the UR plots between the two heavy precipitation events (data not shown). The δ15N and δ18O of N2Odrain were unstable, but generally followed similar courses to those of
drain (). The Δδ18O/Δδ15N of N2Odrain between the two heavy precipitation events ranged between 0.35 and 0.68, except for the SW1 plot with some outliers (). The δ15N values of N2Odrain were 10.1‰ to 39.2‰ lower than those of
drain, whereas δ18O values of N2Odrain were 44.1‰ to 70.0‰ higher.
Vertical comparison of the δ15N and δ18O of N2O
Soil-surface N2O flux and N2O concentration at a depth of 0.15 m in the UR plots reached their highest values in August (). The δ15N values of N2Oflux-c and N2Oflux-s ranged from −17.7‰ to 1.8‰ in the SW plots and from −40.6‰ to −6.7‰ in the UR plots (). Differences in δ15N between N2Oflux-c and N2Oflux-s were generally less than ±5‰. The δ18O values of N2Oflux-c and N2Oflux-s ranged between 35.5‰ and 55.8‰ ().
Figure 8. Detailed time courses of (a) soil-surface nitrous oxide (N2O) flux; (b) N2O concentration at a 0.15 m depth; (c) nitrogen (δ15N) and (d) oxygen (δ18O) of N2Oflux-c; (e) δ15N and (f) δ18O of N2Oflux-s; (g) δ15N and (h) δ18O of N2Osgw; and (i) δ15N and (j) δ18O of N2Odrain from July to August 2007. Arrow in graph (a) indicates urea topdressing in the upland rice (UR) plots (3 g N m−2). Graphs (g) and (h) lack data of the soybean–wheat double cropping (SW) plots due to the lowered groundwater level.
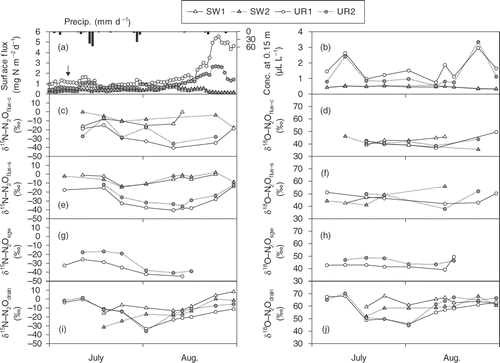
The δ15N of N2Osgw in the UR plots ranged from −44.7‰ to −16.8‰, and were 1.9‰ to 28.9‰ lower than the δ15N of N2Odrain (). The δ15N of N2Odrain in the SW plots ranged from −31.5‰ to 8.4‰ (). The δ18O of N2Osgw in the UR plots ranged from 39.1‰ to 49.4‰, whereas those of N2Odrain in the UR and SW plots ranged from 44.6‰ to 70.2‰ ().
The δ15N values of N2Oflux-c and N2Oflux-s in the SW plots were generally between 5‰ lower and 15‰ higher than δ15N of N2Odrain (, ). The δ18O values of N2Oflux-c and N2Oflux-s in the SW plots were generally 10‰ to 20‰ lower than those of N2Odrain (, ). The time courses of the δ15N values of N2Oflux-c and N2Oflux-s in the UR plots were similar to those of δ15N of N2Osgw, but the δ15N values of N2Oflux-c and N2Oflux-s were generally 5‰ to 10‰ higher than the δ15N of N2Osgw (, ). Differences in δ18O values among N2Oflux-c, N2Oflux-s, and N2Osgw were generally ±5‰ in the UR plots (, ).
Discussion
Denitrification near the groundwater table
The concentration, δ15N, and δ18O of
drain in the SW and UR plots between the two heavy precipitation events () exhibited typical time courses for the progress of denitrification. The high values of δ18O for N2Odrain were probably due to the oxygen in N2O which originated from both
and water (Casciotti et al.
Citation2002; Well and Flessa Citation2009). The Δδ18O/Δδ15N for
drain (0.61 to 0.90) fell within the range observed in groundwater, lake, and pure laboratory culture of denitrifying bacteria (0.47 to 1.02; Lehmann et al.
Citation2003; Singleton et al.
Citation2007; Granger et al.
Citation2008). In addition, dissolved O2 concentration (DO) in the drainage water was generally <1 mg L−1, except for heavy precipitation events (data not shown). These results clearly confirm that denitrification occurred near the groundwater table in the SW and UR plots during the summer.
Apparent differences in δ15N between N2Odrain and
drain (10.1‰ to 39.2‰) fell within the range for N isotope enrichment factor for denitrification in culture experiments and natural environments (0‰ to 40‰; Lehmann et al.
Citation2003, and references therein) though further denitrification process that reduces N2O to N2 may have shrunk the difference in δ15N. The Δδ18O/Δδ15N for N2Odrain (0.35 to 0.68) was comparable to those observed for N2O produced by denitrification (Meijide et al.
Citation2010; Ostrom et al.
Citation2010). In addition, the significant negative linear correlations between the concentrations of
and dissolved N2O in the drainage water of the UR plots can be explained by N2O production by denitrification, even if nitrification had a certain contribution to N2O production. Furthermore, if nitrification is the main process of N2O production, there will be a positive relationship between the concentrations of
and dissolved N2O because N2O and
are an intermediate and the end products of nitrification, respectively. Such a relationship was observed in previous studies (e.g., Ueda et al.
Citation1993; Mühlherr and Hiscock Citation1998), but we did not find it in the SW and UR plots (see ). Mass balance of N between the detected
(<0.1 mg N L−1, data not shown) and the existing N2O in the drainage water that temporally exceeded 0.4 mg N L−1 () also indicates that nitrification was not the main process for N2O production. Therefore, we conclude that denitrification was the main process for N2O production near the groundwater table in our lysimeters during the summer. Although we did not measure the δ15N of
in the shallow groundwater, the observed δ15N of N2Osgw in the UR plots was relatively low in August (<−40‰, ), which may suggest that nitrification had some contribution to N2O production. As for the other seasons, denitrification may also have been the main process for N2O production because of the constant low
and DO through the year, as well as for the reason described below.
Cropping system had strong influences on leaching of and DOC in the soil profile (, , ). The high
concentration in the UR plots was due to the low N uptake by upland rice (). As reported in Kusa et al. (Citation2010), macropores and cracks peculiar to Gray lowland soil would be a cause of rapid leaching of
. It is well known that denitrification in subsoil and groundwater is limited by a lack of readily available C (e.g., Hedin et al.
Citation1998; Hill et al.
Citation2000; von der Heide et al. 2008). From May to July and in the following April, CO2 production and
consumption in the SW plots (see ) were relatively well balanced stoichiometrically for denitrification. During this period, dissolved N2O concentration in the SW plots also decreased correspondingly (). Weymann et al. (Citation2010) observed a significant negative relationship between the highest dissolved N2O concentration and DOC concentration in incubation experiments on the groundwater in a German aquifer. Such relationships were found only in the SW plots (). As reviewed in Kuzyakov and Domanski (Citation2000) and Chantigny (Citation2003), it is obvious that the greater plant biomass is, the greater root exudates and plant-derived C leach. Therefore, the low concentrations of
and dissolved N2O in the SW plots would be explained by appropriate N uptake by crops and enhanced denitrification due to the great wheat biomass.
However, as was the case for the UR plots (), there have been several cases in which DOC was not a significant predictive variable for the magnitude of N2O production by denitrification partly because of its poor bioavailability (e.g., Deurer et al. Citation2008). Observed concentration range of DOC in the UR plots () was narrower and lower than those in the previous studies that showed the significant relationship (e.g., Hill et al. Citation2000; von der Heide et al. 2008), and thus the range in the UR plots may not have developed strict reductive conditions preferable to N2O reduction to N2. More detailed studies of the bioavailability of DOC will be necessary to clarify the difference in N2O production by denitrification between the two cropping systems.
Upward diffusion of N2O produced near the groundwater table
Significant positive correlation between dissolved N2O concentration and soil-surface N2O flux in the summer () strongly supports our hypothesis. The correlation was also observed in the other seasons (, ), suggesting that our hypothesis would apply to the other seasons. As for the seasonal difference in the magnitude of the upward N2O diffusion, Van Groenigen et al. (Citation2005) also observed a similar discrepancy between the summer and winter in a sandy soil field with a shallow groundwater table (0.5 to 1.2 m depth). They argued that high WFPS in the winter would increase the diffusion time for N2O from the subsoil to the atmosphere, thereby increasing the likelihood for further N2O reduction to N2. We expect that this explanation would also be valid in the present study. Therefore, the comparison of the measured data among the seasons indicates that soil water content (WFPS; ) was an important factor determining the magnitude of upward N2O diffusion in our lysimeters.
Calculated diffusive N2O fluxes in the soil profile of the SW plots () indicate that soil-surface N2O flux was mostly derived from the topsoil. In contrast, those in the UR plots indicate that soil-surface N2O fluxes were mainly derived from >0.6 m depth during the summer and autumn. To examine the N2O movement at further depths in the UR plots, we used dissolved N2O concentration in the drainage water, which can also be expressed as the equilibrated N2O concentration in the vapor phase by Henry's law. For example, the highest dissolved N2O concentration in the UR1 plot (0.510 mg N L−1) was supposed to equilibrate with 635 µL L−1 of N2O at 19.2°C of the measured water temperature. Although all the converted N2O concentrations in the UR plots were not shown here, they (2–727 µL L−1) were always higher than the measured N2O concentrations at a depth of 0.75 m through the year (0.3–94.2 µL L−1; ). This indicates that the N2O source for the upward diffusion was at >0.75 m depth.
Inconsistency of δ15N and δ18O values of N2Odrain with those of N2Oflux-c or N2Oflux-s in the SW plots () indicates that the soil-surface N2O flux was mainly derived from other source(s) than the upward diffusion. The δ15N values of N2Oflux-c and N2Oflux-s were similar to but not exactly consistent with those of N2Osgw in the UR plots (, ). This discrepancy may suggest that there were other possible N2O sources and processes that changed the δ15N values of N2Oflux-c and N2Oflux-s. Urea application often induced temporal N2O fluxes (), probably because of nitrification in the topsoil. The δ15N of the N2Oflux-c just after the topdressing was −27.5‰ and −19.0‰ in the UR plots (). In addition, we measured the δ15N of the N2Oflux-c just after urea incorporation in May 2008 (−22.6‰). These values were comparable to those observed in the UR plots in late August when the soil-surface N2O flux remained high. Therefore, from the viewpoint of isotopic signatures of N2O, we could not exclude the topsoil nitrification from possible N2O sources for soil-surface emission during the summer. However, in our lysimeters, Nishimura et al. (Citation2005a) reported that, considering topsoil and
contents, the contribution of topsoil nitrification to soil-surface N2O emission was relatively low. Therefore, we assumed that topsoil nitrification alone could hardly explain the broad and high N2O fluxes in the UR plots from the summer to the autumn. Denitrification in the topsoil was also unlikely during this period because the topsoil WFPS was around 30% to 40% (), which is low enough to significantly impede N2O production by denitrification (Davidson Citation1991). In contrast, N2O reduction to N2 due to entrapment (dissolution) of N2O in soil water (Clough et al.
Citation1999, Citation2005) and in anaerobic microsites (Meijide et al.
Citation2010) would be plausible mechanisms that made heavier the δ15N of N2O that was moving upward. Therefore, our natural abundance method was limited in examining the upward N2O diffusion by itself; however, the obtained results were not inconsistent with our hypothesis, considering other possible sources and processes.
From the combination of the above-mentioned three indirect observations, we conclude that our hypothesis was verified in the present study. It will be difficult to simultaneously explain the three results by other sources and processes. Two kinds of upland cropping systems provided distinctive experimental conditions for the dissolved N2O concentration during the summer. The upward N2O diffusion would have occurred in the UR plots that had high dissolved N2O concentration in the drainage water during the summer. In contrast, the upward N2O diffusion would have been negligible in the SW plots that had the low dissolved N2O concentration during the summer. The δ18O of N2Osgw in the UR plots could not be used as an isotopic signature for the upward movement because these values (approx. 40‰ to 50‰; ) were close to that of the atmospheric N2O. Of course, the usage of 15N-labeled and N2O (e.g., Clough et al.
Citation2006) would provide direct evidence for the upward diffusion. However, we did not adopt it to avoid a long-term residence of 15N-labeled compounds in the soil and groundwater for the following experiments, and also to discuss simultaneously the production process of N2O dissolved in the drainage water by a natural abundance method.
Soil-surface N2O emission from August to October in the UR plots accounted for 63% to 66% of the annual total, versus 25% to 30% in the SW plots. Given that most soil-surface N2O emission in the UR plots during this period was derived from that produced near the groundwater table, its quantitative significance is potentially important, as compared to any previous studies. Weymann et al. (Citation2009) reported that only 0.04–0.28% of the soil-surface N2O flux originated from groundwater-derived N2O. The highest dissolved N2O concentrations in their study were between 0.014 and 0.016 mg N L−1, which were one order of magnitude lower than our study. The N2O concentrations in the soil profile of their study were between 0.297 and 0.427 µL L−1 at depths of 0.3–0.9 m, which were the same order as the atmospheric ambient concentration. The reason for this negligible contribution would be due to high permeability of the soil (Podzols and Gleysols, Deurer et al. 2008). On the other hand, we assume that there were two plausible causes that explain the high contribution in our lysimeters. First, the structure of the bottom of the lysimeter was a closed system that did not allow the resident groundwater to flow out without additional precipitation supply. As indicated by the constant low DO values, such a structure would have developed the redox conditions favorable to denitrification. Another cause is the enhanced upward N2O diffusion during the summer due to the soil drying, as mentioned above. The macropores and cracks could also enhance the upward N2O diffusion as reported in Kusa et al. (Citation2010). It is necessary to investigate the case in actual agricultural fields on lowland soils and poorly drained soils.
Conclusion
The present study examined the following hypothesis: if a large amount of N2O is produced near the shallow groundwater table in the summer, it will diffuse upward to the atmosphere. Dissolved N2O concentration in the drainage water in the UR plots exceeded 0.4 mg N L−1 during the summer, whereas that in the SW plots remained <0.1 mg N L−1. Analyses of the concentrations of and dissolved N2O and their δ15N and δ18O during the summer revealed that denitrification was the main process for N2O production near the groundwater table. There was a significant positive correlation between the dissolved N2O concentration and soil-surface N2O flux in the summer. The course and magnitude of diffusive N2O fluxes at the three depths in the soil profile in the UR plots were almost consistent with those of soil-surface N2O flux during this period. The δ15N values of N2O in the soil-surface flux were similar to those in the shallow groundwater in the UR plots during the summer, whereas such similarity was not found in the SW plots. We conclude that our hypothesis was confirmed by the above results. Comparison of the monitored data with the other seasons indicates that soil water content is a key factor determining the magnitude of upward N2O diffusion as well as the dissolved N2O concentration in our lysimeters.
Acknowlegments
We thank Drs. Hiroko Akiyama and Shigeto Sudo (NIAES) for their help using the GCs. We also thank Drs. Karamat Sistani (USDA Agricultural Research Service) and Sadao Eguchi (NIAES) for valuable comments on the manuscript before submission. This study was funded by the Global Environmental Research Fund (S2-3a) of Japan's Ministry of the Environment.
References
- Akiyama , H , Yan , X and Yagi , K . 2006 . Estimations of emission factors for fertilizer-induced direct N2O emissions from agricultural soils in Japan: Summary of available data . Soil Sci. Plant Nutr. , 52 : 774 – 787 .
- Buckingham , E . 1904 . Contributions to our knowledge of the aeration of soils , Washington D.C : USDA Bureau of Soil, Bulletin 25 .
- Casciotti , KL , Sigman , DM , Hastings , MG , Böhlke , JK and Hilkert , A . 2002 . Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method . Anal. Chem. , 74 : 4905 – 4921 .
- Chantigny , MH . 2003 . Dissolved and water-extractable organic matter in soils: a review on the influence of land use and management practices . Geoderma , 113 : 357 – 380 .
- Clemens , J , Schillinger , MP , Goldbach , H and Huwe , B . 1999 . Spatial variability of N2O emissions and soil parameters of an arable silt loam—A field study . Biol. Fertil. Soils , 28 : 403 – 406 .
- Clough , TJ , Jarvis , SC , Dixon , ER , Stevens , RJ , Laughlin , RJ and Hatch , DJ . 1999 . Carbon induced subsoil denitrification of 15N-labelled nitrate in 1 m deep soil columns . Soil Biol. Biochem. , 31 : 31 – 41 .
- Clough , TJ , Kelliher , FM , Wang , YP and Sherlock , RR . 2006 . Diffusion of 15N-labeled N2O into soil columns: a promising method to examine the fate of N2O in subsoils . Soil Biol. Biochem. , 38 : 1462 – 1468 .
- Clough , TJ , Sherlock , RR and Rolston , DE . 2005 . Review of the movement and fate of N2O in the subsoil . Nutr. Cycl. Agroecosyst. , 72 : 3 – 11 .
- Davidson , EA . 1991: Fluxes of nitrous oxide and nitric oxide from terrestrial ecosystems. In Microbial Production and Consumption of Greenhouse Gases: Methane, Nitrous Oxide, and Halomethanes, Eds. Rogers JE, Whitman WB, pp. 219–235. American Society of Microbiology, Washington D.C
- Deurer , M , von der Heide , C , Böttcher , J , Duijnisveld , WHM , Weymann , D and Well , R . 2008 . The dynamics of N2O near the groundwater table and the transfer of N2O into the unsaturated zone: A case study from a sandy aquifer in Germany . Catena , 72 : 362 – 373 .
- Forster , P , Ramaswamy , V and Artaxo , P . et al. 2007: Changes in atmospheric constituents and in radiative forcing. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Eds. Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, 212pp. Cambridge University Press, Cambridge and New York
- Granger , J , Sigman , DM , Lehmann , MF and Tortell , PD . 2008 . Nitrogen and oxygen isotope fractionation during dissimilatory nitrate reduction by denitrifying bacteria . Limnol. Oceanogr. , 53 : 2533 – 2545 .
- Hedin , LO , von Fischer , JC , Ostrom , NE , Kennedy , BP , Brown , MG and Robertson , GP . 1998 . Thermodynamic constraints on nitrogen transformations and other biogeochemical processes at soil-stream interfaces . Ecology , 79 : 684 – 703 .
- Heincke , M and Kaupenjohann , M . 1999 . Effects of soil solution on the dynamics of N2O emissions: a review . Nutr. Cycl. Agroecosyst. , 55 : 133 – 157 .
- Hill , AR , Devito , KJ , Campagnolo , S and Sanmugadas , K . 2000 . Subsurface denitrification in a forest riparian zone: Interactions between hydrology and supplies of nitrate and organic carbon . Biogeochem. , 51 : 193 – 223 .
- Kawamoto , K , Komatsu , T , Moldrup , P , Yoshikawa , S and Fujimoto , T . 2005 . Development and tests of predictive soil-gas diffusivity models for Japanese undistributed soils . J. Jpn. Soc. Soil Phys. , 101 : 37 – 50 . (in Japanese with English abstract)
- Kusa , K , Sawamoto , T , Hu , R and Hatano , R . 2010 . Comparison of N2O and CO2 concentrations and fluxes in the soil profile between a Gray Lowland soil and an Andosol . Soil Sci. Plant Nutr. , 56 : 186 – 199 .
- Kuzyakov , Y and Domanski , G . 2000 . Carbon input by plants into the soil . Review. J. Plant Nutr. Soil Sci. , 163 : 421 – 431 .
- Lehmann , MF , Reichert , P , Bernasconi , SM , Barbieri , A and McKenzie , JA . 2003 . Modelling nitrogen and oxygen isotope fractionation during denitrification in a lacustrine redox-transition zone . Geochim. Cosmochim. Acta , 67 : 2529 – 2542 .
- Massman , WJ . 1998 . A review of the molecular diffusivities of H2O, CO2, CH4, CO, O3, SO2, NH3, N2O, NO, and NO2 in air, O2 and N2 near STP . Atm. Environ. , 32 : 1111 – 1127 .
- Meijide , A , Cardenas , LM , Bol , R , Bergstermann , A , Goulding , K , Well , R , Vallejo , A and Scholefield , D . 2010 . Dual isotope and isotopomer measurements for the understanding of N2O production and consumption during denitrification in an arable soil . Eur. J. Soil Sci. , 61 : 364 – 374 .
- Minamikawa , K , Nishimura , S , Sawamoto , T , Nakajima , Y and Yagi , K . 2010 . Annual emissions of dissolved CO2, CH4, and N2O in the subsurface drainage from three cropping systems . Glob. Change Biol. , 16 : 796 – 809 .
- Mosier , A , Kroeze , C , Nevison , C , Oenema , O , Seitzinger , S and van Cleemput , O . 1998 . Closing the global N2O budget: nitrous oxide emissions through the agricultural nitrogen cycle . OECD/IPCC/IEA phase II development of IPCC guidelines for national greenhouse gas inventory methodology. Nutr. Cycl. Agroecosyst. , 52 : 225 – 248 .
- Mühlherr , IH and Hiscock , KM . 1998 . Nitrous oxide production and consumption in British limestone aquifers . J. Hydrol. , 211 : 126 – 139 .
- Nishimura , S , Sawamoto , T , Akiyama , H , Sudo , S , Cheng , W and Yagi , K . 2005a . Continuous, automated nitrous oxide measurements from paddy soils converted to upland crops . Soil Sci. Soc. Am. J. , 69 : 1977 – 1986 .
- Nishimura , S , Sudo , S , Akiyama , H , Yonemura , S , Yagi , K and Tsuruta , H . 2005b . Development of a system for simultaneous and continuous measurement of carbon dioxide, methane and nitrous oxide fluxes from croplands based on the automated closed chamber method . Soil Sci. Plant Nutr. , 51 : 557 – 564 .
- Ostrom , NE , Sutka , R , Ostrom , PH , Grandy , AS , Huizinga , KM , Gandhi , H , von Fischer , JC and Robertson , GP . 2010 . Isotopologue data reveal bacterial denitrification as the primary source of N2O during a high flux event following cultivation of a native temperate grassland . Soil Biol. Biochem. , 42 : 499 – 506 .
- Sigman , DM , Casciotti , KL , Andreani , M , Barford , C , Galanter , M and Böhlke , JK . 2001 . A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater . Anal. Chem. , 73 : 4145 – 4153 .
- Singleton , MJ , Esser , BK , Morgan , JE , Hudson , GB , McNab , WW and Harter , T . 2007 . Saturated zone denitrification: Potential for natural attenuation of nitrate contamination in shallow groundwater under dairy operations . Environ. Sci. Technol. , 41 : 759 – 765 .
- Smith , P , Martino , D and Cai , Z . et al. 2007: Agriculture. In Climate Change 2007: Mitigation. Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Eds. Metz B, Davidson OR, Bosch PR, Dave R, Meyer LA, 503pp. Cambridge University Press, Cambridge and New York
- Sudo , S . 2006: Method and instrument for measuring atmospheric gas. Japan Patent No. 2006–275844
- Thorburn , PJ , Walker , GR and Jolly , ID . 1995 . Uptake of saline groundwater by plants: An analytical model for semi-arid and arid areas . Plant Soil , 175 : 1 – 11 .
- Ueda , S , Ogura , N and Yoshinari , T . 1993 . Accumulation of nitrous oxide in aerobic groundwaters . Water Res. , 27 : 1787 – 1792 .
- Van Groenigen , JW , Georgius , PJ , van Kessel , C , Hummelink , EWJ , Velthof , GL and Zwart , KB . 2005 . Subsoil 15N-N2O concentrations in a sandy soil profile after application of 15N-fertilizer . Nutr. Cycl. Agroecosyst. , 72 : 13 – 25 .
- Von der Heide , C , Böttcher , J , Deurer , M , Weymann , D , Well , R and Duijnisveld , WHM . 2008 . Spatial variability of N2O concentrations and of denitrification-related factors in the surficial groundwater of a catchment in northern Germany . J. Hydrol. , 360 : 230 – 241 .
- Well , R , Augustin , J , Davis , J , Griffith , SM , Meyer , K and Myrold , DD . 2001 . Production and transport of denitrification gases in shallow ground water . Nutr. Cycl. Agroecosyst. , 60 : 65 – 75 .
- Well , R and Flessa , H . 2009 . Isotopologue signatures of N2O produced by denitrification in soils . J. Geophys. Res. , 114 : G02020
- Weymann , D , Geistlinger , H , Well , R , von der Heide , C and Flessa , H . 2010 . Kinetics of N2O production and reduction in a nitrate-contaminated aquifer inferred from laboratory incubation experiments . Biogeosci. , 7 : 1953 – 1972 .
- Weymann , D , Well , R , von der Heide , C , Böttcher , J , Flessa , H and Duijnisveld , WHM . 2009 . Recovery of groundwater N2O at the soil surface and its contribution to total N2O emissions . Nutr. Cycl. Agroecosyst. , 85 : 299 – 312 .
- Yanai , J , Sawamoto , T , Oe , T , Kusa , K , Yamakawa , K , Sakamoto , K , Naganawa , T , Inubushi , K , Hatano , R and Kosaki , T . 2003 . Spatial variability of nitrous oxide emissions and their soil-related determining factors in an agricultural field . J. Environ. Qual. , 32 : 1965 – 1977 .
- Zhang , L , Dawes , WR , Slavich , PG , Meyer , WS , Thorburn , PJ , Smith , DJ and Walker , GR . 1999 . Growth and ground water uptake responses of lucerne to changes in groundwater levels and salinity: lysimeter, isotope and modelling studies . Agric. Water Manage. , 39 : 265 – 282 .