Abstract
Lime-nitrogen (calcium cyanamide, CaCN2) is used as a nitrogenous fertilizer, pesticide, and herbicide. During the process of decomposition of lime-nitrogen in the soil, dicyandiamide (DCD), a nitrification inhibitor, is formed. Therefore, lime-nitrogen application may mitigate nitrous oxide (N2O) emission from the soil. We conducted a field experiment to investigate the effect of lime-nitrogen on nitrification and N2O emission in fertilized soils, and a soil incubation experiment for further analysis of the effect of the lime-nitrogen. In a field experiment we compared four nitrogen (N) fertilizer treatments: CF (chemical fertilizer), LN100 (application of all N fertilizer as lime-nitrogen), LN50 (application of 50% of N as lime nitrogen and the remainder as chemical fertilizer), and CFD (chemical fertilizer with DCD). In a soil incubation experiment, we also studied two nitrogen treatments: CF and lime-nitrogen. Soil nitrification activity was lower in the LN100, LN50, and CFD plots than in the CF plot. The duration of this reduction in soil nitrification activity was longer in the LN100 plot than in the other plots. We found an apparent decrease in the N2O emission rate between 7 and 14 days after fertilization in the LN100, LN50, and CFD plots compared with that in the CF plot. This period of decreased N2O emission paralleled that when DCD was detected in the topsoil layers of the former three plots. Moreover, in the soil incubation experiment, cumulative N2O emission was significantly lower in the lime-nitrogen treatment than in the CF treatment, although the difference in cumulative N2O emission among the plots was not significant in the field experiment. Correlation analysis suggested that application of lime-nitrogen affects N2O emission by controlling both the first (ammonium to nitrite) and the second (nitrite to nitrate) soil nitrification reactions, whereas DCD blocks only the first nitrification reaction.
Introduction
Nitrous oxide (N2O), which has 298 times the global warming potential of carbon dioxide (CO2), is an important greenhouse gas that also contributes to the destruction of stratospheric ozone (IPCC Citation2007). Moreover, Ravishankara et al. (Citation2009) showed that N2O is the most important ozone-depleting substance and is expected to remain most important throughout the 21st century. Therefore, in recent years, accurate estimation of N2O emission, elucidation of the mechanisms of N2O emission, and reduction of N2O emission have become increasingly important.
Two microbial processes contribute to most of the N2O production in soil: nitrification and denitrification. There are several anthropogenic and natural sources of N2O emission. Notably, agriculture is responsible for 42% of current anthropogenic N2O emission (IPCC Citation2007). Application of nitrogen (N) fertilizer to the soil enhances nitrification and denitrification activity.
The use of nitrification inhibitors (NIs) has been studied extensively as a mitigation option for N2O emission (Weiske et al. Citation2001; Singh et al. Citation2008; Chen et al. Citation2010). NIs have been developed to increase the nitrogen-use efficiency of crops. There are many types of NIs, such as dicyandiamide (DCD), nitrapyrin, and 3,4-dimethylpyrazole phosphate. Akiyama et al. (Citation2010) reported that application of NIs instead of conventional fertilizers significantly reduces N2O emission, by an average of 38%. The effect on N2O emission differs among the different types of NIs (e.g., Akiyama et al. Citation2010). Therefore, understanding the effect of NIs on N2O emission requires in situ studies that compare emissions resulting from the application of different types of NIs and of fertilizers applied with a nitrification inhibitor.
Lime-nitrogen (calcium cyanamide) is used as a nitrogenous fertilizer, pesticide, and herbicide. Lime-nitrogen is produced by N2 fixation to calcium carbide (CaC2). Lime-nitrogen contains approximately 55% calcium cyanamide (CaCN2), with calcium oxide (CaO) and carbon (C). In the soil, CaCN2 is hydrolyzed into cyanamide (H2CN2) and lime [Ca(OH)2]. Thereafter, H2CN2 is converted into urea and DCD. The DCD formed during the process of decomposition of lime-nitrogen may affect N2O emission from soils, as does the direct application of DCD to soils (e.g., Di et al. Citation2010). However, there have been few studies of the effectiveness of lime-nitrogen in reducing N2O emission.
Haenseler and Moyer (Citation1937) and Klasse (Citation1996) reported that CaCN2 has a marked influence on populations of soil bacteria and fungi. Therefore, lime-nitrogen may mitigate N2O emission via mechanisms different from those of common NIs, which inhibit only the first stage of nitrification [ammonium () to nitrite (
)]. Lime-nitrogen may also affect CO2 emission by altering the activity of a wide range of soil bacteria and fungi, and it may affect methane (CH4) uptake by the soil by altering methanotroph activity.
By means of field and soil incubation experiments, we therefore aimed to quantify the effect of lime-nitrogen in mitigating N2O emission from an Andosol field and to investigate the environmental factors controlling N2O emission. The effects of lime-nitrogen on CO2 and CH4 fluxes were also investigated.
Materials and Methods
Field experiment
Site description
The study site was located at the National Institute for Agro-Environmental Sciences (NIAES), Tsukuba, Ibaraki, Japan (36°01′N, 140°07′E). On the basis of 30 years of observations (1971–2000, Japan Meteorological Agency), the annual mean air temperature was 13.5°C and the total annual precipitation averaged 1236 mm. The study site had an area of 600 m2 (15 × 40 m). The soil type was an Andosol. The pH (H2O) of the topsoil (0 to 10 cm) was 6.31 and the bulk density was 0.59 g cm−3.
Experimental design
We established 12 study plots. Each plot had an area of 30 m2 (5 × 6 m). The field experiment was conducted from 5 October 2010 to 24 December 2010. Komatsuna (Brassica rapa L. var. perviridis L.H. Bailey) was cultivated. Each plot had 20 rows. The rows were spaced at 30 cm. Plots were laid out in a randomized block design based on four nitrogen fertilizer treatments:
| 1. | CF plot: All fertilizer was applied as chemical compound fertilizer, which contained 8% nitrogen (N, as ammonium nitrogen), 8% phosphorus (P2O5), and 8% potassium (K2O), by weight. | ||||
| 2. | LN100 plot: All N fertilizer was applied as lime-nitrogen (containing 20% N by weight). P2O5 and K2O were applied as calcium superphosphate and chloride of potash, respectively. | ||||
| 3. | LN50 plot: Fifty percent of the N fertilizer was applied as lime-nitrogen and the rest was applied as chemical compound fertilizer (8% N, as ammonium nitrogen, plus 8% P2O5 and 8% K2O). The remaining P2O5 and K2O were applied as calcium superphosphate and chloride of potash, respectively. | ||||
| 4. | CFD plot: All fertilizer was applied as chemical compound fertilizer containing DCD. The fertilizer contained 15% N (as ammonium nitrogen), 15% P2O5, 15% K2O, and 2.4% DCD, by weight. | ||||
Total application rates of N, P2O5, and K2O were 120 kg N ha−1, 120 kg P2O5 ha−1, and 120 kg K2O ha−1 in each treatment. Each fertilizer was applied by incorporation into the soil (to a depth of 15 cm) using a rotary tiller. The dates of fertilization, seeding, and harvest were 5 October, 15 October, and 6 December 2010, respectively.
Measurement of N2O, CO2, and CH4 fluxes and of environmental factors
We measured N2O, CO2, and CH4 fluxes using the closed static-chamber technique. Measurements were taken four times per week during the first two weeks after fertilization, and thereafter three times per week until harvest and once per week after harvest. We inserted a collar (25 cm diameter, 10 cm height) 5 cm into the soil at the center of each plot the day before the first gas sampling to reduce the influence of soil disturbance on the gas fluxes. The collars were positioned between the rows, with no seeds inside the collars. For each gas flux measurement, gas samples were withdrawn from the headspace into 15-mL evacuated vials, 0, 5, 10, 15, 20, and 30 min after the systems had been closed (at 0, 10, 20, and 30 min for N2O, and at 0, 5, 10, and 15 min for CO2 and CH4). The rate of increase in the concentration of each gas in the chambers was determined by linear regression analysis. Only those samples with a regression correlation coefficient greater than 0.80 were used for further correlation analysis between gas fluxes and environmental factors. Cumulative gas losses were calculated using a trapezoidal method. All gas samples were analyzed with an automated gas analysis system (Sudo Citation2006), which consisted of two gas chromatographs (GC-14B, Shimadzu, Kyoto, Japan) equipped with a thermal conductivity detector, a flame ionization detector, and an electron capture detector. For more information on the gas chromatographs, see the work of Minamikawa et al. (Citation2010). We adopted the sign convention of CH4 emission as positive and CH4 uptake as negative.
From 5 October to 24 December 2010, we monitored the daily changes in soil temperature at a depth of 5 cm, and the volumetric soil water content at a depth of 5 cm, every 30 min using a thermistor and an ECH2O probe (soil temperature ECT; Decagon, Pullman, WA, USA; volumetric soil water content, EC-5; Decagon). The calibration curve for the Andosol was determined by adding a known amount of water to a container packed with oven-dried soil. The absolute value of the volumetric soil water content was then determined using a calibration curve (y = 0.95x + 0.086, r 2 = 0.99). Volumetric soil water content was converted to the water-filled pore space (WFPS) using the soil porosity value.
Soil samples were collected to a depth of 5 cm for analysis of pH and the contents of inorganic N [ammonium-nitrogen (NH4-N), nitrite-nitrogen (NO2-N), and nitrate-nitrogen (NO3-N)], cyanamide (H2CN2), and dicyandiamide (DCD). The pH of the soil samples was measured at a 1:2.5 weight/volume (w/v) soil to water ratio with an electrode-type pH meter (model FE20, Mettler Toledo AG, Schwerzenbach, Switzerland). Analytes were extracted from the soil samples in 10% potassium chloride (KCl) or distilled water (soil to 10% KCl = 1:10 w/v for soil inorganic N content; soil to water = 1:4 w/v for contents of H2CN2 and DCD). The contents of inorganic N were measured with a continuous-flow analyzer (model TRAACS2000, Bran + Luebbe, Norderstedt, Germany). The soil H2CN2 and DCD contents were analyzed using high-performance liquid chromatography (model L-6200, Hitachi High-Technologies Co. Ltd., Tokyo, Japan) with a UV-VIS detector (model L-4200 H, Hitachi High-Technologies Co. Ltd., detection wavelength 195 nm). Separation was achieved in a polymer-based column (model RSpak DE-613, Showa Denko Co. Ltd., Tokyo, Japan) with H2O as the mobile phase (Owa Citation2010). Soil nitrification activity (ammonium oxidation activity) was determined in accordance with the methods of Belser and Mays (Citation1980), with slight modifications.
Before fertilization, there were small differences among the plots in the N2O, CO2, and CH4 fluxes; in soil pH and the soil inorganic-N, H2CN2, and DCD contents; or in soil ammonium oxidation activity ( and ). However, these differences were not statistically significant.
Figure 1. Temporal variations in (a) daily precipitation (bars) and soil temperature (line) at a depth of 5 cm, (b) water-filled pore space (WFPS), (c) nitrous oxide (N2O) emission, (d) carbon dioxide (CO2) emission, and (e) methane (CH4) flux over the course of the field experiment in the chemical fertilizer (CF), application of all nitrogen (N) fertilizer as lime-nitrogen (LN100) treatment, application of 50% of N as lime-nitrogen and the remainder as CF (LN50) treatment, and CF with dicyandiamide (CFD) plots. Error bars represent standard deviation (n = 3).
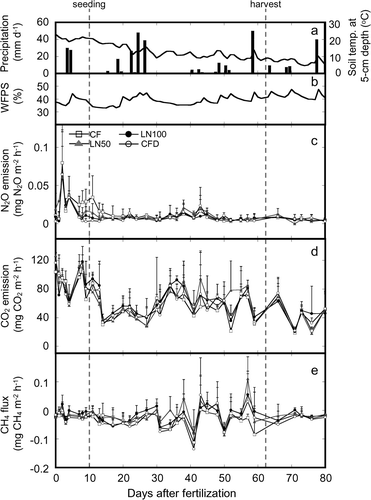
Figure 2. Relationships between nitrous oxide (N2O) emission and soil temperature at 5 cm depth in the chemical fertilizer (CF) treatment, application of all nitrogen (N) fertilizer as lime-nitrogen (LN100) treatment, application of 50% of N as lime-nitrogen and the remainder as CF (LN50) treatment, and CF with dicyandiamide (CFD) treatment plots.
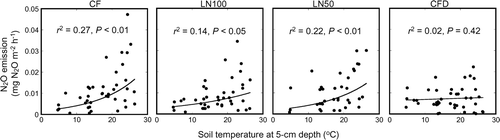
Figure 3. Temporal variations in (a) pH, and in the contents of (b) cyanamide (H2CN2), (c) dicyandiamide (DCD), (d) ammonium-nitrogen (NH4-N), (e) nitrite-nitrogen (NO2-N), and (f) nitrate-nitrogen (NO3-N), and in (g) ammonium oxidation activity in the topsoil layer (0 to 5 cm) over the course of the field experiment in the chemical fertilizer (CF) treatment, application of all nitrogen (N) fertilizer as lime-nitrogen (LN100) treatment, application of 50% of N as lime-nitrogen and the remainder as CF (LN50) treatment, and CF with DCD (CFD) treatment plots. Error bars represent standard deviation (n = 3). DW, dry weight.
Incubation experiment
A soil incubation experiment was conducted to further analyze the effect of lime-nitrogen on N2O emission. Soil was collected from a depth of 0 to 10 cm from the same site used in the field experiment, before N fertilization. Soil was sieved through a 2-mm mesh and stored at 4°C until the incubation experiment. For the incubation experiment, 10 g of soil (oven-dry equivalent) was placed in a 125-mL glass bottle to achieve a bulk density of 0.60 g cm−3 (similar to field conditions) and the soil water content was adjusted to 60% WFPS. Two nitrogen treatments were studied: chemical fertilizer (CF treatment, containing 8% N by weight) and lime-nitrogen (LN treatment, containing 21% N by weight). Each treatment received 2 mg N per 10 g dry weight (DW) of soil. In total, 36 soil bottles were prepared (i.e., 18 samples for each treatment). After a 3-day pre-incubation at 25°C, chemical fertilizer or lime-nitrogen was applied to each soil bottle, and the soil bottles were incubated at 25°C for 60 days. During the incubation experiment, the WFPS was maintained by monitoring the water loss (based on changes in weight of the bottles) and adding deionized water.
For gas measurement, three samples were selected at random from each treatment. Gas samples were withdrawn from the headspace of each glass bottle and injected into a 15-mL evacuated vial 0 and 30 min after the glass bottle had been closed with a butyl-rubber stopper. After gas sampling, soil in one of the glass bottles was used for analysis of pH, inorganic-N, H2CN2, and DCD, and the other two glass bottles were incubated for the next samplings. The gaseous N2O concentration, pH, and contents of inorganic-N, H2CN2, and DCD were determined as described above.
Statistical analysis
The significance of the differences among the four fertilized plots was assessed by one-way analysis of variance (ANOVA, P = 0.05). After one-way ANOVA, differences in gas fluxes and environmental factors among the four fertilized plots were analyzed using Tukey's test. Pearson's correlation coefficient was used to identify significant associations between the N2O emission rate and environmental factors. The significance of the difference in cumulative N2O emissions between the CF treatment and the LN treatment in the incubation experiment was tested with a t-test. All statistical analyses were performed with SPSS ver. 11.0 (SPSS Inc., Chicago, IL, USA).
Results
Temporal variations in N2O emission
Mean soil temperature at a depth of 5 cm, WFPS, and total precipitation over 80 days were 12.4°C, 39.5% and 351 mm, respectively ().
N2O emission rates increased after fertilization and then either decreased or remained relatively high during the first 2 weeks after fertilization, depending on the fertilized plot (). N2O emission peaks were observed 2 days after fertilization in all fertilized plots. The highest N2O emission rate was observed in the CF plot (0.079 mg N2O m−2 h−1). Peak N2O emission rates in the LN100, LN50, and CFD plots were 0.065, 0.061, and 0.064 mg N2O m−2 h−1, respectively. There were clear but not significant (P > 0.05) differences in N2O emission rates from 7 to 14 days after fertilization among the fertilized plots: a greater decrease in N2O emission rate was observed in the LN100, LN50, and CFD plots than in the CF plot. Moreover, N2O emission from the CFD plot was lower than that from the LN100 and LN50 plots during this period. After 17 days, there were no apparent differences in N2O emissions among the fertilized plots.
Correlation analysis showed no consistent pattern of correlation between the N2O emission rate and environmental factors across the four fertilized plots (). N2O emission responded differently to changes in the environmental factors in each fertilized plot. There was a significant positive correlation between N2O emission and soil content in the CF plot. N2O emission in the CF, LN100, and LN50 plots was significantly and exponentially correlated with soil temperature at 5 cm depth, and soil temperature accounted for approximately 14% to 27% of the variation in N2O emission in these plots (). In addition, there was a significant positive correlation between N2O emission and soil
content in the CF and LN50 plots. N2O emission in the LN100 and LN50 plots was significantly positively correlated with the soil H2CN2 content. The pH, WFPS, and contents of
and DCD in the soil were not significantly correlated with N2O emission.
Table 1 Pearson's correlation coefficients for the relationships between the nitrous oxide (N2O) emission rate and soil environmental factors [pH, soil temperature at a depth of 5 cm (ST5), water-filled pore space (WFPS)] and the contents of ammonium-nitrogen (NH4-N), nitrite-nitrogen (NO2-N), nitrate-nitrogen (NO3-N), cyanamide (H2CN2), and dicyandiamide (DCD) in each fertilized plot
Cumulative N2O emissions over 80 days differed among the fertilized plots. Cumulative N2O emissions in the CF (15.3 ± 12.8 mg N2O m−2; mean ± SD) and LN50 (15.1 ± 4.5 mg N2O m−2; mean ± SD) plots were higher than those in the LN100 (13.2 ± 6.1 mg N2O m−2; mean ± SD) and CFD (10.6 ± 2.7 mg N2O m−2; mean ± SD) plots. Cumulative N2O emissions in the LN100, LN50, and CFD plots were 13.8%, 1.3%, and 30.9% lower, respectively, than those in the CF plot. However, the differences in cumulative N2O emissions among the fertilized plots were not significant.
Fluxes of CO2 and CH4
There were no apparent differences in the temporal variation in CO2 emission and CH4 flux among the fertilized plots (). Moreover, both cumulative CO2 emission and cumulative CH4 flux over 80 days did not differ significantly among the fertilized plots [CF plot (mean ± SD): 105.3 ± 22.8 mg CO2 m−2, −0.06 ± 0.007 mg CH4 m−2; LN100 plot: 116.7 ± 4.2 mg CO2 m−2, −0.03 ± 0.005 mg CH4 m−2; LN50 plot: 93.9 ± 14.4 mg CO2 m−2, −0.07 ± 0.05 mg CH4 m−2; CFD plot: 91.1 ± 13.6 mg CO2 m−2, −0.08 ± 0.04 mg CH4 m−2].
Changes in soil pH and H2CN2 and DCD contents
The temporal variation in soil pH was similar in all fertilized plots (). Soil pH varied within a range of 1.0 units in all plots (CF plot: 5.4–6.3; LN100 plot: 5.9–6.5; LN50 plot: 5.7–6.3; CFD plot: 5.8–6.5). In the LN100 plot, soil pH from 9 to 76 days after fertilization was higher than in the other plots. Soil pH was lower in the CF plot than in the other plots until the end of the experiment, except at 48 and 76 days after fertilization. After 34 days, there was little temporal variation in soil pH in any of the plots.
H2CN2 was detected in the LN100 and LN50 plots (). The soil H2CN2 content was highest 1 day after fertilization, and then decreased rapidly. The timing of the disappearance of H2CN2 differed between the LN50 plot (7 days after fertilization) and the LN100 plot (9 days after fertilization). DCD was detected in all plots except the CF plot (). In the LN100 and LN50 plots, soil DCD content increased after fertilization and then decreased. In contrast, the highest soil DCD content was observed in the CFD plot, immediately after fertilization. The timing of the disappearance of DCD differed among the fertilized plots (LN100 plot: 66 days after fertilization; LN50 plot: 55 days after fertilization; CFD plot: 48 days after fertilization).
Soil mineral nitrogen
The soil content in all fertilized plots peaked 1 to 3 days after fertilization (). The highest
value was observed in the LN50 plot. In the LN100 plot, soil
content remained high until 55 days after fertilization, unlike in the other plots, in which the soil
content decreased to 0 within around 40 days. There were three peaks in soil
content in all fertilized plots (). In the LN100 plot, soil
content tended to be higher than that in the other plots, particularly at 20 days after fertilization [LN100 plot (mean ± SD): 0.05 ± 0.02 mg N kg DW soil−1; CF plot: 0.03 ± 0.02 mg N kg DW soil−1; LN50 plot: 0.03 ± 0.03 mg N kg DW soil−1; CFD plot: 0.03 ± 0.02 mg N kg DW soil−1]. A soil
content peak was observed after the soil
content peak in all plots (). The soil
content differed among plots. It was higher in the CF plot than in other plots until 27 days after fertilization; it then declined and remained lower than in the other plots until the end of the experiment. The correlations between soil mineral nitrogen contents and the soil H2CN2 and DCD contents differed among plots (). In the LN100 plot, the soil H2CN2 content was significantly positively correlated with the soil
content and significantly negatively correlated with the soil
content. In the LN50 plot, the soil H2CN2 content was significantly positively correlated with the soil
and
contents. In the CFD plot, soil H2CN2 could not be detected, so there were no correlations. Soil DCD content was positively correlated with soil
content in the CFD plot. Soil DCD content was positively correlated with soil
content in all plots except the CF plot. In the LN100, LN50, and CFD plots, soil DCD content accounted for approximately 37% to 56% of the variation in soil
content ().
Figure 4. Relationships between soil ammonium-nitrogen (NH4-N) content and soil dicyandiamide (DCD) content in the application of all nitrogen (N) fertilizer as lime-nitrogen (LN100) treatment, application of 50% of N as lime-nitrogen and the remainder as chemical fertilizer (LN50) treatment, and chemical fertilizer with DCD (CFD) plots. DW, dry weight.
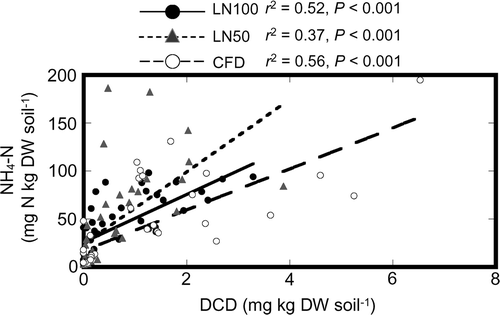
Table 2 Pearson's correlation coefficient between soil ammonium-nitrogen (NH4-N), nitrite-nitrogen (NO2-N), and nitrate-nitrogen (NO3-N) contents and soil cyanamide (H2CN2) and dicyandiamide (DCD) contents
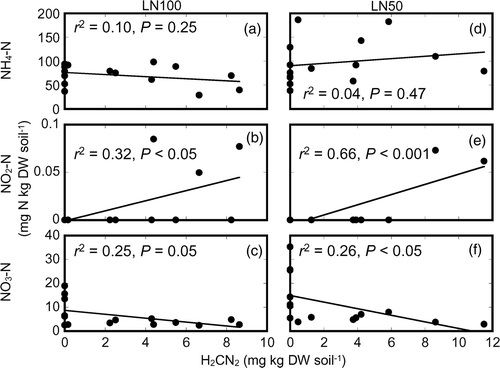
shows the relationships between the soil mineral nitrogen contents and the soil H2CN2 content during the period in which H2CN2 was detected in the soil of the LN100 and LN50 plots (LN100 plot: from days 1 to 9 after fertilization; LN50 plot: from days 1 to 7 after fertilization). Soil content was not significantly correlated with soil H2CN2 content in either plot. The soil
content in the LN100 and LN50 plots was significantly correlated with the soil H2CN2 content (). Soil
content tended to decrease with increasing soil H2CN2 content in the LN100 and LN50 plots (; LN100 plot: r
2 = 0.25, P = 0.05; LN50 plot: r
2 = 0.26, P < 0.05).
Figure 5. Relationships between soil mineral nitrogen (N) content and the soil cyanamide (H2CN2) content during the restricted period in which H2CN2 was detected in the soil of the application of all N fertilizer as lime-nitrogen (LN100) treatment plot (a, b, c) and the application of 50% of N as lime-nitrogen and the remainder as chemical fertilizer (LN50) treatment plot (d, e, f). NH4-N, ammonium-N; NO2-N, nitrite-N; NO3-N, nitrate-N; DW, dry weight.
Soil ammonium oxidation activity
In the CF plot, soil ammonium oxidation activity increased after fertilization. It was higher in the CF plot than in the other plots until the end of the experiment (). In the LN100, LN50, and CFD plots, soil ammonium oxidation activity decreased until 7 days after fertilization and then increased. In the LN100 plot, soil ammonium oxidation activity was lower than in the other fertilized plots from 14 to 76 days after fertilization.
Crop yield
Average aboveground crop yields in the CF, LN100, LN50, and CFD plots were 195.7 ± 20.2, 246.4 ± 32.9, 233.5 ± 9.1, and 222.3 ± 36.8 g DW m−2 (mean ± SD), respectively. The aboveground crop yields in the LN100, LN50 and CFD plot were higher (by 20% ± 6.9%, mean ± SD) than in the CF plot. However, there were no significant differences in aboveground crop yield among the treatments.
Incubation experiment
There was an apparent difference between the CF and LN treatments in the N2O emission and in its pattern of change until 10 days after N application (). The N2O emission rates peaked in the CF and LN treatments 10 and 13 days, respectively, after N application. In addition, there was a significant difference in N2O emissions between the CF and LN treatments from days 1 to 3 after N application (P < 0.05); during this time, N2O emission was higher in the CF treatment. Furthermore, cumulative N2O emission over 60 days in the LN treatment (9.80 ± 0.94 µg N2O g DW soil−1, mean ± SD) was significantly lower (by 16.2%; P < 0.05) than in the CF treatment (11.7 ± 0.32 µg N2O g DW soil−1, mean ± SD).
Figure 6. Temporal variations in (a) nitrous oxide (N2O) emission, (b) pH, and contents of (c) cyanamide (H2CN2), (d) dicyandiamide (DCD), (e) ammonium-nitrogen (NH4-N), (f) nitrite-nitrogen (NO2-N), and (g) nitrate-nitrogen (NO3-N) in the soil during the incubation experiment in the chemical fertilizer (CF) and lime-nitrogen (LN) treatments. Error bars represent standard deviation (n = 3). DW, dry weight.
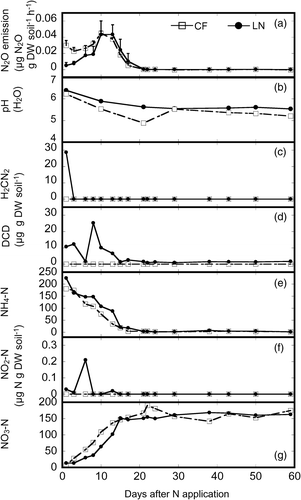
Both H2CN2 and DCD were observed in the soil only in the LN treatment (). The content of H2CN2 peaked 1 day after N application; H2CN2 then declined rapidly and was not detected for the remainder of the experiment. The soil DCD content peaked 8 days after N application and then decreased to around zero by 15 days after N addition. The soil DCD content remained constant and near zero from 22 days until the end of the experiment.
The soil content was highest immediately after N application and then decreased steadily in both treatments (). In the CF treatment, the soil
content had decreased to the baseline level by 17 days after N application, whereas it took 21 days to reach the baseline level in the LN treatment. The highest soil
content was observed 6 days after N application in the LN treatment (); in contrast, no soil
was detected in the CF treatment. The soil
content gradually increased after N application in both treatments; peaks occurred 22 and 38 days after N application in the CF and LN treatments, respectively ().
Discussion
Application of lime-nitrogen or DCD inhibited nitrification in the soil (). This finding was supported by our measurements of soil ammonium oxidation activity (). Thus, DCD derived from the lime-nitrogen in the LN100 and LN50 plots had nitrification-inhibition effects similar to those that occurred when DCD was directly applied to soil in the CFD plot. In the LN100 plot, however, nitrification was inhibited for longer than in the CFD plot. There was no apparent difference in the soil DCD content between the LN100 and CFD plots, except just after fertilization. Therefore, it is probable that the effects of CaCN2 itself and/or of H2CN2 derived from the lime-nitrogen were responsible for the differences in the magnitude and duration of nitrification inhibition between the LN100 and CFD plots.
In addition, we found an apparent decrease in N2O emission from 7 to 14 days after fertilization in the LN100, LN50, and CFD plots compared with N2O emission in the CF plot (). This period of lower N2O emission paralleled the period of DCD detection in the soil of the LN100, LN50, and CFD plots. Therefore, inhibition of nitrification in the soil by applied DCD or by DCD derived from the lime-nitrogen is likely to be an important factor in reducing N2O emission. Many previous studies have reported that the presence of DCD in soils reduces N2O emission by controlling nitrification (e.g., Li et al. Citation2009; Di et al. Citation2010). However, in the CFD plot, there was little decrease in N2O emission from days 1 to 3 after fertilization, when the highest soil DCD content was observed. Furthermore, soil DCD content was not significantly correlated with N2O emission in any of the fertilized plots. These results suggest that there is a time lag before DCD begins to decrease N2O emission.
It is also probable that the presence of H2CN2 in the soil, in addition to DCD derived from the lime-nitrogen, affected the decrease in N2O emissions from the LN100 and LN50 plots, because N2O emission from the LN treatment in the incubation experiment was lower than that in the CF treatment when the H2CN2 content was high in the LN treatment (). In the LN100 and LN50 plots, the some high soil contents were observed when the H2CN2 content was high (). The soil
content was significantly negatively correlated with the H2CN2 content (; ). Moreover, the soil
content was significantly positively correlated with the soil DCD content in the LN100, LN50, and CFD plots (). These results suggest that the presence of lime-nitrogen affects both the first (
to
) and the second (
to
) nitrification reactions in the soil, whereas the presence of DCD affects only the first reaction. Thus, components other than DCD that were derived from lime-nitrogen, such as lime and H2CN2, may have both directly and indirectly contributed to the difference in reduction of N2O emission between the LN plots (LN100 and LN50) and the CFD plot.
One possibility for the difference in reduction of N2O emission between the LN plots (LN100 and LN50) and the CFD plot is the production of N2O by chemodenitrification, a non-biological process, along with an increase in accumulation (van Cleemput and Samater Citation1996; Bremner Citation1997). Chemodenitrification is an important chemical process that is involved in N2O production in soils (Mørkved et al.
Citation2007), and intermediates between
and
—and
itself—can chemically decompose into N2O (Granli and Bøckman Citation1994). Our results suggest that lime-nitrogen may also affect
accumulation by controlling both the first and the second nitrification reactions. Moreover, the effects of CaCN2 on populations of various soil bacteria have been reported by Haenseler and Moyer (Citation1937). In the LN100 and LN50 plots, it is possible that decreased nitrite oxidizer activity as a result of the presence of CaCN2 itself or of H2CN2 derived from the lime-nitrogen affected
accumulation. Thus, the increase in production of N2O by chemodenitrification as a result of
accumulation could have affected N2O emission in the LN100 and LN50 plots.
accumulation in the soil of the LN100 and LN50 plots was consistent with the results of the incubation experiment ().
Arora et al. (Citation1987) reported that accumulation of in the soil is due to partial inhibition of the activity of Nitrobacter sp. Therefore, in our study, inhibition of the action of nitrifying bacteria as a result of lime-nitrogen fertilization could have affected both
accumulation and N2O emission. In addition, cumulative CH4 uptake in the LN100 plot was lower than that in the CF plot, although this difference was not significant. This result suggests that the CaCN2 and H2CN2 derived from the lime-nitrogen could also affect the oxidation of methane by bacteria, such as methane-oxidizing bacteria and ammonia-oxidizing bacteria (Arp and Stein Citation2003). In contrast, cumulative CO2 emissions did not differ among the treatments, and the effect of lime-nitrogen on soil microorganisms was therefore not clear from the soil respiration data. Further experimental approaches are needed to clarify the effects of lime and H2CN2 derived from lime-nitrogen on N2O emission and
accumulation and on fluxes of other greenhouse gases.
Furthermore, cumulative N2O emissions from the LN100 plot were lower than those from the LN50 plot, although the difference was not significant. The proportions of lime-nitrogen applied to the LN100 and LN50 plots were 100% and 50%, respectively, of the total N fertilizer applied. Thus, there was half as much lime and CaCN2 in the LN50 plot as in the LN100 plot. The decrease in soil DCD content occurred earlier in the LN50 plot than in the LN100 plot (). These differences between the LN100 and LN50 plots, in the proportion of lime-nitrogen and in the timing of the decrease in DCD content, may have contributed to the differences in cumulative N2O emissions from these plots.
Among the environmental factors that affect soil nitrogen, soil temperature and soil pH have been considered key controllers of N2O emissions (Conen et al. Citation2000; Dalal et al. Citation2003). In the CF, LN100, and LN50 plots, N2O emissions were significantly and exponentially correlated with soil temperature, although there was no significant correlation in the CFD plot (). This result suggests that N2O emissions were partly limited by soil temperature. Similar results have been reported in previous studies (e.g., Clayton et al. Citation1997; Smith et al. Citation1998). Moreover, in the LN100 and LN50 plots, there was no apparent effect of lime derived from the lime-nitrogen on the temporal variation in soil pH, and there was no significant relationship between soil pH and N2O emission. This result can be attributed to the usually high pH-buffering capacity of Andosols (Baba et al. Citation1995; Takahashi et al. Citation2001). Tokuda and Hayatsu (Citation2001) reported a negative exponential relationship between soil pH and N2O emission in an Andosol field. In contrast, Mkhabela et al. (Citation2006) reported a positive correlation between soil pH and N2O emission in a Regosol field. Thus, the relationship between soil pH and N2O emission seems to differ among soil types; therefore, in another soil type, the lime-nitrogen could have had a substantial impact on N2O emission rate by changing the soil pH.
Our results showed that lime-nitrogen or DCD application inhibited nitrification in the soil. Cumulative N2O emissions were lower from the plots that received lime-nitrogen or DCD than from the CF plot, but in this Andosol field the differences were not significant, probably because of large spatial variation in N2O emission from the CF plot compared with the other plots. Further studies of the effects of lime-nitrogen on N2O emission and of the mechanisms that underlie these effects under various environmental conditions are needed.
Acknowledgments
We are grateful to Dr. Shigeto Sudo and Dr. Syuntaro Hiradate (both of NIAES, Japan) for their help in using the gas chromatography and liquid chromatography systems. Part of this study was financially supported by Denki Kagaku Kogyo Kabushiki Kaisha.
References
- Akiyama , H , Yan , X and Yagi , K . 2010 . Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: meta-analysis . Global Change Biol. , 16 : 1837 – 1846 .
- Arora , Y , Singh , L and Nnadi , LA . 1987 . Transformation of calcium cyanamide and its inhibitory effect on urea nitrification in some tropical soils . Fertilizer Res. , 12 : 3 – 9 .
- Arp , DJ and Stein , LY . 2003 . Metabolism of inorganic N compounds by ammonia-oxidizing bacteria . Crit. Rev. Biochem. Mol. Biol. , 38 : 471 – 495 .
- Baba , M , Okazaki , M and Hashitani , T . 1995 . Effect of acidic deposition on forested Andisols in the Tama Hill region of Japan . Environ. Pollut. , 89 : 97 – 106 .
- Belser , LW and Mays , EL . 1980 . Specific inhibition of nitrite oxidation by chlorate and its use in assessing nitrification in soils and sediments . Appl. Environ. Microbiol. , 39 : 505 – 510 .
- Bremner , JM . 1997 . Sources of nitrous oxide in soils . Nutr. Cycl. Agroecosys. , 49 : 7 – 16 .
- Chen , D , Suter , HC , Islam , A and Edis , R . 2010 . Influence of nitrification inhibitors on nitrification and nitrous oxide (N2O) emission from a clay loam soil fertilized with urea . Soil Biol. Biochem. , 42 : 660 – 664 .
- Clayton , H , McTaggart , LP , Parker , J , Swan , L and Smith , KA . 1997 . Nitrous oxide emissions from fertilized grassland: A 2-year study of the effects of N fertiliser form and environmental conditions . Biol. Fertil. Soils , 25 : 252 – 260 .
- Conen , F , Bobble , KE and Smith , KA . 2000 . Predicting N2O emissions from agricultural land through related soil parameters . Global Change Biol. , 6 : 417 – 426 .
- Dalal , RC , Wang , W , Robertson , GP and Parton , WJ . 2003 . Nitrous oxide emission from Australian agricultural lands and mitigation options: a review . Aust. J. Soil Res. , 41 : 165 – 195 .
- Di , HJ , Cameron , KC , Sherlock , RR , Shen , J , He , J and Winefield , CS . 2010 . Nitrous oxide emissions from grazed grassland as affected by a nitrification inhibitor, dicyandiamide, and relationships with ammonia-oxidizing bacteria and archaea . J. Soil. Sed. , 10 : 943 – 954 .
- Granli , T and Bøckman , OC . 1994 . Nitrous oxide from agriculture . Norw. J. Agric. Sci. , 12 : 7 – 127 .
- Haenseler , CM and Moyer , TR . 1937 . Effect of calcium cyanamide on the soil microflora with special reference to certain plant parasites . Soil Sci. , 43 : 133 – 151 .
- IPCC 2007: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change Eds. Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, Cambridge, UK and New York: Cambridge University Press
- Klasse , HJ . 1996 . Calcium cyanamide – An effective tool to control clubroot – A review . Acta Hort. , 407 : 403 – 409 .
- Li , X , Zhang , G , Xu , H , Cai , Z and Yagi , K . 2009 . Effect of timing of joint application of hydroquinone and dicyandiamide on nitrous oxide emission from irrigated lowland rice field . Chemosphere , 75 : 1417 – 1422 .
- Minamikawa , K , Nishimura , S , Sawamoto , T , Nakajima , Y and Yagi , K . 2010 . Annual emissions of dissolved CO2, CH4, and N2O in the subsurface drainage from three cropping systems . Global Change Biol. , 16 : 796 – 809 .
- Mkhabela , MS , Gordon , R , Burton , D , Madani , A and Hart , W . 2006 . Effect of lime, dicyandiamide and soil water content on ammonia and nitrous oxide emissions following application of liquid hog manure to marshland soil . Plant Soil , 284 : 351 – 361 .
- Mørkved , PT , Dörsch , P and Bakken , LR . 2007 . The N2O product ratio of nitrification and its dependence on long-term changes in soil pH . Soil Biol. Biochem. , 39 : 2048 – 2057 .
- Owa , N . 2010 . The analysis of the influence of cyanamide, derived from lime nitrogen, on the inhibition of nitrification . Niigata Agron. , 46 : 39 – 43 . (in Japanese)
- Ravishankara , AR , Daniel , JS and Portman , RW . 2009 . Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century . Science , 326 : 123 – 125 .
- Singh , J , Saggar , S , Giltrap , DL and Bolan , NS . 2008 . Decomposition of dicyandiamide (DCD) in three contrasting soils and its effect on nitrous oxide emission, soil respiratory activity, and microbial biomass – an incubation study . Aust. J. Soil Res. , 46 : 517 – 525 .
- Smith , KA , Thomson , PE , Clayton , H , McTaggart , IP and Conen , F . 1998 . Effects of temperature, water content and nitrogen fertilisation on emissions of nitrous oxide by soils . Atmos. Environ. , 19 : 3301 – 3309 .
- Sudo , S . 2006: Method and Instrument for Measuring Atmospheric Gas. Industrial Property Digital Library, Patent of Japan, no. 2006-275844. http://www.ipdl.inpit.go.jp/home-pg_e.ipdl (Accessed 1 June 2010)
- Takahashi , M , Sakata , T and Ishizuka , K . 2001 . Chemical characteristics and acid buffering capacity of surface soils in Japanese forests . Water Air Soil Poll. , 130 : 727 – 732 .
- Tokuda , S and Hayatsu , M . 2001 . Nitrous oxide emission potential of 21 acidic tea field soils in Japan . Soil Sci. Plant Nutr. , 47 : 637 – 642 .
- van Cleemput , O and Samater , AH . 1996 . Nitrite in soils: accumulation and role in the formation of gaseous N compounds . Fert. Res. , 45 : 81 – 89 .
- Weiske , A , Benckiser , G , Herbert , T and Ottow , JCG . 2001 . Influence of the nitrification inhibitor 3,4-dimethylpyrazole phosphate (DMPP) in comparison to dicyandiamide (DCD) on nitrous oxide emission, carbon dioxide fluxes and methane oxidation during 3 years of repeated application in field experiments . Biol. Fertil. Soils , 34 : 109 – 117 .