Abstract
The inorganic nitrogen (N) cycle and its dynamics in the soil were observed in the ecosystem at the Spasskaya Pad experimental forest near Yakutsk in northeastern Siberia in order to estimate the N availability for the larch (Larix cajanderi Mayr.) The soil pool of bulk N in the forest accounted for up to 866 g N m−2 (0–50 cm), whereas up to 1.7% (14.6 g N m−2) was potassium chloride (KCl)-extractable inorganic N. Ammonium was a dominant form of inorganic N. The size of the soil inorganic N pool fluctuated seasonally. It was small in the beginning of the summer and increased rapidly once the cumulative degree day of the soil temperature at 20 cm reached more than 300 (°C days), indicating that active N mineralization began at some point from the middle of July to the beginning of August. This soil pool of inorganic N that had accumulated at the end of the previous summer was consumed by the beginning of the next summer through microbial immobilization, which may have begun in September. Recycling of N in the soil was important because the input of inorganic N by deposition was very small (48 mg N m−2 year−1). A tracer 15N experiment showed that larch did not uptake organic N in the form of amino acid (alanine); rather, it used ammonium and nitrate as N sources. The amount of available N for plants was assessed as water-extractable inorganic N in the soil solution that was transported to the rooting area by mass flow driven by plant transpiration, and it accounted for 1.9 × 103 (1.2 × 103 for trees) mg N m−2 during the growing season−1 (0–50 cm of the mineral soil layer in June, July, and August). Microorganisms in the soil are also expected to be competing for this available N. Our results show that despite a large amount of inorganic N production, N availability for plants is low as the soil pool of inorganic N is built up at the same rate as larch senescence.
1. INTRODUCTION
One of the hottest topics at present is the increase in the concentration of greenhouse gases in the atmosphere, which contribute to global warming (Solomon et al. Citation2007). There is evidence that boreal forests act as a global carbon (C) sink (Schulze et al. Citation1999; Dolman et al. Citation2004). Russian Siberia covers 13 million km2, which is almost 10% of the total land mass of the Earth. Russian forests are a global sink accounting for 0.13 Pg C per year (Goodale et al. Citation2002). This means that the amount of sequestrated carbon dioxide (CO2) in the Siberian taiga is rather significant, and its changes could affect the C cycle on a global scale.
It is known that the availability of nitrogen (N) is one of the important limiting factors for plants in the northern hemisphere (Aerts et al. Citation1999; Vitousek et al. Citation2002). Therefore, understanding the dynamics of N is fundamental to knowing the effects of global climate change on C cycles in boreal forests. N exists in several forms in soil pools, and the major component is expected to be organic matter. Inorganic N is formed by the microbial processes of decomposition of organic matter in the soil (mineralization). Generally, the rates of mineralization and nitrification depend on several factors, including soil temperature, moisture conditions, and the availability of labile organic matter (Cassman and Munns Citation1980; Nadelhoffer et al. Citation1991; Sierra Citation1997). Leaching and denitrification processes are negligible or not significant in our study area, located on the permafrost (Shugalei and Vedrova Citation2004, Koide et al. Citation2010).
N availability for plants is a complex question that has a long history in research. Several large-scale studies have been conducted in boreal and tundra areas in Europe and North America. These reports showed that N availability in northern ecosystems is generally very limited (Schulze et al. Citation1994; Bauer et al. Citation2000), and proposed various hypotheses to fill the gap between soil N availability and plant demand (Kielland Citation1994; Harrison et al. Citation2000; Lisuzzo et al. Citation2008; Koyama and Kielland Citation2011). Direct uptake of organic N in the form of amino acids was found to be an important source of N for some deciduous and conifer plants, although the uptake rate varied greatly among species (Jones et al. Citation2005). The soil inorganic N pool is known to regulate N availability for plants (Schulze et al. Citation1999); however, there are several competitors such as bacteria and fungi, and the proportion readily used by plants needs to be determined further. It is also believed that global warming may increase the fluxes of C and N cycling (Nadelhoffer et al. Citation1991; Borner et al. Citation2008; Chapin et al. Citation2009), whereas the mechanisms and possible effects of such changes on plant N availability are still not clear.
There have been many reports from European and North American boreal ecosystems. However, only a few studies have been conducted in Siberia, where the larch, a deciduous conifer, dominates the area. The majority of these works have been reported in Russian; therefore, up to now, these studies have not been available to most scientists outside Russia. Based on accessible information, the available inorganic N was likely to be a limiting factor for plant growth in central (Tokuchi et al. Citation2010) and northeastern parts of Siberia (Schulze et al. Citation1995). In addition, the amount of N in the soil pools, the plant N (Matsuura and Hirobe Citation2010), the soil inorganic N, and the decomposition rate (Shugalei and Vedrova Citation2004) in central Siberia, have been observed.
The Siberian taiga consists of larch forests that include several species. The dominant species in the eastern part of the Siberian taiga is Larix cajanderi Mayr (Pozdnyakov Citation1975). Previous studies performed in northeastern Siberia demonstrated the importance of water and temperature for plant growth (Maximov et al. Citation2010). The permafrost system was shown to be important because it provides a source of water for the larch trees (Sugimoto et al. Citation2002). In addition, the seasonal dynamics of soil respiration have also been observed (Kononov Citation2006). Only one report has been published on the N cycle in the forest at the study site (Koide et al. Citation2010), and no study on N dynamics has yet been performed.
This study is a pioneering study of N dynamics in this area. Therefore, the main objectives of the current research are to monitor the seasonal fluctuation of soil N availability in the taiga forest ecosystem in northeastern Siberia, to estimate N demand of the tree stands, and to identify the factors that regulate N availability.
2. STUDY SITE AND METHODS
2.1. Study site
Observations were conducted at the Spasskaya Pad experimental forest (62.15°N, 129.37°E, 220 m above sea level) of the Institute for Biological Problems of Cryolithozone, Siberian Branch of the Russian Academy of Sciences, which is located on the first terrace (left bank) of the Lena river, about 25 km northwest of Yakutsk, Russia, in a continuous permafrost area. The soil type is classified as Gelisols according to the USDA. The period of observation of soil N was mostly from 2005 to 2011 and intensive observation was conducted during the period from 2009 to 2011. The climate in the study area is sharply continental, with monthly mean temperatures ranging from +19°C in July to −40°C in January. The annual mean precipitation in the study area is quite low, i.e., on average 238 mm (from 1971 to 2000), of which two-thirds occurs in the summer. The active layer depth (defined as the soil layer that thaws during the warm period) is usually 1.2–1.4 m. Soil thaw starts from the surface layer at the end of April, and the thaw depth increases until October, whereas the surface soil layer starts to freeze in September and the soil stays frozen for seven months of the year.
Larix cajanderii Mayr is the dominant species at the study site, and Betula platyphylla Sukaczev is found in a gap. The forest floor is covered by various plant species: Vaccinium vitis-idaea L., Pyrola rotundifolia L., Ledum palustre L., and Arctostaphylos uva-ursi (L.) Spreng are typically found. The growing season is short: It begins with leaf unfolding (from mid-May to the beginning of June), when the surface layer of the soil thaws, and it ends with senescence at the end of August or the beginning of September; litterfall takes place in the middle of September.
The plot (20 × 20 m) for samplings and installation of observation materials was established in a non-disturbed area of a larch forest that is located about 100 m northeast of the observation tower where meteorological and hydrological observations have been conducted. The forest floor is covered with a thin layer of litter and an organic layer (A0), typically 8 cm thick; a mineral soil layer lies underneath. Mineral soil is typically sandy loam or sandy clay loam and is rich in carbonates. Total (inorganic and organic) C content sharply decreases with depth. It is about 2–3%, with the C/N ratio of the soil at 22, at the border with an organic layer, and it increases to 34 at a depth of 50 cm. The plant roots are mainly distributed down to 20 cm in mineral soil layer and rarely found below 50 cm.
2.2. Observation and analyses
2.2.1. Soil moisture and temperature and meteorological conditions
Volumetric soil water content was manually observed at the study site using time domain reflectometry (Moisture Point, Environmental Sensors Inc.). The soil temperature was measured using a thermometer with a data-logger (Tidbit, Onset, USA). Automatic measurements of soil moisture and temperature in the larch forest were initiated by GAME-Siberia [GEWEX (The Global Energy and Water Cycle Experiment) Asian Monsoon Experiment] (Ohta et al. Citation2008), and continued by JAMSTEC (Japan Agency for Marine-Earth Science and Technology). The soil moisture-water equivalent was calculated with the same method by Sugimoto et al. (Citation2003) using both manual and automatic measurement data. Air temperature and precipitation data were obtained through an NCDC NOAA (National Climatic Data Center, National Oceanic and Atmospheric Administration) summary-of-the-day web database.
2.2.2. Inorganic N in throughfall
For determining the amount of N deposited via throughfall (wet and dry), samples were collected from 29 May 2009 to 27 August 2009, from 5 June 2010 to 1 August 2010, from 25 May 2011to 29 July 2011, and from 15 October 2011 to 28 February 2012. During summer observations, sampling containers were placed under the canopy in the forest so that dry particulate matter and rainwater were collected together (n = 9). Throughfall samples were collected after a major rainfall and weighed. An aliquot was transferred to a 50-mL plastic tube and kept frozen at –18°C until analysis. To observe the amount of N deposited during the winter, a sampling tube (diameter 0.1 m) was used to collect samples at various depths of snow cover under the canopy (n = 3). Snow samples were liquefied and stored as described above.
2.2.3. Soil inorganic and amino acid N pools
Soil sampling was done for the organic layer, and at depths of 5, 15, and 50 cm for the mineral layer. To calculate the amount of N per unit area, the soil densities from previous observations were assumed. For the organic layer, a value of 0.30 g cm−3 was used; as for the mineral layer, values of 1.34, 1.45 and 1.65 g cm−3 were used for depths of 0–10, 10–30, and 30–50 cm, respectively.
Bulk N concentration in the soil pool was determined using samples taken between 4 and 8 August 1999, and on 11 August 2004, 10 August 2005, and 15 August 2006. Soil samples were collected in the forest, oven-dried at 60°C, and separated from debris. The soils were finely ground in a ceramic bowl and analyzed for bulk N.
For studying the soil inorganic N pool, soil samples were collected from June to August in 2005, 2009, 2010, and 2011. Fresh soil samples were sieved (2 mm mesh), and N was immediately extracted using a 2 M potassium chloride (KCl) solution or ion exchange water [soil:solvent ratio was 4:40 (w/v)] on a reciprocating shaker (1 h), then filtrated with a prewashed cellulose filter. Extracts were frozen and maintained at −18°C until analysis. 10 g of each sample was oven dried at 105°C and used for dry weight estimation.
Total free amino acids were extracted from samples collected on 15 June, 1 July, and 21 July 2011 using ion exchange water [soil:solvent ratio was 4:40 (w/v)]. The samples were filtered using a 0.45-µm syringe filter and were then frozen.
2.2.4. Ion exchange resins
The ion exchange resin bags method has been used frequently in situ to evaluate nutrient availability in various types of forests (Giblin et al. Citation1994). Binkley and Matson (Citation1983) have suggested that the ion exchange resin bags method gives a comprehensive picture of the soil status of plant-available nutrients, especially because membranes resemble the structure of a plant root. The ion-exchange resin bags method was reported to overcome the disadvantage of chemical extractions accounting for the kinetics of nutrient release and transport (Curtin et al. Citation1987; Abrams and Jarrell Citation1992) and mobilizing nutrient forms that are truly available for plants (Skogley and Dobermann Citation1996). Using this method, the relative amounts of inorganic N percolating through the soil profile with the soil solution can be determined; this is especially informative in a permafrost area with a dry climate, where water moves vertically upward and downward seasonally.
Ammonium and nitrate percolating in the soil solution were collected using ion-exchange resin plates. A mixture of cation and anion exchange resins (5 g each) was packed in a plate (5 cm diameter, 1 cm thick). Three plates were installed at depths of 5 and 15 cm in the observation plot during the following periods: 13 August 2005 to 6 August 2006, 7 August 2006 to 7 July 2007, 6 June 2009 to 21 August 2009, 22 August 2009 to 17 August 2010, and 18 August 2010 to 25 July 2011. After recovering the plates from the soil, they were oven dried at 40°C and kept in plastic bags until analysis. For the analysis, extraction of ions from resins was done using 2 N hydrochloric acid (HCl).
2.2.5. Production of inorganic N
Production of inorganic N was observed using the in situ incubation method. Observation of the mineral soil layer was conducted from 21 August 2009 to 17 August 2010 and from 18 August 2010 to 21 July 2011 at depths of 5, 15, and 50 cm (for each depth, n = 12). The method was described (Nadelhoffer et al. Citation1984) with the modification suggested in Tokuchi et al. (Citation2010). Briefly, soil was placed in 30-mL loosely capped bottles to keep the plant roots out and at the same time to allow the exchange of water and gases. To obtain the initial condition, the same soil was used for extraction of inorganic N with 2 M KCl. Bottles filled with soil were returned to the original position in the soil. After about 1 year of incubation, the bottles were recovered and then inorganic N was extracted using 2 M KCl.
2.2.6. Ammonium, nitrate and amino acid tracer experiment
15N-ammonium and nitrate tracer labeling experiments were carried out to determine the preferred form of inorganic N. An experimental plot (15 m × 15 m) was established in the larch forest site 200 m southwest of the tower site. A total of 18 trees with an average total height of about 1.5 m were used for the experiment. Nine trees were treated with ammonium sulfate (15NH4 +)2SO4 (min. 60 at% of 15N) and another nine trees with sodium nitrate Na15NO3 (min. 60 at% of 15N), which were dissolved in ion exchange water. One hundred milliliters of a tracer containing 0.02 M-N was applied under the organic layer around the main root of each tree. Labeling was done on 28 May 2009. On Day 26, branches were sampled from the middle part of the labeled trees, and oven dried at 60°C; the needles were then separated. Needle samples were milled for analyzing C and N content and isotope ratios.
Tracer experiments with dual labels, 13C15N-alanin and 15N-ammonium, were conducted to detect the possibility of organic and inorganic N uptake by the larch trees. Since the ratio of 13C:15N of alanine was 3:1, if this alanine was assimilated directly, the increase of heavy isotopes in the labeled trees would follow the same proportion. Experiments were held twice: from 30 June to 2 July, and from 27 to 29 July 2011. For each experiment nine larch seedlings (about 40 cm total height) were chosen at the tracer experimental plot: Three were used as the control, three for the 15N-ammonium treatment and three for the 13C15N-alanine treatment. Control trees were watered with 100 mL of ion exchange water and did not receive any nutrition. Amino acid treated trees received 100 mL of 0.018 M of 13C-15N-alanine (min. 99.9 at% of 13C and 15N) and 0.010 M of non-labeled ammonium sulfate solution, whereas ammonium labeled trees received 100 mL 0.010 M of 15N-ammonium sulfate (min. 60 at% of 15N) and 0.018 M of non-labeled alanine. Needles were collected from each tree at intervals of 1, 2, 4, 8, 12, 24, and 48 h.
2.2.7. Laboratory analyses
Ammonium and nitrate concentrations in the throughfall and soil extracts were analyzed using a continuous flow spectrophotometer autoanalyzer (Bran & Luebbe, Norderstedt, Germany). Amino acid content in soil extract was assessed using ninhydrin (Moore Citation1968) with a modified method by Lipson and Monson (Citation1998) with alanine as the standard. The ammonium interference effect was subtracted using ammonium concentration data. Bulk C and N content and isotopic ratios were assessed using an elemental analyzer (Flash EA, Thermo Scientific, Billerica, MA) coupled with an isotope ratio mass-spectrometer (Delta V, Thermo Scientific). Results are expressed in delta notation (δ13C or δ15N):
3. RESULTS
3.1. N budget
3.1.1. Inorganic N deposition
The total amounts of N in throughfall or deposition for three summer seasons and one winter season are presented in . During the summers, ammonium in throughfall varied from 8.6 to 27.2 mg N m−2, respectively, which was larger than that of nitrate (from 0.18 to 8.1 mg N m−2). Nitrite (less than 0.31 mg N m−2) was not significant (not shown). Each observational period presented in consists of 1 to 10 individual samplings, although one was a snow sample taken during the winter after the snow cover stabilized. The concentration of inorganic N (the sum of ammonium and nitrate) in throughfall ranged from 0.32 to 139.88 µmol L−1. The amount of water during rain events ranged from 1 to 21 mm. Among the samples collected during the period from 25 May to 29 July 2011, the highest deposition rate of N (35.3 mg N m−2) was observed in the sample taken for 17 to 22 July 2011, which overlapped a state of emergency declared by the local government due to a forest fire. This was probably caused by complete and incomplete combustion of organic matter (Olivier et al. Citation1994; Bouwman et al. Citation1997; Anderson et al. Citation2003; Coheur et al. Citation2009; Polischuk et al. Citation2011).
Table 1 Observed and estimated total inorganic nitrogen (N) in throughfall and deposition
Winter deposition for the period from 15 October 2011 to 28 February 2012 was observed as deposited N in the snow cover. This snow cover sample accumulated 8.2 mg N m−2 of ammonium, 11.0 mg N m−2 of nitrate, and 0.07 mg N m−2 of nitrite. For each sample, including that from the winter data, a positive correlation between total deposited N and sampling duration (r = 0.92) was observed, except for the summer of 2011, when very high deposition was observed as a result of the forest fire described above. An increase in ammonium deposition during the warm period and an increase in nitrate deposition during the winter are consistent with the observation made in central Yakutia (Makarov Citation2007).
Since the observation of deposited inorganic N did not cover a full year, the annual deposition was estimated using a daily average total N deposition (131.2 µg N m−2 day−1) calculated except for the data from the summer of 2011. Estimated annual total N deposition was 48 mg N m−2year−1 (), which was similar to that reported in Makarov (Citation2007) for central Yakutia (1–40 mg N m−2 year−1) and lower than that reported for the Asian part of Russia (50–2.95 × 103 mg N m−2 year−1) (Polischuk et al. Citation2011). The amount of N deposited annually appeared to be very low compared to that observed in Europe (less than 100 to greater than 7500 mg N m−2 year−1) and North America (from 310 to 3200 mg N m−2 year−1) (Aber et al. Citation1989; Dise and Wright Citation1995; Fenn et al. Citation1998; Galloway et al. Citation2004).
3.1.2. Soil N pool
Bulk, inorganic, and organic N pools observed in the organic and mineral soil layers are summarized in , and vertical profiles are presented in the supporting information (Table S1). The amount of bulk N observed in the organic soil layer was 99 × 103 and 116 × 103 mg N m−2, while the amount in the mineral soil layer was much larger, ranging from 608 × 103 to 866 × 103 mg N m−2. N content in the soil decreased markedly with depth from the organic layer (0.007–0.008% in dry weight) down to 5 cm of the mineral layer (0.002% in dry weight), after which it exhibited a small variation around 0.001% dry weight (data not shown). Such a distribution is consistent with studies performed in the larch forests in central Siberia (Shugalei and Vedrova Citation2004).
Table 2 Soil nitrogen (N) pools in the organic layer and mineral soil layer 0 to 50 cm: average with standard deviation (SD) and repeated number of samples (in parentheses) for bulk N, potassium chloride (KCl)-extractable and water-extractable ammonium (g N m−2), nitrate and total free amino acid (mg N m−2).
The amount of KCl-extractable ammonium dominated the soil inorganic N pool irrespective of seasons or year-to-year variation, and it was up to 75 times larger than nitrate. Large seasonal variation in the soil KCl-extractable inorganic N pool was found in the soil mineral layer. KCl-extractable ammonium in the mineral soil layer increased from 2.5 on 6 June to 10.5 g N m−2 on 21 August in 2009, from 0.354 on 7 June to 14.3 g N m−2 on 17 August in 2010, and from 1.5 on 15 June to 3.0 g N m−2 on 21 July in 2011. As seen in , in the beginning of the growing season (June until the middle of July), the inorganic N content in the soil was low. It increased by August due to increase in the amount of ammonium in the KCl-extractable soil N pool.
Water-extractable ammonium was two to ten times less than KCl-extractable ammonium. In the beginning of the growing season, the amount of water-extractable ammonium in the soil mineral layer was low (151 mg N m−2 on 15 June 2011), and it increased to 1.4 × 103 mg N m−2 on 21 July 2011. Unlike water-extractable ammonium, nitrate did not show a clear increase from June to August.
The total free amino acid in the soil pool observed in 2011 in the mineral layer was higher than that in the organic layer; however, the difference was statistically not significant. Soil pools of amino acid tended to decrease (from 53 to 10 mg N m−2) in the organic layer and increase in the mineral soil layer (from 221 to 432 mg N m−2) from June to July.
3.1.3. N collected by ion exchange resins
Similarly to soil extracts, the amount of collected total N varied greatly from 50 to 475 mg N m−2 period−1 (Table S2). The amount of ammonium captured by ion exchange resins was also larger than that of nitrate in most of samples. Ammonium to nitrate ratio was from 1.7 to 2.8 except for the ratio of 18.8 observed for the period from 3 August 2005 to 6 August 2006. The observations that covered late summer and fall (August and September) showed larger amounts of inorganic N than those observed during other periods.
3.1.4. Inorganic N production
A high production of ammonium (26.0 g N m−2 period−1) was observed in the first incubation period from 22 August 2009 to 17 August 2010 covering a full year, and the rate decreased with depth ( and Table S3). On the other hand, in the second incubation from 18 August 2010 to 21 July 2011, ammonium was consumed (−13.6 g N m−2 period−1). Consumption rate was the highest at the top of the mineral soil layer, and it decreased with depth. The second incubation experiment did not cover the whole year, particularly late July and August. This indicates the relative importance of late summer for N production.
Table 3 Soil inorganic nitrogen (N) pools before and after incubation experiments and calculated production calculated for the mineral soil layer 0–50 cm for the period of incubation (g N m−2)
3.2. Tracer experiments
Results of the amino acid tracer labeling experimental results are shown in . No significant change in needle δ15N was observed in the control and amino acid-treated trees during both experiments. On the other hand, δ15N of needles from 15N-ammonium treated trees gradually increased beginning 1 h after the experiment started. In the experiment performed in June, the maximum change observed in δ15N was +5.5‰, whereas it was +5.0‰ in July (e and f). The needle δ13C of individual trees showed no significant change in experiments for both control and labeled trees with alanine and ammonium (data not shown).
Figure 1 The change in larch (Larix cajanderi Mayr.) needle δ15N observed during amino acid and ammonium tracer experiments held from 30 June to 2 July (a, c, and e) and from 27 to 29 July 2009 (b, d, and f), calculated from needle δ15N of each sample tree before the tracer application, ranged from −5.2 to 0.2‰. Different markers (open or closed square, triangle, or circle) indicate each sample tree. A broken line indicates the average δ15N change (e and f). Although the same markers were used to indicate individual trees in two experiments, the trees are not the same.
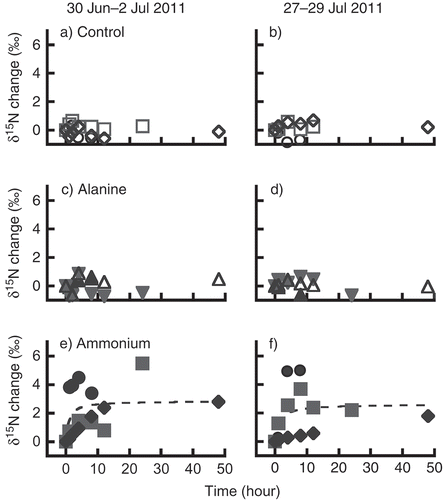
Figure 2 The relationship between inorganic nitrogen (N) soil pool and soil temperature cumulative degree day. Open squares indicate potassium chloride (KCl)-extractable inorganic N pool (mineral soil layer 0–50 cm) observed from 2009 to 2011 (data shown in ). A fitted line was also shown. Degree days were calculated for each observation date from the soil temperatures above 0°C observed at 20 cm from 2009 to 2011.
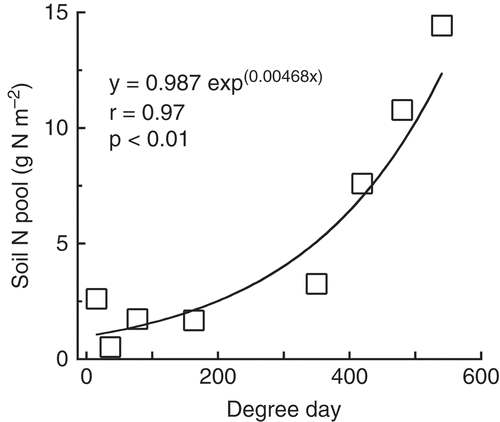
Figure 3 The seasonal changes in the size of the soil inorganic nitrogen (N) pool observed in 2009 (a), 2010 (b), and 2011 (c). A solid line represents daily average soil temperature observed at 20 cm depth, open squares denote potassium chloride (KCL)-extractable soil inorganic N pool size. “Layer” indicates the soil mineral layer, 0–50 cm.
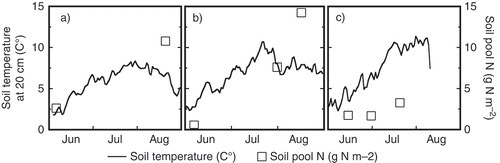
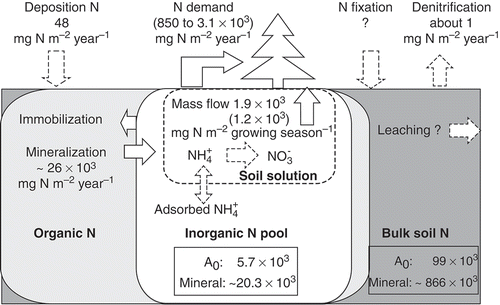
Figure 4 The schematic figure for nitrogen (N) pools in the soil (mg N m−2) and ecosystem fluxes. Areas outlined with solid lines represent the following: dark grey, bulk soil N pool; light grey, organic N pool, white, potassium chloride (KCL)-extractable inorganic N pool. Areas outlined with dashed lines represent the water-extractable part of the inorganic N pool in the soil solution. The maximum sizes of the pools are described in the figure as outlined values. “A0 ” and “Mineral” denote pools in organic layer and mineral soil layer (0–50 cm). respectively. Fluxes within the ecosystem or larch (Larix cajanderi Mayr.) trees (in parentheses) are shown by arrows with open values. Solid arrows are major fluxes, whereas dashed arrows are those expected to be minor or negligible. The maximum net mineralization rate () shown here was calculated from the incubation that covered almost one year. N demand and mass flow estimation were explained in sections 4.2 and 4.3, respectively. “Growing season” indicates the period from 1 June to 31 August. The value for denitrification was calculated from Koide et al. (Citation2010). Values of N fixation and leaching are not indicated here but are expected to be minor (Shugalei and Vedrova Citation2004).
The results of the inorganic 15N labeling experiments are summarized in . The δ15N of needles from trees labeled with 15N-ammonium (127 ‰) was about 1.5 times larger than that of trees labeled with 15N-nitrate (84‰) on Day 26 after application of the tracer, although the difference was statistically insignificant.
Table 4 Needle δ15N observed in ammonium and nitrate tracer experiments and soil nitrogen (N) pool.
4. DISCUSSION
4.1. Preference of N form
The results of amino acid tracer labeling experiments showed no change in δ15N (). This means that larch trees did not uptake the amino acid (alanine) directly in this site, and it also showed that the amino acid did not turn into an inorganic form available for plants within 48 h, which means inorganic N production was still slow in the middle of the growing season or amino acid was immobilized by microorganisms. In contrast, inorganic N in the form of ammonium was quickly assimilated and was transported to the needles of larch saplings within 2 h. Since these tracer experiments were conducted with the addition of ammonium, we cannot rule out the possibility of interference of added ammonium in the uptake rate of amino acid, because it has been suggested that direct uptake of amino acid may take place favorably under limited supply of inorganic N (Jones et al. Citation2005). However, even if the uptake of amino acid were suppressed by the addition of ammonium, the importance of amino acid as a source of N would have been minor, because the natural abundance of amino acid was one order smaller than that of ammonium in the earlier half of the growing season. In addition, it should be noted that the level of ammonium concentration in the tracer experiment is naturally observed in the latter half of the growing season, as we describe in the next section. Therefore, from results of these tracer experiments no uptake of amino acid is expected.
From the inorganic 15N labeling experimental results (), it can be said that larch trees uptake both forms of inorganic N. These results also indicate another important fact: Added 15N-labeled ammonium was possibly diluted only by the soil solution. Because of the considerable amounts of ammonium and nitrate in the soil pool, dilution of the tracers must be considered. Generally, it is not easy to determine the exact extent of the volume of dilution in the soil. Therefore, we assumed that the labeling solution was uniformly spread in the volume of mineral soil with diameter of 30 cm and 10 cm deep. Using the data observed on 6 June 2009, one week ahead of the tracer application, the amount of KCl-extractable ammonium and nitrate in this volume of soil described above were calculated to be 72.0 and 2.1 mg N, respectively (). These data showed that 34 times more ammonium than nitrate existed in the soil. Since the same amount of tracer was applied in the form of ammonium and nitrate, a small difference in δ15N of needles indicated a similar magnitude of uptake of labelled material from the soil pool. There are three possibilities to explain these observations: (1) dilution occurs only in the soil solution; (2) ammonium was taken up 34 times more than nitrate, or (3) nitrate was immobilized by microbes 34 times more intensively than ammonium. As we describe in Section 4.2, microbial activities are expected to be low in the first half of the growing season. Although we cannot exclude the possibility of a 34-fold increase in the uptake of ammonium or 34 times more intensive immobilization of nitrate than ammonium, we expect that the reason of dilution of the tracer label only in the soil solution is the most likely explanation.
Thus, contrary to our expectation, 15N-ammonium was not diluted by the large KCl-extractable soil N pool, but rather by that in the soil solution (9.5 mg N). Apparently, the exchange and thus equilibration between adsorbed ammonium and ammonium in the soil solution were slow. Although we cannot reject the possibility that nitrate was taken up much more quickly than ammonium, an equal preference for ammonium and nitrate, and a slow exchange between adsorbed ammonium and ammonium in the soil solution, seem to be explanations that are more reasonable. Dörr et al. (Citation2012) also reported a long retention period of added ammonium in a temperate Norway spruce (Picea abies (L.) Karst.) forest, which is consistent with the idea of a slow release of adsorbed ammonium.
4.2. Seasonal dynamics of N in soil
The existence of permafrost affects the temperature and moisture in the surface soil layer and, as a result, many physiological processes. In our study, the soil inorganic N pool seemed to exhibit seasonality, depending on the soil temperature. To confirm this, the observed inorganic N soil pool was plotted against the cumulative degree days of soil temperatures at 20 cm (°C day). The inorganic N soil pool demonstrated clear non-linear seasonality, and it increased with soil temperature cumulative degree days (). In the beginning of the summer, the soil inorganic N pool was small. The increase in the pool started at some point in August, when the soil temperature degree days reached 300°C day. The soil inorganic N pool increased rapidly in August; then by the next June it was small again. The soil temperatures reach about 9–10°C when soil inorganic N pool begins to increase. Nadelhoffer et al. (Citation1991) reported that in a laboratory experiment, between 3 and 9°C N mineralization rates of arctic soils in Alaska were relatively unaffected by temperatures but they increased significantly between 9 and 15°C. Dependency of inorganic N production on temperature has also been shown by Hobbie (Citation1996). Therefore, it is quite reasonable that active inorganic N production begins in the late summer and causes a rapid increase in the inorganic N in August at our site. Microbial activity in the late summer was also supported by a result of high soil respiration rate observed in August by Kononov (Citation2006) at our study site.
Microbial consumption (immobilization) should be taken into consideration to explain the seasonality observed at our site. A large decrease of inorganic N pool size was observed in the second incubation experiment, which ended on 21 July 2011 (), indicating that microbial immobilization occurred during the period of incubation. As soil inorganic N pool is a result of two opposite processes – production and consumption of inorganic N – the size of the pool changes depending on their relative rates. As described above, the rate of production of inorganic N increased in late summer (mid-July and August), while the rate of microbial consumption is expected to increase subsequently after that. Ivanova et al. (Citation2006) reported an increase in number of cellulolytic microorganisms (actinomycetes and fungi) from the beginning of September at our study site. The number of those was increasing until the beginning of October. Since the N source for those microorganisms would be inorganic N from the soil pool, a continuous increase in rate of consumption of inorganic N is expected in fall. A similar seasonal pattern for production and consumption was observed in the soil in a temperate beech (Fagus sylvatica L.) forest and explained by the availability of dissolved C for microorganisms (Kaiser et al. Citation2011). When the rate of consumption of inorganic N exceeds the rate of its production, the size of the inorganic N soil pool decreases. We observed the increase in the soil inorganic N pool in the late summer, which means the rate of production of inorganic N exceeded the rate of its consumption. In the fall the rate of inorganic N consumption is expected to prevail, leading to an observed decrease in the size of inorganic N pool in the beginning of each growing season. It should be noted that the year-to-year variations of inorganic N production and consumption rates affect the size and the timing of build-up of the soil inorganic N pool.
Microbial activity during the winter has not been well understood so far. However, some researchers have suggested there is microbial activity in the winter. According to Kielland et al. (Citation2006) microbial processes continue in the soil even after the soil temperatures dip below zero degrees. At our site, some microorganisms were found in the beginning of the spring (Ivanova et al. Citation2006). Although microbial activity during the winter has not been proven, a large soil inorganic N pool observed in late summer was consumed until the early summer of the following year.
It has been reported that the inorganic N pool showed year-to-year variation depending on mean air temperature in July in Central Siberia (Tokuchi et al. Citation2010). Although a year-to-year variation in the soil inorganic N pool is expected in our site, the contribution of seasonal variation () might be of greater magnitude than that of the year-to-year variation (). It should be noted that this pattern of seasonality makes it inconvenient for plants to uptake N because of the discrepancy in timing between inorganic N production (starting from late summer) and active transpiration or growth of plants. The build-up of a large amount of inorganic N in the soil in fall is too late for plants, resulting in a decrease in the availability of N for plants.
4.3. Availability of N for plants
4.3.1. N budget
The main fluxes and pools of N observed in our study are summarized in . To describe variations of pools and fluxes in the ecosystem, we used the average annual N throughfall shown in , the highest observed values of bulk N and the soil inorganic N pool presented in , and the inorganic N production observed in the first incubation experiment, which covered almost one year (). The amount of N in the litterfall and the amount of available N for plants is estimated later in this section.
Our results showed that a large amount of bulk N, up to 866 and 99 g N m−2 for the 0–50 cm mineral layer and the surface organic layer, respectively (), is stored in the soil mostly as organic matter. The inorganic N pool was up to 14.6 and 5.7 g N m−2 for the mineral layer and the organic layer, respectively, which accounts for 1.7 and 5.8% of bulk N in each layer. A major component of the soil inorganic N pool was ammonium in our study site (), and most of it exists in a form adsorbed on soil particles extractable with KCl. The large variation that is observed in the soil inorganic N pool () indicates dynamic production and consumption of inorganic N in the soil. It has been reported that the contribution of N fixation to the inorganic N soil pool was minor in the larch forests in central Siberia (Shugalei and Vedrova Citation2004), and the rate of N loss due to denitrification was also very low (Koide et al. Citation2010; Matsuura and Hirobe Citation2010).
4.3.2. Plant N demand
In Lambers et al. (Citation2008) and Shaver and Chapin (Citation1991), nutrient recycling before senescence and new uptake were considered to supply new growing parts. To evaluate the amount of recycled nutrients, we estimated N demand for our 150-year-old larch stand. In previous reports (Schulze et al. Citation1995; Shibuya et al. Citation2004), in northeastern Siberia, forest aboveground biomass becomes saturated at the age of 120–130 yr and does not change much after that. Therefore, we assume that the biomass production reported for 120 and 125-year-old stands (Schulze et al. Citation1995; Shibuya et al. Citation2004) was similar to that of our 150-year-old study site. The annual production of woody biomass required 240 to 1.0 × 103 mg N m−2 year−1, and for the production of new needles 3.1 × 103 to 4.0 × 103 mg N m−2 year−1 was additionally needed. However, considering the reallocation of N from senescing larch needles, which has been reported to be about 70% (Gower and Richards Citation1990; Matsuura and Hirobe Citation2010), the estimated annual loss of N with litterfall would be only 930 to 1.2 × 103 mg N m−2 year−1. The amount of N lost with the litterfall observed at our study site was about 500 mg N m−2 year−1 (A. Sugimoto, unpublished data), which was much lower than the estimated range above. Belowground production was not considered in many of the previous works, mainly because of the difficulty of such estimation in the field. Calculation of N demand based on the estimation of larch belowground production (Pozdnyakov et al. Citation1969) accounted for about 110 mg N m−2 year−1. On the other hand, for the 105-year-old larch stand in central Siberia, the demand of N for belowground production was about 850 mg N m−2 year−1 (Tokuchi et al. Citation2010), and no recovery of N from tree roots has been reported so far. Using the minimum and maximum values described above, in total roughly 850 to 3.1 × 103 mg N m−2 year−1 is the range of demand for above- and belowground production annually by a larch stand only (requirements for needles, above-, and belowground production), which has to be covered by nutrient uptake. This range is wide but still provides an understanding of the magnitude of larch N demand and it covers somewhat that estimated for the central Siberia L. gmelinii stand (1.1 × 103 mg N m−2 year−1) (Tokuchi et al. Citation2010).
4.3.3. N availability
Nutrient supply occurs mainly via mass flow and diffusion (Lambers et al. Citation2008). It is expected that mass flow driven by evapotranspiration is an important force to supply nutrients to plants. Here we assessed the available N for plants using observed data on water-extractable inorganic N as shown in . The idea of assessment with water-extractable N is also supported by the observed fact that the ammonium-to-nitrate ratio collected by ion exchange resin (Table S2) functioning as plant roots was close to that in the soil solution. The amount of water-extractable N in the mineral soil layer (0–50 cm) was expected to increase from June (512 mg N m−2 on 15 June 2011) to August (1.6 × 103 mg N m−2 on 17 August 2010) as the KCl-extractable ammonium pool increased with soil temperature. To estimate the seasonal variation of water-extractable N, the relationship between water-extractable inorganic N () and the cumulative degree day of soil temperature at 20 cm was obtained:
The concentration of water-extractable N in the soil solution was estimated as follows:
The amount of inorganic N supplied by mass flow barely covered the annual demand of the larch (850 to 3.1 × 103 mg N m−2 year−1) estimated from annual biomass production. Despite the large amount of inorganic N produced in the soil, there are certain limitations of N availability for the larch. First, very little inorganic N exists in the soil pool in the beginning of the growing season, which is when plants require large amounts of nutrients. At the same time, a majority of the inorganic N exists in the form of ammonium adsorbed on soil particles. Aoki et al. (Citation2012) showed the use of exudation of organic acids by plants to derive non-labile phosphate from phosphorus-poor soil. A similar mechanism might be used by plants to obtain adsorbed N, although the high expense for organic acids production required for it would limit the process. On the other hand, the inorganic N pool becomes large by the end of the growing season, when senescence starts and transpiration rate decreases, which also equates to a decrease in mass transport. Thus, there is a discrepancy between the timing of inorganic N production by microorganisms and the seasonal development of the larch, which leads to limitations in N availability for plants. In addition, competition for N among microorganisms and understory plants is expected to be very strong and is not considered in our estimation. It is obvious that the N budget for the larch is very tight and N availability must be limited.
The amount of N available for plants estimated in various boreal forests in the northern hemisphere at similar latitudes is presented in . N uptake estimated for various boreal ecosystems differs from forest to forest, but it is somehow dependent on longitude. It is likely that in the Eurasian continent, the amount of N availability decreases from west to east or from spruce forest to larch forest. Differences in the methods for estimation of N availability make direct comparison difficult among the estimated values. This is because such processes as recovery of N from needles before litterfall, which accounted for a large amount of N at the scale of individual trees in this study, is not always taken into consideration in the methods based on net primary production. Nonetheless, all compared estimations are within the same order of magnitude, regardless of the method used for calculation. Among the ecosystems compared, our study in eastern Siberia and the study performed in Central Siberia showed the lowest utilization of N.
Table 5 Nitrogen (N) availability estimated in various types of boreal forest
In the current study, we demonstrated the strong dependence of inorganic N production by microbiota on soil temperatures. According to the Intergovernmental Panel on Climate Change (IPCC) Fourth Assessment Report (Solomon et al. Citation2007), it is expected that in the area of our study, the mean summer air temperatures are going to increase over the coming 70 to 80 years by 3–3.5°C. This considerable change would lead to an increase of soil temperatures and thus the production of inorganic N by microbes. On the other hand, the same report suggests a prospective significant increase in precipitation, especially during the winter (up to 50% of the current amounts). If this leads to later snowmelt, the timing of microorganism development and inorganic N production may be delayed.
5. CONCLUDING REMARKS
N transformation in soil is a very dynamic process with large seasonal variation: There was a rapid increase in the soil inorganic N pool in late summer followed by consumption by soil microbiota in the fall. Larch trees could not uptake organic N directly from the soil. The main source of N for plants was the inorganic N (either ammonium or nitrate) that existed in the soil solution. The amount of N supplied by mass flow was barely enough to meet the demand of the larch trees. Soil temperature was one of the main factors limiting inorganic N availability.
6. SUPPLEMENTARY MATERIAL
Supplementary material for this article is available online at http:////dx.doi.org/10.1080/00380768.2013.772495
Supplementary Table S1-S3
Download PDF (51.3 KB)ACKNOWLEDGEMENTS
We thank all Institute for Biological Problems of Cryolithozone members working with us at the Spasskaya Pad experimental forest station. We also thank Y. Hoshino, K. Tanaka, and K. Saito and other members of our laboratories. This work was financially supported by Integrated Field Environmental Science-Global Center of Excellence (IFES-GCOE) and Grant-in-Aid for Scientific Research (Kakenhi) 21403011 granted by Japan Society for the Promotion of Science.
REFERENCES
- Aber , JD , Nadelhoffer , KJ , Steudler , P and Melillo , JM . 1989 . Nitrogen saturation in Northern forest ecosystems . BioSci. , 39 : 378 – 386 .
- Abrams , MM and Jarrell , WM . 1992 . Bioavailability index for phosphorus using ion-exchange resin impregnated membranes . Soil Sci. Soc. Am. J. , 56 : 1532 – 1537 .
- Aerts , R , Chapin , III FS , Fitter , AH and Raffaelli , DG . 1999 . The mineral nutrition of wild plants revisited: a re-evaluation of processes and patterns . Adv. Ecol. Res. , 30 : 1 – 67 .
- Anderson , N , Strader , R and Davidson , C . 2003 . Airborne reduced nitrogen: Ammonia emissions from agriculture and other sources . Environ. Int. , 29 : 277 – 286 .
- Aoki , M , Fujii , K and Kitayama , K . 2012 . Environmental control of root exudation of low-molecular weight organic acids in tropical rainforests . Ecosystems , 15 : 1194 – 1203 .
- Archegova , IB , Zabolotskaya , TG and Kononenko , AV . 1976 . Productivity and Cycle of Elements in Phytocenoses of North , Leningrad (in Russian : Nauka .
- Bauer , GA , Persson , H , Persson , T , Mund , M , Hein , M , Kummetz , E , Matteucci , G , Hv , Oene , Scarascia-Mugnozza , G and Schulze , ED . 2000 . “ Linking plant nutrition and ecosystem processes ” . In Carbon and Nitrogen Cycling in European Forest Ecosystems , Edited by: Schulze , ED . 63 – 98 . Heidelberg : Springer Verlag .
- Binkley , D and Matson , P . 1983 . Ion-exchange resin bag method for assessing forest soil-nitrogen availability . Soil Sci. Soc. Am. J. , 47 : 1050 – 1052 .
- Borner , AP , Kielland , K and Walker , MD . 2008 . Effects of simulated climate change on plant phenology and nitrogen mineralization in Alaskan arctic Tundra . Arct. Antarct. Alp. Res. , 40 : 27 – 38 .
- Bouwman , AF , Lee , DS , Asman , WAH , Dentener , FJ , VanderHoek , KW and Olivier , JGJ . 1997 . A global high-resolution emission inventory for ammonia . Global Biogeochem. Cycl. , 11 : 561 – 587 .
- Cassman , KG and Munns , DN . 1980 . Nitrogen mineralization as affected by soil moisture, temperature, and depth . Soil Sci. Soc. Am. J. , 44 : 1233 – 1237 .
- Chapin , FS , McFarland , J , McGuire , AD , Euskirchen , ES , Ruess , RW and Kielland , K . 2009 . The changing global carbon cycle: Linking plant-soil carbon dynamics to global consequences . J. Ecol. , 97 : 840 – 850 .
- Coheur , PF , Clarisse , L , Turquety , S , Hurtmans , D and Clerbaux , C. 2009 . IASI measurements of reactive trace species in biomass burning plumes . Atmos. Chem. Phys. , 9 : 5655 – 5667 .
- Curtin , D , Syers , JK and Smillie , GW . 1987 . The importance of exchangeable cations and resin-sink characteristics in the release of soil phosphorus . J. Soil Sci. , 38 : 711 – 716 .
- Dise , NB and Wright , RF . 1995 . Nitrogen leaching from European forests in relation to nitrogen deposition . For. Ecol. Manage. , 71 : 153 – 161 .
- Dolman , AJ , Maximov , TC , Moors , EJ , Maximov , AP , Elbers , JA , Kononov , AV , Waterloo , MJ and van der Molen , MK . 2004 . Net ecosystem exchange of carbon dioxide and water of far eastern Siberian Larch (Larix cajanderii) on permafrost . Biogeosci. , 1 : 133 – 146 .
- Dörr , N , Kaiser , K , Sauheitl , L , Lamersdorf , N , Stange , CF and Guggenberger , G . 2012 . Fate of ammonium 15N in a Norway spruce forest under long-term reduction in atmospheric N deposition . Biogeochem. , 107 : 409 – 422 .
- Fenn , ME , Poth , MA , Aber , JD , Baron , JS , Bormann , BT , Johnson , DW , Lemly , AD , McNulty , SG , Ryan , DF and Stottlemyer , R . 1998 . Nitrogen excess in North American ecosystems: Predisposing factors, ecosystem responses, and management strategies . Ecol. Appl. , 8 : 706 – 733 .
- Galloway , JN , Dentener , FJ and Capone , DG . 2004 . Nitrogen cycles: Past, present, and future . Biogeochem. , 70 : 153 – 226 .
- Giblin , AE , Laundre , JA , Nadelhoffer , KJ and Shaver , GR . 1994 . Measuring nutrient availability in Arctic soils using ion-exchange resins: A field-test . Soil Sci. Soc. Am. J. , 58 : 1154 – 1162 .
- Goodale , CL , Apps , MJ and Birdsey , RA . 2002 . Forest carbon sinks in the Northern Hemisphere . Ecol. Appl. , 12 : 891 – 899 .
- Gordon , AG . 1979 . “ Productivity and nutrient cycling in natural forests ” . In Report of the Biomass Strategy Consultation , 34 – 49 . Ottawa , , Canada : CANADA/MAB .
- Gower , ST and Richards , JH . 1990 . Larches – deciduous conifers in an evergreen world . BioSci. , 40 : 818 – 826 .
- Harrison , AF , Schulze , ED , Gebauer , G and Bruckner , G . 2000 . “ Canopy uptake and utilization of atmospheric pollutant nitrogen ” . In Carbon and Nitrogen Cycling in European Forest Ecosystems , Edited by: Schulze , ED . 171 – 188 . Berlin and Heildelberg, Germany : Springer-Verlag .
- Hobbie , SE . 1996 . Temperature and plant species control over litter decomposition in Alaskan tundra . Ecol. Monogr. , 66 : 503 – 522 .
- Ivanova , TI , Kononova , NP , Nikolaeva , NV and Chevychelov , AP. 2006 . Microorganisms in forest soils of Central Yakutia . Eurasian Soil Sci. , 39 : 661 – 666 .
- Jones , DL , Healey , JR , Willett , VB , Farrar , JF and Hodge , A . 2005 . Dissolved organic nitrogen uptake by plants – An important N uptake pathway? . Soil Biol. Biochem. , 37 : 413 – 423 .
- Kaiser , C , Fuchslueger , L and Koranda , M . 2011 . Plants control the seasonal dynamics of microbial N cycling in a beech forest soil by belowground C allocation . Ecology , 92 : 1036 – 1051 .
- Kelliher , FM , Hollinger , DY and Schulze , ED . 1997 . Evaporation from an eastern Siberian larch forest . Agr. For. Meteorol. , 85 : 135 – 147 .
- Kielland , K . 1994 . Amino acid absorption by Arctic plants – Implications for plant nutrition and nitrogen cycling . Ecology , 75 : 2373 – 2383 .
- Kielland , K , Olson , K , Ruess , RW and Boone , RD . 2006 . Contribution of winter processes to soil nitrogen flux in taiga forest ecosystems . Biogeochem. , 81 : 349 – 360 .
- Koide , T , Saito , H , Shirota , T , Iwahana , G , Lopez c , ML , Maximov , TC , Hasegawa , S and Hatano , R . 2010 . Effects of changes in the soil environment associated with heavy precipitation on soil greenhouse gas fluxes in a Siberian larch forest near Yakutsk . Soil Sci. Plant Nutr. , 56 : 645 – 662 .
- Kononov AV 2006: Carbon dioxide emission from frozen soils of Central Yakutia larch forests depending on hydrothermal conditions (PhD thesis). Institute for Biological Problems of Cryolithozone from the Siberian Branch of the Russian Academy of Sciences (IBPC SB RAS), Yakutsk, Russia (in Russian).
- Koyama , L and Kielland , K . 2011 . Plant physiological responses to hydrologically mediated changes in nitrogen supply on a boreal forest floodplain: A mechanism explaining the discrepancy in nitrogen demand and supply . Plant Soil , 342 : 129 – 139 .
- Lambers , H , Chapin , FS and Pons , TL . 2008 . “ Mineral nutrition ” . In Plant Physiological Ecology , 255 – 320 . New York : Springer .
- Lipson , DA and Monson , RK . 1998 . Plant-microbe competition for soil amino acids in the alpine tundra: Effects of freeze–thaw and dry-rewet events . Oecologia , 113 : 406 – 414 .
- Lisuzzo , NJ , Kielland , K and Jones , JB . 2008 . Hydrologic controls on nitrogen availability in a high-latitude, semi-arid floodplain . Ecoscience , 15 : 366 – 376 .
- Lukina , NV and Nikonov , VV . 1996 . Biological Cycles in Northern Forests under Aerotechnogenic Pollution , Russia (in Russian) : RAS Publishing House, Apatity .
- Makarov , VN. 2007 . “ Atmospheric deposition fluxes of hydrogen, sulfur and nitrogen in Central Yakutia ” . In The 3rd International Conference on “The role of permafrost ecosystems in global climate change” Edited by: Ivanov , BI and Maximov , TC . 175 – 180 . Yakutsk , , Russia YSC Publishing House SB RAS,
- Matsuura , Y and Hirobe , M . 2010 . “ Soil carbon and nitrogen, and characteristics of soil active layer in Siberian permafrost region ” . In Permafrost Ecosystems. Siberian Larch Forests , Edited by: Osawa , A , Zyryanova , OA , Matsuura , Y , Kajimoto , T and Wein , RW . 149 – 163 . Netherlands : Springer .
- Maximov , TC , Kononov , AV , Petrov , KA and Ivanov , BI . 2010 . “ Structural and functional peculiarities of the plants of Yakutia ” . In The Far North , Edited by: Troeva , EI , Isaev , AP , Cherosov , MM and Karpov , NS . 317 – 355 . Netherlands : Springer .
- Moore , S . 1968 . Amino acid analysis. Aqueous dimethyl sulfoxide as solvent for the ninhydrin reaction . J. Biol. Chem. , 243 : 6281 – 6283 .
- Nadelhoffer , KJ , Aber , JD and Melillo , JM . 1984 . Seasonal patterns of ammonium and nitrate uptake in nine temperate forest ecosystems . Plant Soil , 80 : 321 – 335 .
- Nadelhoffer , KJ , Giblin , AE , Shaver , GR and Laundre , JA . 1991 . Effects of temperature and substrate quality on element mineralization in six arctic soils . Ecology , 72 : 242 – 253 .
- Ohta , T , Hiyama , T , Tanaka , H , Kuwada , T , Maximov , TC , Ohata , T and Fukushima , Y . 2001 . Seasonal variation in the energy and water exchanges above and below a larch forest in eastern Siberia . Hydrol. Processes , 15 : 1459 – 1476 .
- Ohta , T , Maximov , TC and Dolman , AJ . 2008 . Interannual variation of water balance and summer evapotranspiration in an eastern Siberian larch forest over a 7-year period (1998–2006 . Agr. For. Meteorol. , 148 : 1941 – 1953 .
- Olivier , JGJ , Bouwman , AF , Vandermaas , CWM and Berdowski , JJM . 1994 . Emission database for global atmospheric research (EDGAR . Environ. Monit. Assess. , 31 : 93 – 106 .
- Polischuk , AI , Svistov , PF , Pavlova , MT and Pershina , MA . 2011 . “ Background levels of ionic contents of atmospheric deposition ” . In Review of Background Environmental Situation in CIS for 2009–2010 , Edited by: Izrael , YA . 48 – 57 . Moscow, Russia (in Russian : Roshydromet .
- Pozdnyakov LK 1975: The Daur Larch (Larix dahurica), Nauka, Moscow (in Russian).
- Pozdnyakov , LK , Protopopov , VV and Gorbatenko , VM . 1969 . Biological Productivity of Forests of Central Siberia and Yakutia , Krasnoyarsk (in Russian : Sukachev Institute of Forest SB RAS USSR .
- Ruess , RW , VanCleve , K , Yarie , J and Viereck , LA . 1996 . Contributions of fine root production and turnover to the carbon and nitrogen cycling in taiga forests of the Alaskan interior . Can. J. For. Res./Rev. Can. Rech. For. , 26 : 1326 – 1336 .
- Schulze , ED , Chapin , FS and Gebauer , G . 1994 . Nitrogen nutrition and isotope differences among life forms at the Northern treeline of Alaska . Oecologia , 100 : 406 – 412 .
- Schulze , ED , Lloyd , J and Kelliher , FM . 1999 . Productivity of forests in the Eurosiberian boreal region and their potential to act as a carbon sink–A synthesis . Global Change Biol. , 5 : 703 – 722 .
- Schulze , ED , Schulze , W and Koch , H . 1995 . Aboveground biomass and nitrogen nutrition in a chronosequence of pristine Dahurian Larix stands in eastern Siberia . Can. J. For. Res./Rev. Can. Rech. For. , 25 : 943 – 960 .
- Shaver , GR and Chapin , FS . 1991 . Production–Biomass relationships and element cycling in contrasting arctic vegetation types . Ecol. Monogr. , 61 : 1 – 31 .
- Shibuya , M , Saito , H , Sawamoto , T , Hatano , R , Yajima , T , Takahashi , K , Cha , JY , Isaev , AP and Maximov , TC . 2004 . Time trend in aboveground biomass, net primary production and carbon storage of natural Larix gmelinii stands in Eastern Siberia . Eurasian J. For. Res. , 7 : 67 – 74 .
- Shugalei , LS and Vedrova , EF . 2004 . Nitrogen pool in northern-taiga larch forests of central Siberia . Biol. Bull. , 31 : 200 – 208 .
- Sierra , J . 1997 . Temperature and soil moisture dependence of N mineralization in intact soil cores . Soil Biol. Biochem. , 29 : 1557 – 1563 .
- Skogley , EO and Dobermann , A . 1996 . Synthetic ion-exchange resins: Soil and environmental studies . J. Environ. Qual. , 25 : 13 – 24 .
- Solomon , S , Qin , D and Manning , M , eds. 2007 . “ Climate Change 2007: The Physical Science Basis Contribution of Working Group I to the Fourth Assessment. Report of the Intergovernmental Panel on Climate Change. ” . In Cambridge University Press , UK and New York, NY : Cambridge .
- Sugimoto , A , Naito , D , Yanagisawa , N , Ichiyanagi , K , Kurita , N , Kubota , J , Kotake , T , Ohata , T , Maximov , TC and Fedorov , AN . 2003 . Characteristics of soil moisture in permafrost observed in East Siberian taiga with stable isotopes of water . Hydrol. Processes , 17 : 1073 – 1092 .
- Sugimoto , A , Yanagisawa , N , Naito , D , Fujita , N and Maximov , TC . 2002 . Importance of permafrost as a source of water for plants in east Siberian taiga . Ecol. Res. , 17 : 493 – 503 .
- Tokuchi , N , Hirobe , M , Kondo , K , Arai , H , Hobara , S , Fukushima , K and Matsuura , Y. 2010 . “ Soil nitrogen dynamics in larch ecosystem ” . In Permafrost Ecosystems. Siberian Larch Forests , Edited by: Osawa , A , Zyryanova , OA , Matsuura , Y , Kajimoto , T and Wein , RW . 229 – 243 . Netherlands : Springer .
- Vitousek , PM , Hattenschwiler , S , Olander , L and Allison , S . 2002 . Nitrogen and nature . Ambio , 31 : 97 – 101 .