Abstract
Tea (Camellia sinensis (L.) O. Kuntze) is a globally important crop and is unusual because it both requires and acidifies the soil in which it grows. In spite of the low pH, high nitrate () accumulates in tea soils, resulting in a great potential for diffuse pollution. Nitrification in tea soils remains poorly understood. The purpose of this study was to investigate net and gross nitrification in three tea soils with varying productivity, their adjacent forest and vegetable soils. The results showed that neither net nor gross nitrification rates were significantly related to soil pH. The net nitrification rate increased with increasing tea production, and when soils were incubated with ammonium sulfate. The ammonium (
) added to the vegetable soil was immediately and completely nitrified, but that in the tea and forest soils was nitrified more slowly. About 60–80% of the
in the tea and forest soils was nitrified during 35 d of incubation, indicating nitrifiers do exist in these soils. Significantly higher gross nitrification rates were observed in vegetable and tea soils with high and medium production than in forest and tea soils with low production. Microbial consumption (immobilization and nitrate respiration) accounted for 30 to 70% of the mineralized nitrate nitrogen (
−N) with the lowest in the forest soil, and no significant difference between the tea and vegetable soils. Nitrogen fertilizer stimulated soil nitrification. In conclusion, the types of land use and their management are more important than soil pH for soil nitrification. High nitrogen application rate is the main cause of
accumulation in tea soils and should be reduced to minimize
pollution of water resources.
INTRODUCTION
Nitrification is the soil microbial process responsible for the transformation of ammonium () into nitrite and then to nitrate (
). It is a critical part of the nitrogen (N) cycle with great agricultural and environmental importance. Nitrification is affected by soil pH. In the early 20th century, nitrification was generally considered not to occur in soils below about pH 5, since nitrate concentrations are low in such soils and nitrate accumulation requires liming or other pH-raising measures (De Boer and Kowalchuk 2001). The optimum soil pH for nitrification is from about 7.5 to 8.0 (Paul and Clark Citation1989). Soil acidification has been proposed as a management strategy to decrease nitrification and nitrate loss from agricultural land (Kemmitt et al. Citation2005). However, there is considerable evidence that nitrification could occur in acid soils including agricultural fields, coniferous and deciduous forests, heathlands and natural grasslands (Walker and Wickramasinghe Citation1979; Robertson Citation1982; Troelstra et al. Citation1990; Pansombat et al. Citation1997; Pennington and Ellis Citation1993; Xue et al. Citation2006).
Nitrification is generally measured by two distinct experimental means: net and gross nitrification rates. The net nitrification rate is the rate of accumulation during a certain incubation period. This is the most commonly measured rate in laboratory and field conditions (Norton and Stark Citation2011). However, N can turn over quickly because of the high biological demand for N by which
consumption and production can occur during soil incubation (Davidson et al. Citation1992). The
consumption includes
immobilization by microbes, denitrification and other
consuming processes. The denitrification may be inhibited in aerobic incubations. The gross nitrification rate is the actual rate of conversion of
to
. It is determined by use of isotopes (15N) to give distinct estimates of simultaneous production and consumption of
. High gross nitrification rate can be observed even in soils with negligible net nitrification rate (Stark and Hart Citation1997). Measurements of these different rates are often combined to give a clearer view of the nitrification process (Norton and Stark Citation2011).
Tea (Camellia sinensis (L.) O. Kuntze) is an unusual crop because it both requires an acid soil and also acidifies soil. Tea can be grown within a pH range of 3.0 to 6.8 with the optimal level from 4.5 to 5.5 (Yang Citation2005). Soil pH is less than 4.5 in about 50% of the typical tea producing regions in China (Han et al. Citation2002). Soil becomes strongly acidified following the planting of tea and soil pH generally continues to decrease with the increase in stand age and tea productivity (Song and Liu Citation1990; Han et al. Citation2007). The soil is acidified by the tea plant itself and by fertilization, especially with ammonium sulfate ((NH4)2SO4) and urea (Shi et al. Citation1999). Tea soils also receive large amounts of tea litter and prunings, which contain high concentrations of aluminum (Al) and polyphenols. Al is beneficial for the growth and development of tea plants, but it is toxic to most soil microorganisms (Tate Citation1995). Polyphenols lower soil urease activity and N mineralization (Sivaplan et al. 1983, 1985). Therefore, tea cultivation intensity and duration could have a strong impact on the microbial biomass and its community structure and functioning, including nitrification (Han et al. Citation2007).
Tea is a leaf harvested crop and preferentially takes up rather than
when both N forms are available in soils (Morita et al.
Citation1998; Yang Citation2005). Nitrogen fertilizers can improve both the yield and quality of tea, especially green tea. Therefore, farmers apply large amount of N fertilizers, usually as (NH4)2SO4, ammonium phosphate, and urea, in tea gardens. Nitrogen application rates range from 0–2600 kg ha−1 with an average of 553 kg ha−1 in the main tea producing areas in China (Han et al. Citation2002). N rates often exceeded 1000 kg ha−1 in Japan in the 1980 s (Tokuda and Hayatsu Citation2000). The high N fertilizer application rate not only causes accumulation of
−N in tea soils from 10 to 444 mg kg−1 (Nioh et al. Citation1993; Pansombat et al. Citation1997), but reduces N use efficiency and diffuses pollution. It was reported that 457 and 155 kg N ha−1 were leached as
−N when 900 and 500 kg N ha−1 y−1 respectively were applied in tea fields (Kiml et al. Citation2002; Watanabe et al. Citation2002). The
−N concentration in spring water near tea gardens is around 50 and even as high as 100 mg L−1 (Li et al. Citation1997; Kumazawa Citation2002), far in excess of the World Health Organization standard of 50 mg L−1 for
. Obviously, nitrification proceeds in tea soils despite their low pH. Hayastu and Kosuge (1993) had found that nitrification occurred in strongly acid tea soils with pH values ranging from 3 to 5.
The area under tea cultivation in China is currently about 1.85 million ha, accounting for 52% of the world's total tea lands (ITC 2010). Tea plays a significant role in poverty alleviation and rural economic development. However, nitrification in acid tea soils is not well understood. In this study, we determined net and gross nitrification rates and the effect of N fertilizer on the nitrification potential in soils of varying tea productivity, and compared nitrification with the adjacent forest and vegetable soils. Our aim was to provide information on nitrification in different soils, and the effect of N fertilizer on nitrification and concentration, which could help to improve N fertilizer use efficiency and reduce environmental risks derived from tea ecosystems.
MATERIALS AND METHODS
Field sites
The study area was in Hangzhou city (120°09′ E, 30°14′ N), Zhejiang province, eastern China, where the most famous Chinese premium tea, Westlake Longjin, is produced. The annual mean temperature is 17°C ranging from 1.7°C in January to 33.0°C in July. The annual mean precipitation is 1533 mm with 74% of rainfall occurring during the tea-growing season from March to September. The soils were taken from experimental fields with high, middle and low tea production levels (HTP, MTP and LTP) with Longjin tea (made by one bud and one leaf in spring season) of 280, 200 and 150 kg ha−1, respectively, at the Tea Research Institute of the Chinese Academy of Agricultural Sciences. The different tea production levels mainly resulted from different long-term fertilizer applications. Approximately 900, 600 and 300 kg N ha−1 y−1 of chemical fertilizers (mainly urea) were applied in 3–4 split dressings in the HTP, MTP and LTP fields respectively during the last decade. Organic fertilizer (farmyard manure or rapeseed cake) was applied in the HTP field at 2250 kg ha−1 and at half that rate in the MTP and LTP fields. The annual accumulated ratio of nitrogen:phosphorous pentoxide:potassium oxide (N:P2O5:K2O) was 3:1:1. Phosphorus (P) and potassium (K) (mainly compound fertilizer) and organic fertilizer were applied as basal fertilizer once a year in September or October. The three fields had similar crop management and the stand age was approximately 35 y. A forested area (the land use prior to clearing and planting of tea) and a farmyard vegetable field adjacent to the tea fields were sampled for comparison. The evergreen latifoliate vegetation was dominated by Schima crenata Korthals, Cinnamonum camphora and Castanopsis sclerophylla, and no fertilizer was applied in the forest. About 300 kg N ha−1 y−1 (mainly urea and compound fertilizer) with an N:P2O5:K2O ratio of 2:1:1, 10 t ha−1 y−1 farmyard manure and biogass slurry were applied in the vegetable field. The vegetables planted were mainly Chinese cabbages (Brassica rapa chinensis) and radishes (Raphanus sativus). The residues after vegetable harvest were left in the field. The soils are classified as an Ultisol and the parent material is Anshan quartz-free porphyry.
Soil sampling and treatment
In each field, 3 replicate samples were taken using a soil auger between rows of tea plants in September 2009 before basal fertilizers. Each replicate consisted of 10 random sub-samples of 0–20 cm depth. Plant residues, roots, stones and obvious macrofauna were removed by hand, then the soil was sieved at field moisture < 2 mm and stored at 4°C until analysis. Sub-samples were air-dried and ground to < 160 µm for physical and chemical analysis. Wet soils were adjusted to ca. 40% of water holding capacity and pre-incubated aerobically at 25°C in the dark with water and soda-lime for 7 d before use. The soda-lime was to absorb carbon dioxide (CO2) evolved during incubation, to prevent over-concentration of CO2 from affecting soil respiration.
Net nitrification
Net nitrification was determined by aerobic incubation for 35 d. The soils were kept in drums containing distilled water and soda-lime at a constant temperature of 25°C and relative humidity of above 95%. After 0, 3, 10, 20 and 35 d, sub-samples were extracted with 0.5 mol L−1 potassium sulfate (K2SO4) for 30 min (soil:extractant ratio 1:4) and analyzed for mineral N (,
) by Flow Injection Analysis (Skalar SAN++ system, Netherlands). Net nitrification rates were calculated from the changes in
−N pool sizes during the incubations.
To assess the effect of N fertilizer application on net nitrification, 200 mg N kg−1 soil using (NH4)2SO4 solution was added to the soils before incubation. The volume of solution was taken into consideration during adjustment of soil water holding capacity.
Gross nitrification and nitrate consumption
Gross nitrification was determined by 15N isotopic dilution (Kirkham and Bartholomew Citation1954; Hart et al. Citation1994). Each soil sample of 150 g was spread as a 2-mm homogeneous layer on a plastic film and sprayed with 9 mL of high enrichment (99 atom% 15N) potassium nitrate-15N (K15NO3) solution and mixed. The 15N concentration in the solution was 18 mg L−1. Moist soil equivalent to 10 g dry soil for HTP, MTP and vegetable soils, and 20 g of dry soil for LTP and forest soils (due to low concentrations of in these 2 soils) were incubated for 24 h at 25°C and a relative humidity above 95%. Mineral N was extracted immediately (within 15 min) after spraying of the labeled solutions (n = 3 per soil), and after the incubation period (n = 3). Mineral N was extracted with 100 mL 0.5 mol L−1 K2SO4 for 30 min. Ammonium ion and
concentrations were determined by Flow Injection Analysis. A mason-jar diffusion method (Khan et al.
Citation1998) was used to collect the mineral N for isotopic analysis. About 70 mL of the extract was incubated with added base [magnesium oxide (MgO)] for 7 d, to convert the
to NH3. The
was converted to
in a sealed jar by addition of 0.2 g of Devarda's alloy. The released NH3 was trapped in acidified paper disks which were suspended above the extract and analyzed for % atom excess 15N by a gas isotope mass spectrometer (Finnigan MAT-271, Germany). The gross nitrification rate and gross
consumption rate were calculated from the following equations:
Soil analysis
Soil pH was determined using a combined glass electrode in 1:1 (w:v) ratio of soil:distilled water. Soil total N and C were determined by a Vario Max CN Analyzer (Elementar Analysensysteme GmbH, Germany). Available soil P was extracted with 0.03 mol L−1 ammonium fluoride (NH4F) + 0.025 mol L−1 hydrochloric acid (HCl) and exchangeable K with 1 mol L−1 NH4OAC (both extractions used a 1:10 soil-solution ratio) for 0.5 h. The P and K in the solutions were determined using inductively coupled plasma atomic emission spectroscopy (ICP-AES) (JAC IRIS/AP, Thermo Jarrell Ash Corporation, Franklin, USA).
Soil microbial biomass carbon (C) was determined by the fumigation-extraction method (Vance et al. 1987). Three replicates of both fumigated and non-fumigated soils were extracted with 0.5 mol L−1 K2SO4 for 30 min (1:4 soil:extractant ratio). Organic C was measured by automated liquid organic C analysis. Biomass C was calculated from: BC = Ec/kc, where Ec = [(organic C extracted from fumigated soil) minus (organic C extracted from non-fumigated soil)] and kc = 0.45.
Statistical analyses
A one-way or two-way analysis of variance (ANOVA) was used to compute means and the least significant differences (LSD) with different soils or treatments as factors by SPSS 13 for Windows. The significance level was set at p < 0.05. The values in tables and figures are mean values and the standard deviation (SD) of three replicates.
RESULTS
Basic soil properties
The pH of the tea soils decreased gradually with increasing tea production. The soils from the fields of HTP and MTP had significantly lower pHs than LTP and forest soils. The adjacent vegetable soil had the highest pH (). Both soil C and N increased with increasing tea production. Tea soils were significantly higher than the forest soil, and the vegetable soil was between soils of HTP and MTP in total C and N. The soil C/N ratio in the vegetable fields was significantly higher than those in tea gardens and forest, which were all very similar. The soil microbial biomass C concentration was the highest in the vegetable field, followed by HTP and forest fields. It decreased with decreasing tea production (). Significant differences were found between vegetable and HTP soils, and between HTP and MTP and LTP soils, respectively. The majority of mineral N was −N in all tested soils. The vegetable soil had the highest
−N concentration, followed by HTP and MTP soils; LTP and forest soils were relatively lower. Available P had the largest variation among the selected soil parameters. It was not detected in the forest soil. There were significant differences between all soils. Exchangeable K had the same trend as
−N concentrations, being highest in the vegetable soil and lowest in the forest soil. The soil nutrients generally increased with increasing tea production due to higher long-term fertilizer application rate in fields of higher production.
Table 1. Selected basic properties of the tested soils
Net nitrification and the effect of N fertilizer on net nitrification
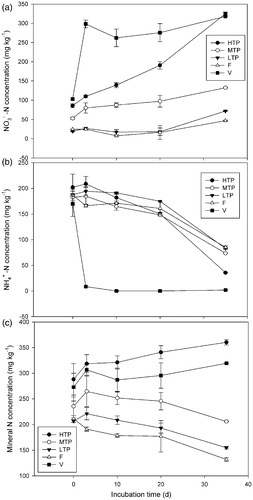
The soils were incubated with and without N fertilizer for 35 d. To better elucidate different nitrification patterns between tea and vegetable soils, the net nitrification rates were calculated during the first 3 d and over the whole period of incubation. Without N fertilizer, the net nitrification rate in HTP soil was significantly higher than those in other soils, despite smallest pH. It was 3.01 and 15.10 times higher than those of the vegetable and forest soils, respectively in the first 3 d of incubation (). The −N concentrations steadily increased with the incubation time in HTP, Vegetable and MTP soils, and only slightly increased in the LTP and forest soils (). The
−N concentrations were low, less than 5 mg kg−1 and relatively stable in all the soils during the incubation (data not shown).
Figure 1. Nitrate change in different soils incubated without nitrogen (N) fertilizer. Soil types: HTP, high tea production; MTP, middle tea production; LTP, low tea production; F, forest; V, vegetable. –N, nitrate N. Vertical bars show standard deviations.
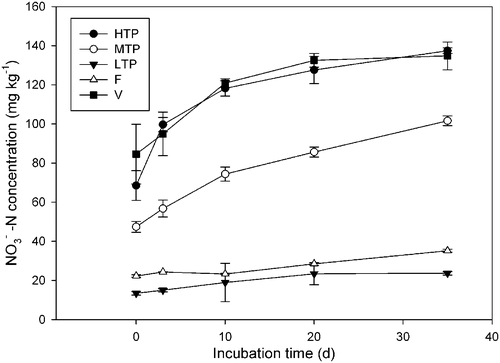
Table 2. Net nitrification rates (mg kg−1 d−1) with nitrogen (N) fertilizer (ammonium sulfate) and without N, incubated for 3 and 35 d in different types of soils
Following the addition of (NH4)2SO4, the net nitrification rate (nitrification potential) was quite different among the soils. It suddenly and significantly increased from 3.45 mg kg−1 d−1 without N addition to 65.13 mg kg−1 d−1 with N addition during the first 3 d of incubation in the vegetable soil. It also significantly increased in MTP and LTP soils. However, the net nitrification rate was even slightly lower in HTP and forest soils (). During the whole period of incubation, all the soils had significantly higher net nitrification rates than those incubated without N fertilizer (). A two-way ANOVA test with type of soil and treatment (with and without N fertilizer) as factors show that very significant differences were found among the soils and between the treatments. The added to the vegetable soil was completely nitrified during the first 3 d, while that in the tea and forest soils was slowly and steadily nitrified ( a and b). About 82% of the
in HTP soil and 60% in MTP, LTP and forest soils was nitrified, respectively, during the 35 d of incubation. The mineral N significantly increased in HTP and vegetable soils after incubation for 35 d, but slightly decreased in MTP soil, and significantly decreased in LTP and forest soils ().
Gross nitrification and microbial consumption
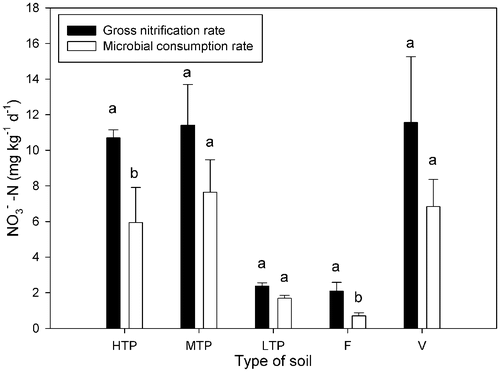
The gross nitrification rates were both high and comparable between HTP, MTP and vegetable soils, and significantly higher than the low rates in LTP and forest soils, respectively (). There were no significant differences between the rates in HTP, MTP and vegetable soils and between LTP and forest soils. About 30 to 70% of the N mineralized to −N was consumed by soil microbes. It was lowest in the forest soil, and around 60% in the other soils. There were significant differences between gross nitrification and microbial consumption in HTP and forest soils.
Figure 3. Gross nitrification rate and nitrate consumption in different soils. Soil types: HTP, high tea production; MTP, middle tea production; LTP, low tea production; F, forest; V, vegetable. –N, ammonium N. Vertical bars show standard deviations. Different letters denote significant differences (p < 0.05) between gross nitrification rate and microbial consumption rate in the same soil.
DISCUSSION
Our survey of different soil types shows that soil pH did not influence net and gross nitrification rates. The HTP soil, at pH 3.32, had a similar nitrification rate to the vegetable soil at pH 7.18 ( and ). The net nitrification rate in HTP soil incubated without N fertilizer was even higher than that in the vegetable soil (), indicating that nitrifying microorganisms exist in the very acid tea soils. This result coincides with previous findings that strong nitrification occurred in acid soil with a pH range from 3.71 to 4.22 (Xue et al. Citation2006), and the lowest limit for nitrification could be around pH 2.9 (Hayatsu Citation1993). Substantial evidence supports the role of chemolitho-autotrophic bacteria as the main nitrifying agents in most acid soils (De Boer and Kowalchuk 2001) and other microorganisms such as archaea and heterotrophs also make great contribution to nitrification in acid soils (Hayatsu et al. Citation2008). Acidophilic autotrophic ammonia oxidizing bacteria have been isolated in tea soils (Walker and Wickramasinghe Citation1979; Hayatsu Citation1993). Recent research shows that ammonia-oxidizing archaea play a significant role in a wide range of soil ecosystems (He et al. Citation2007; Nicol et al. Citation2008; Di et al. Citation2009). There was a significant relationship between ammonium-oxidizing archaea abundance and nitrification potential, but not between ammonia-oxidizing bacteria abundance and nitrification potential, and an exponential increase in the ratio of amoA ammonia-oxidizing archaea to bacterial gene copies with decreasing soil pH values in the tea soils (Yao et al. Citation2011). The high abundance of ammonia-oxidizing archaea could compensate for the relatively low number of ammonia-oxidizing bacteria in highly acid tea soils.
However, probably due to the different levels of soil pH and sizes of the nitrifying microorganisms, the nitrification pattern was quite different between the low pH tea and forest soils and the high pH vegetable soil. The as (NH4)2SO4 added to the tea and forest soils was slowly and steadily nitrified, and that added to the vegetable soil was immediately nitrified (). Under aerobic conditions, nitrification in high pH soils is generally rapid during the initial period of incubation, and ultimately decreases due to the limitation of
availability (Khalil et al. Citation2007). This can be described by first-order kinetics (Aulakh et al. Citation1996). The nitrification pattern in low pH soils is approximately linear and Ross et al. (Citation2004) further classified the net nitrification rates into three temporal patterns: high net nitrification with minimal
accumulation, high net nitrification and high
accumulation, and minimal net nitrification and moderate
accumulation.
We found all tea and forest soils had very low accumulation with varying nitrification rates. The vegetable soil had the optimum pH for producing maximum activities of microbial populations, including the nitrifying and other microorganisms. The highest microbial biomass concentration was in the vegetable soil (). A significantly and positively exponential relationship was found between the gross nitrification rate and microbial biomass C (R
2 = 0.271, n = 15, p < 0.05). The relatively low nitrification rates in tea and forest soils were due to the limited number of nitrifiers, especially the chemolitho-autotrophic microbes (De Boer and Kowalchuk 2001) and probably other soil characteristics, such as nutrient availability, C/N ratio and plant species (Ste-Marie and Pare Citation1999; Khalil et al. Citation2007). The present study shows that both net and gross nitrification rates were significantly related to soil total organic C and total N regardless of soil pH. The net nitrification rate during 35 d of incubation were significantly correlated with total C and N (R2 = 0.681 and 0.742 respectively, n = 15, p < 0.001). The gross nitrification rate was also significantly correlated with soil total organic C and total N (R2 = 0.528 and 0.456 respectively, n = 15, p < 0.01), but not with soil C/N ratio. The slower nitrification rate in tea soils was also probably due to the higher Al and polyphenol concentrations caused by incorporation of tea litter and prunings (Tate Citation1995; Hättenschwiler and Vitousek Citation2000; Kraus et al. Citation2004; Peng et al. Citation2008). The slower nitrification rate would be beneficial to increase N use efficiency in tea soils since tea prefers
to
as a source of N. It will also reduce
lost by leaching and denitrification.
According to Stark and Hart (Citation1997), acid forest soils have high gross nitrification rates because of the substantial microbial assimilation of , despite net measurements showing low
accumulation and even negative nitrification rates. The present study, in agreement with this, also found that the acid tea soils had high gross nitrification and microbial
consumption rates. Microbial N consumption in tea soils was similar to the vegetable soil, accounting for 39 to 73% of the mineralized
−N, which is even higher than the adjacent frost soil (34%). The decreased extractable mineral N concentrations after incubation for 35 d in MTP, LTP and forest soils () also indicate the possibility of net N immobilization in these soils. Of course, it would be possibly due to the loss of mineral N through volatilization (NH3) and denitrification (N2O and N2) since tea soils had relatively higher N2O emission, especially when soils were very acid and large quantities of N fertilizers were applied (Tokuda and Hayatsu Citation2000; Yokoyama and Ohama Citation2005; Lin and Han Citation2009).
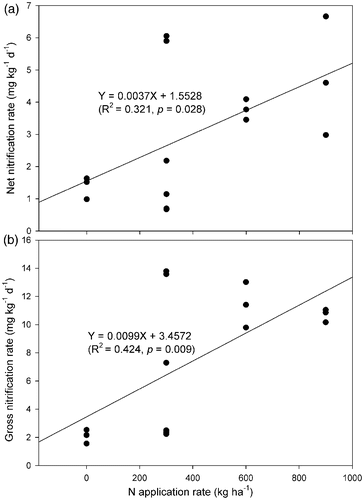
In contrast to the forest soil, the tea soils had higher net nitrification rates and accumulation, which is in line with Hayatsu (Citation1993) and Xue et al. (Citation2006). This is probably due to a high N fertilizer application rate in tea soils. Previous studies showed that fertilized soils had higher nitrifying activity due to the increased release of ammonium from fertilizers and the accelerated growth of nitrifying populations (Martikainen Citation1985; Hayastu and Kosuge 1993; Aarnio and Martikainen Citation1996; Mendum et al. Citation1999; Chu et al. Citation2008; Xue et al. Citation2009). The present study also shows that N fertilizer had a strong stimulating effect on soil nitrification. The net nitrification rates after incubation for 35 d showed a very significant difference between the treatments with and without N fertilizer addition in all tested soils (). Further analysis shows that the higher N fertilizer application rates, the higher the nitrification rates. The net and gross nitrification rates were significantly correlated with N application rates, the correlation coefficients (R
2) were 0.321 and 0.424 (n = 15), respectively (). If vegetable soils are excluded, as they are quite different from tea and forest soils, the correlation coefficients (R
2) between net and gross nitrification rates and N application rate would increase to 0.653 and 0.758 (n = 12, p < 0.001), respectively, indicating that soil nitrification was significantly influenced by N management. In addition, the high accumulation of
in tea soils can be partly attributed to the plant itself as tea is a crop preferring
to
. Tea plants assimilated twice as much
as
in a solution with same concentrations of
and
during 24 h hydroculture (Morita et al.
Citation1998). And the significant difference between the gross nitrification and microbial consumption in HTP soil () indicates microbial N consumption is relatively lower compared to the vegetable soil. Due to the large amount N fertilizer applied in this soil,
accumulation would occur, resulting in leaching to the nearby water resources and possible release of greenhouse gases into the atmosphere by denitrification.
Figure 4. Relationships between nitrogen (N) application rate and net (a) and gross (b) nitrification rates.
We conclude that the types of land use and their management are more important than soil pH for soil nitrification. High N application rate in tea fields increases soil nitrification rate, accumulation and the risk of environmental pollution. Therefore, the strategy of high application of N fertilizer in tea soils should be changed and a more appropriate N fertilizer application, in balance with plant N requirements, should be adopted.
ACKNOWLEDGEMENTS
This work was supported by the Ministry of Science and Technology of China (No. 2011BAD01B02, 2011CB100502) and the National Natural Science Foundation (No. 40771113, 41171218). We appreciate Prof. P. C. Brookes in Rothamsted Research and Dr. C. Abeysinghe in Wayamba University of Sri Lanka for their meaningful comments and suggestions.
REFERENCES
- Aarnio , T and Martikainen , PJ . 1996 . Mineralization of carbon and nitrogen, and nitrification in Scots pine forest soil treated with fast- and slow-release nitrogen fertilizers . Biol. Fertil. Soils , 22 : 214 – 220 .
- Aulakh , MS , Kuldip , S , Bijay , S and Doran , JW . 1996 . Kinetics of nitrification under upland and flooded soils of varying texture . Commun. Soil Sci. Plant Anal. , 27 : 2079 – 2089 .
- Chu , HY , Fujii , T , Morimoto , S , Lin , XG and Yagi , K . 2008 . Population size and specific nitrification potential of soil ammonia oxidizing bacteria under long-term fertilizer management . Soil Biol. Biochem. , 40 : 1960 – 1963 .
- Davidson , EA , Hart , SC and Firestone , MK . 1992 . Internal cycling of nitrate in soils of a mature coniferous forest . Ecology , 73 : 1148 – 1156 .
- De Boer , W and Kowalchuk , GA . 2001 . Nitrification in acid soils: microorganisms and mechanisms . Soil Biol. Biochem. , 33 : 853 – 866 .
- Di , HJ , Cameron , KC , Shen , JP , Winefield , CS , Bowatte , S and He , JZ . 2009 . Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils . Nat. Geosci. , 2 : 621 – 624 .
- Han , WY , Kemmitt , S and Brookes , P . 2007 . Soil microbial biomass and its activities in tea (Camellia sinensis) garden with relation to the different stand age and productivity . Soil Biol. Biochem. , 39 : 1468 – 1478 .
- Han , WY , Ruan , JY and Lin , Z . 2002 . The major nutrient limiting factors in tea soils and development of series tea speciality fertilizers . J. Tea Sci. , 22 : 70 – 74 . (in Chinese with English abstract)
- Hart , SC , Stark , JM , Davidson , EA and Firestone , MK . 1994: Nitrogen mineralization, immobilization, and nitrification. In Methods of Soil Analysis Part 2. Microbiological and Biochemical Properties, Eds. Weaver RW, Angle S, Bottomley P, Bezdiecek D, Smith S, Tabatabai A, Wollum A, Mickelson SH, Bigham JM, pp. 985–1018. Soil Science Society of America, Madison
- Hättenschwiler , S and Vitousek , PM . 2000 . The role of polyphenols in terrestrial ecosystem nutrient cycling . Trends Ecol. Evol. , 15 : 238 – 243 .
- Hayatsu , M . 1993 . The lowest limit of pH for nitrification in tea soil and isolation of an acidophilic :ammonia oxidizing bacterium . Soil Sci. Plant Nutr. , 39 : 219 – 226 .
- Hayatsu , M and Kosuge , N . 1993 . Autotrophic nitrification in acid tea soils . Soil Sci. Plant Nutr. , 39 : 209 – 217 .
- Hayatsu , M , Tago , K and Saito , M . 2008 . Various players in the nitrogen cycle: Diversity and functions of the microorganisms involved in nitrification and denitrification . Soil Sci. Plant Nutr. , 54 : 33 – 45 .
- He , J , Shen , J , Zhang , L , Zhu , Y , Zheng , Y , Xu , M and Di , HJ . 2007 . Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices . Environ. Microbiol. , 9 : 2364 – 2374 .
- International Tea Committee (ITC) 2010: Annual Bulletin of Statistics. International Tea Committee, London
- Kemmitt , SJ , Wright , D and Jones , DL . 2005 . Soil acidification used as a management strategy to reduce nitrate losses from agricultural land . Soil Biol. Biochem. , 37 : 867 – 875 .
- Khalil , MI , Rahman , MS , Schmidhalter , U and Olfs , HW . 2007 . Nitrogen fertilizer–induced mineralization of soil organic C and N in six contrasting soils of Bangladesh . Soil Sci. Plant Nutr. , 170 : 210 – 218 .
- Khan , SA , Mulvaney , RL and Brooks , PD . 1998 . Diffusion methods for automated nitrogen-15 analysis using acidified disks . Soil Sci. Soc. Am. J. , 62 : 406 – 412 .
- Kiml , YG , Ryul , HS and Leel , JH . 2002 . Nitrogen leaching volume based on soil property and fertilization method in Jeju Island's tea gardens . Proceedings of International Tea Symposium, compiled by the organizing committee, Shizuoka , Japan : 187 – 190 .
- Kirkham , D and Bartholomew , WV . 1954 . Equations for following nutrient transformations in soil, utilizing tracer data . Soil Sci. Soc. Am. Proc. , 18 : 33 – 34 .
- Kraus , TEC , Zasoski , RJ , Dahlgren , RA , Horwath , WR and Preston , CM . 2004 . Carbon and nitrogen dynamics in a forest soil amended with purified tannins from different plant species . Soil Biol. Biochem. , 36 : 309 – 321 .
- Kumazawa , K . 2002 . Nitrogen fertilization and nitrate pollution in groundwater in Japan: Present status and measures for sustainable agriculture . Nutr. Cycl. Agroecosys. , 63 : 129 – 137 .
- Li , H , Hirata , T , Matsuo , H , Nishikawa , M and Tase , N . 1997 . Surface water chemistry, particularly concentrations of and DO and δ15N values, near a tea plantation in Kyushu, Japan . J. Hydrol. , 202 : 341 – 352 .
- Lin , YD and Han , WY . 2009 . N2O emission from soils with different stands . J. Tea Sci. , 29 : 456 – 464 . (in Chinese with English abstract)
- Martikainen , PJ . 1985 . Numbers of autotrophic nitrifiers and nitrification in fertilized forest soil . Soil Biol. Biochem. , 17 : 245 – 248 .
- Mendum , TA , Sockett , RE and Hirsch , PR . 1999 . Use of molecular and isotopic techniques to monitor the response of autotrophic ammonia-oxidizing populations of the β subdivision of the class Proteobacteria in arable soils to nitrogen fertilizer . Appl. Environ. Microbiol. , 66 : 4155 – 4162 .
- Morita , A , Ohta , M and Yoneyama , T . 1998 . Uptake, transport and assimilation of 15N-nitrate and 15N-ammonium in tea (Camellia sinensis L.) plants . Soil Sci. Plant Nutr. , 44 : 647 – 654 .
- Nicol , GW , Leininger , S , Schleper , C and Prosser , JI . 2008 . The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria . Environ. Microbiol. , 10 : 2966 – 2978 .
- Nioh , I , Isobe , T and Osada , M . 1993 . Microbial biomass and some biochemical characteristics of a strongly acid tea field soil . Soil Sci. Plant Nutr. , 39 : 617 – 626 .
- Norton , JM and Stark , JM . 2011: Regulation and measurement of nitrification in terrestrial systems. In Methods in Enzymology, Vol. 486, Ed. Klotz MG, pp. 343–368. Elsevier, Oxford
- Pansombat , K , Kanazawa , S and Horiguchi , T . 1997 . Microbial ecology in tea soils I. soil properties and microbial populations . Soil Sci. Plant Nutr. , 43 : 317 – 327 .
- Paul , EA and Clark , FE . 1989 . Soil Microbiology and Biochemistry , San Diego : Academic Press .
- Peng , R , Guo , SM and Sun , ZY . 2008 . Effect of tea polyphenols on soil nitrification . Soil Fertil. Sci. China , 4 : 50 – 52 . (in Chinese with English abstract)
- Pennington , PI and Ellis , RC . 1993 . Autotrophic and heterotrophic nitrification in acidic forest and native grassland soils . Soil Biol. Biochem. , 25 : 1399 – 1408 .
- Robertson , GP . 1982 . Factors regulating nitrification in primary and secondary succession . Ecology , 63 : 1561 – 1573 .
- Ross , DS , Lawrence , GB and Fredriksen , G . 2004 . Mineralization and nitrification patterns at eight northeastern USA forested research sites . Forest Ecol. Manag. , 188 : 317 – 335 .
- Shi , S , Ding , R , Liu , Y and Sun , Y . 1999 . Acidification of soil by urea and fallen tea leaves . J. Tea Sci. , 19 : 7 – 12 . (in Chinese with English abstract)
- Sivaplan , K , Fernando , V and Thenabadu , MW . 1983 . Humified phenol-rich plant residues and soil urease activity . Plant Soil , 70 : 143 – 146 .
- Sivaplan , K , Fernando , V and Thenabadu , MW . 1985 . Mineralization in polyphenol-rich plant residues and their effect on nitrification of applied ammonium sulfate . Soil Biol. Biochem. , 17 : 547 – 551 .
- Song , M and Liu , Y . 1990 . Effect of biogeochemical cycle in tea garden on the soil acidification . J. Tea Sci. , 10(2) : 19 – 26 . (in Chinese with English abstract)
- Stark , JM and Hart , SC . 1997 . High rates of nitrification and nitrate turnover in undisturbed coniferous forests . Nature , 385 : 61 – 64 .
- Ste-Marie , C and Pare , D . 1999 . Soil, pH and N availability effect on net nitrification in the forest floors of a range of boreal forest stands . Soil Biol. Biochem. , 31 : 1579 – 1589 .
- Tate , RL . 1995 . Soil Microbiology , New York : Wiley .
- Tokuda , S and Hayatsu , M . 2000 . Nitrous oxide production from strongly acid tea field soils . Soil Sci. Plant Nutr. , 46 : 835 – 844 .
- Troelstra , SR , Wagenaar , R and De Boer , W . 1990 . Nitrate production in Dutch heathland soils . I. General soil characteristics and nitrification in undisturbed soil cores. Plant Soil , 127 : 179 – 192 .
- Vance , ED , Brookes , PC and Joergensen , RG . 1987 . An extraction method for measuring soil microbial biomass C . Soil Biol. Biochem. , 19 : 703 – 707 .
- Walker , N and Wickramasinghe , KN . 1979 . Nitrification and autotrophic nitrifying bacteria in acid tea soils . Soil Biol. Biochem. , 11 : 231 – 236 .
- Watanabe , I , Tokuda , S and Nonaka , K . 2002 . Nutrients leaching losses from lysimeter-grown tea plants fertilized at two rates of nitrogen . Tea Res. Rep. , 94 : 1 – 6 .
- Xue , D , Gao , YM , Yao , HY and Huang , CY . 2009 . Nitrification potentials of Chinese tea orchard soils and their adjacent wasteland and forest soils . J. Environ. Sci. , 21 : 1225 – 1229 .
- Xue , D , Yao , HY and Huang , CY . 2006 . Microbial biomass, N mineralization and nitrification, enzyme activities, and microbial community diversity in tea orchard soils . Plant Soil , 288 : 319 – 331 .
- Yang , Y . 2005: China Tea Cultivation, Shanghai Scientific and Technical Publishers, Shanghai
- Yao , H , Gao , Y , Nicol , GW , Campbell , CD , Prosser , JI , Zhang , L , Han , W and Singh , BK . 2011 . Links between ammonia oxidizer community structure, abundance, and nitrification potential in acidic soils . Appl. Environ. Microbiol. , 77 : 4618 – 4625 .
- Yokoyama , K and Ohama , T . 2005 . Effect of inorganic N composition of fertilizers on nitrous oxide emission associated with nitrification and denitrification . Soil Sci. Plant Nutr. , 51 : 967 – 972 .