Abstract
Electrolyte concentration changes the surface tension of the water-air interface, which is expected to affect the solid-water contact angle. In the present study, we focused on examining the effects of aqueous electrolyte concentration on the contact angle of soil samples with different hydrophobicities using sodium chloride (NaCl) and calcium chloride (CaCl2) solutions. Japanese Andisol and silica sand were hydrophobized using stearic acid to obtain different hydrophobicities. The contact angle of all the samples increased with increasing electrolyte concentration. This was attributed to the increasing surface tension of the solutions. The response of contact angle to the electrolyte concentration was positively correlated with soil surface free energy fitting into a linear relationship in Andisol, but not in silica sand. Surface tension of electrolyte solutions increases linearly with electrolyte concentration up to high salinities, and the contact angle can be expected to increase with increasing electrolyte concentration in a corresponding pattern. The relationship observed in the present study was non-linear, where more prominent increase of contact angle was observed at low electrolyte concentrations. The response of contact angle to the increasing electrolyte concentration was almost negligible at concentrations higher than 0.06 mol L−1, [ionic strength (I): 0.06 mol L−1] for NaCl, and 0.1 mol L−1, (I: 0.3 mol L−1) for CaCl2 solutions. The lowered solid-liquid interfacial free energy and its effects on contact angle with increasing adsorption of ions at the interface was considered to be the reason for decreasing slopes of the curves. Initial increase in contact angle with increasing ionic strength of CaCl2 was lower than that of NaCl. This might be because the adsorption of ions on soil surfaces, and the consequent effects on lowering the free energy of the solid-liquid interface and the contact angle, are more pronounced for calcium ions (Ca2+) than for sodium ions (Na+).
INTRODUCTION
Dissolved salts in irrigation water cause numerous challenges in the agriculture sector. They may provoke problems such as water stress in plants, toxicity, reduced availability of micronutrients, etc. All irrigation water contains dissolved mineral salts, where the concentration and composition of salts vary depending on the sources of the irrigation water. The most common cations found in irrigation water are calcium (Ca2+), magnesium (Mg2+), and sodium (Na+), whereas the most commonly found anions are chloride (Cl−), sulfate (SO4 2–), and bicarbonate (HCO3 −). Potassium (K+) and nitrate (NO3 −) ions are usually observed in minor amounts (Pratt and Suarez Citation1996; Grattan Citation2002).
High levels of Na+ can impede the infiltration rate of water into soil by dispersing soil aggregates and forming crusts on the soil surface. According to Ayers and Westcot (Citation1985), irrigation water with a high electrical conductivity (EC) would have fewer problems with infiltration compared with low-EC water. The quality of water affects both the aggregation and dispersion of soil colloidal material, and has been linked with many important soil phenomena including surface sealing and crusting, infiltration, runoff, illuviation and erosion. Dissolved salts in water may also influence the wettability of soils as a result of the strong interaction between water quality and various constituents in soils and on particle surfaces.
The wettability of soils is a dynamic surface property that is interlinked with numerous biological, chemical and physical soil properties, and various environmental factors. Contact angle, being the quantitative measurement of wettability, is therefore influenced by many environmental and physicochemical factors such as relative humidity, temperature, presence of organic matter, roughness and heterogeneity of the surfaces, shape and size of the particles, liquid properties, drop size, etc. (Dekker et al. Citation1998; De Jonge et al. Citation1999; Lam et al. Citation2001; Doerr et al. Citation2005; Hamlett et al. Citation2011; Leelamanie and Karube Citation2012). In spite of the fact that determining contact angles is challenging as there are too many factors affecting the measurements, the contact angle is of practical importance in understanding the hydrologic functions of unsaturated soils during natural wetting and drying processes. A significant amount of effort has been made to find the correlations between these various factors and the contact angle. For a good understanding of ion transport and wetting behavior in an unsaturated porous medium, determining the effects of aqueous electrolytes on soil-water contact angle is required.
The surface tension of any pure liquid (water or organic liquid) will change when another substance (solute) is dissolved in it. The change in surface tension will depend on the characteristics of the solute added. In general, the surface tension of water increases when inorganic salts (such as sodium chloride (NaCl), potassium chloride (KCl), sodium sulfate (Na2SO4), calcium chloride, (CaCl2), etc.) are dissolved (Jones and Ray Citation1941; Bostrom et al. Citation2001; Ozdemir et al. Citation2009; Matubayasi et al. Citation2011; Holthusen et al. Citation2012), while it decreases when organic substances (ethanol, methanol, fatty acids, soaps, detergents, etc.) are added. The increase in surface tension, Δγ, with dissolved salts is proportional to the concentration of salt, c, Δγ = Ωc, where Ω is usually positive and depends on both the anion and the cation (Bostrom et al. Citation2001). At high ionic strengths, the effect of ions on water's structure may be responsible for the variation of surface tension with salinity (Horne and Young Citation1972; Ralston and Healy Citation1973; Manciu and Ruckenstein Citation2003, Citation2005). Dissolved salts in water form cations and anions. Each ionic species experiences different attractive or repulsive forces near the air-water interface, which together lead to an increase or decrease of concentration near the interface. This is considered to be the change in concentration that leads to the surface tension increment. Leroy et al. (Citation2010) explained that structure-making ions such as Na+ flee from the air-water interface to organize in the bulk water. The increase in surface tension of water upon the addition of electrolytes has been explained by the repulsion or negative adsorption of ions from the gas/water interface. According to the Gibbs adsorption equation, any solute that increases the surface tension of water may exhibit a negative total adsorption at the air/water interface (Weissenborn and Pugh Citation1996).
Wetting of a solid surface by a liquid is associated with molecular attractions at the solid-liquid interface. The surface molecules of a liquid with low surface tension have a stronger attraction to molecules of a solid surface than to each other, which would result in surface wetting showing lower contact angles. Alternatively, large contact angles can be observed in the case of liquids with high surface tensions, because the liquid molecules are more strongly attracted to each other than to the molecules of the solid surface. Accordingly, changes in the liquid surface tension related to the changes in aqueous electrolyte concentration are expected to affect the solid-water contact angle.
Gomari and Hamouda (Citation2006) observed advancing contact angles of n-decane drops on calcite surfaces in the presence of different salts. Sghaier et al. (Citation2006) reported the influence of sodium chloride (NaCl) concentration on the equilibrium contact angle of droplets of NaCl solution on various solid surfaces, including hydrophilic and hydrophobic glass plates, Plexiglas, Rhodorsil® RTV-2 (a silicone elastomer) (Sghaier et al. Citation2006), and silicon. They reported that the contact angle significantly increases with NaCl concentration on all hydrophilic surfaces, but not on the tested hydrophobic surfaces. In their experiment to find the role of divalent fatty acid salts in soil water repellency Graber et al. (Citation2009) studied the relation of calcium chloride (CaCl2) and NaCl concentration to water repellency for different kinds of soils. They used not the contact angle but the drop penetration time as the repellence index and reported that the concentration did not affect repellency up to 0.1 mol L−1, whereas it did affect repellency at 1 mol L−1. To the best of our knowledge, the effects of the aqueous electrolyte concentration on the soil-water contact angle has not been addressed so far. Therefore, the objective of this study was to examine the effects of aqueous electrolyte concentration on contact angle of soil samples with different hydrophobicities using aqueous solutions of NaCl and CaCl2.
MATERIALS AND METHODS
Sample preparation
Fine silica sand, bought from Tohoku Keisha Co., Yamagata, Japan (bulk density 1.30 Mg m−3, particle density 2.65 Mg m−3, fine sand 98.7%, silt 1.3%), and Andisol (surface soils 0–5 cm) obtained from Field Science Center, Faculty of Agriculture, Ibaraki University, Japan, were used in the experiment. The basic properties of the Andisol are given in . Stearic acid (Wako pure chemical industries, Osaka, Japan), which is considered to be a common organic acid in natural soil (Deng and Dixon Citation2002), was used to hydrophobize silica sand and Andisol into different initial hydrophobicities. Andisol was air-dried and passed through a 2-mm sieve before the hydrophobization with stearic acid.
Table 1 Basic properties of Andisol used in the experiment
Silica sands with 0.2, 0.5, 1.0, 2.0, and 5 g kg−1 of stearic acid and Japanese Andisol with 0, 0.2, 1.0, 2.0, 5, and 10 g kg−1 of stearic acid were used for the experiment. Since stearic acid is insoluble in water, it was dissolved in diethyl ether and mixed with the sand and soils in a fume hood. Samples were placed in the hood for 2 h to allow the complete volatilization of diethyl ether, and then kept in a thermostated room with 25 ± 1°C and 75 ± 5% of relative humidity for 1 d. Each soil sample was fixed on a double-sided 1.5 cm × 1.5 cm adhesive tape adhered on a glass slide (Leelamanie et al. Citation2008a) with six replicates.
Electrolyte solutions
Electrolyte solutions were prepared by dissolving NaCl and CaCl2·2H2O (Wako pure chemical industries, Osaka, Japan) separately in deionized water. Seven NaCl solutions and six CaCl2 solutions were prepared with concentrations ranging from 0 to 0.13 mol L−1 and 0 to 0.2 mol L−1, respectively. As the surface tension and EC of the electrolyte solutions vary with the temperature, all solutions were kept in a thermostated room at 25 ± 1°C.
The ionic strength, I, of each solution was calculated using:
Experimental conditions
All prepared samples were kept in a sealed chamber under 75% relative humidity, maintained using saturated NaCl solution (Leelamanie et al. Citation2008b), for 1 week before the contact angle measurement. The experiment was conducted in a thermostated room at 25 ± 1°C and 75 ± 5% relative humidity.
Contact angle measurement
The contact angle on each surface was measured using the sessile drop method (Bachmann et al. Citation2000). An electrolyte solution drop of 10 µL (±1% accuracy) was placed on the each prepared surface using a micro-pipette (PipetGene, TGK, Tokyo, Japan), in six replicates per surface, per electrolyte type, per electrolyte concentration, and a digital micro-photograph of the horizontal view of the drop was taken within 1 s using a digital microscopic camera (Leelamanie et al. Citation2008a). Using the microphotographs, the contact angle of each sample was directly measured considering the average of the dihedral angles at both three-phase contact points of the drop.
As the measurement takes about 1 s and was performed at a temperature of 25°C, droplet evaporation was considered negligible over the measurement duration.
RESULTS AND DISCUSSION
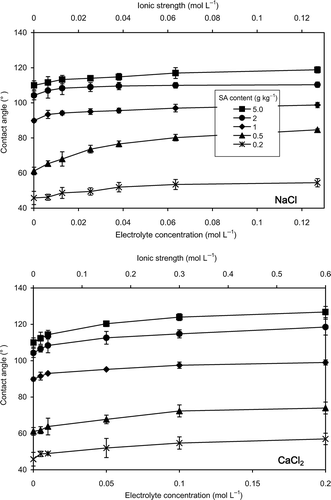
Effects of electrolyte concentration on contact angle of Andisol and silica sand are presented in and , respectively. The contact angles of all samples increased with increasing electrolyte concentration. The relationship was non-linear, where more prominent increase of contact angle was observed at low electrolyte concentrations.
Figure 1 Effects of the electrolyte concentration of sodium chloride (NaCl) and calcium chloride (CaCl2) on contact angle of Japanese Andisol. Error bars indicate ± standard deviation. SA, stearic acid.
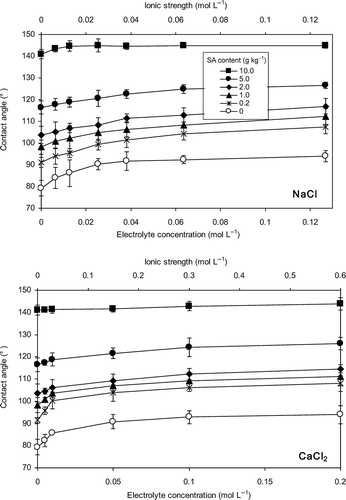
Figure 2 Effects of the electrolyte concentration of sodium chloride (NaCl) and calcium chloride (CaCl2) on contact angle of silica sand. Error bars indicate ± standard deviation. SA, stearic acid.
The addition of electrolytes into pure water would increase the surface tension of the solutions (Jones and Ray Citation1941; Bostrom et al. Citation2001; Ozdemir et al. Citation2009; Matubayasi et al. Citation2011; Holthusen et al. Citation2012). These surface tension increments can be related to the force balance at the three-phase contact line formed by the drops of electrolyte solutions on the soil surfaces, and therefore to the contact angle.
Contact angle describes the edge of the two-phase boundary (e.g., soil-water) where it ends at a third phase (e.g., gas). It is the dihedral angle formed at the interface among the three phases. The relationship between surface tension and contact angle was first recognized by Young (Citation1805). In principle, the contact angle is determined by the free energies of solid-gas, liquid-gas, and solid-liquid interfaces. Considering the force balance at the mechanical equilibrium:
As stated by EquationEq. 2(2), it is clear that the surface free energy of the solid (γsg) will favor, whereas the solid-liquid interfacial free energy (γsl) and the surface free energy (surface tension) of the liquid (γlg) will oppose, the spreading of the liquid on the plane of the solid surface. In a situation where the surface free energy of the solid is a constant, increase in liquid surface tension would increase the contact angle. Accordingly, increase in the contact angle of Andisol and silica sand with increasing electrolyte concentration was attributed to the increasing surface tension of the solutions.
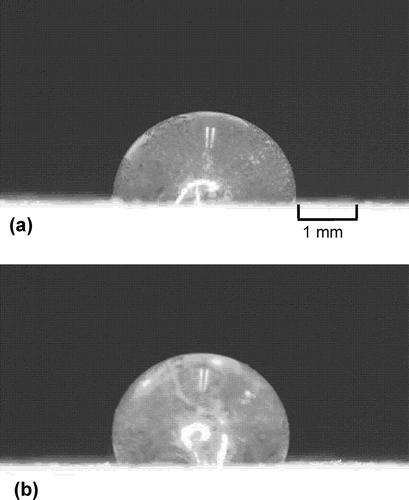
and b respectively present the microphotographs of the drops of deionized water (contact angle: 91°) and 0.2 mol L−1 CaCl2 solution (contact angle: 108°) on Andisol treated with 0.2 g kg−1 stearic acid. The difference in contact angle at the three-phase boundary, with two predetermined phases (solid and gas) and a varying liquid phase by means of the electrolyte concentration, can be clearly observed in the microphotographs.
Figure 3 Microphotographs showing the drops of (a) deionized water (contact angle: 91°) and (b) 0.2 mol L−1 calcium chloride (CaCl2; contact angle: 108°) on Andisol treated with 0.2 g kg−1 stearic acid.
Surface tension of electrolyte solutions increases with electrolyte concentration up to high salinities (Ozdemir et al. Citation2009; Leroy et al. Citation2010). Abramzon and Gaukhberg (Citation1993) reported that the surface tension increases with NaCl concentration up to 3 mol L−1 showing a near-linear tendency. Matubayasi et al. (Citation2011) found that the surface tension of aqueous CaCl2 solution increased with the electrolyte concentration up to 1 mol L−1 showing a linear correlation. Bostrom et al. (Citation2001) explained that the increase in surface tension, Δγ, is proportional to the concentration of salt, c, Δγ = Ωc, where Ω is usually positive. The contact angle, being positively related with surface tension of the solution, can also be expected to increase with increasing electrolyte concentration up to high salinities. In the present study, the contact angle-electrolyte concentration curves demonstrated a decreasing slope with increasing electrolyte concentration. The response of the contact angle to the increasing electrolyte concentration was almost negligible at concentrations higher than 0.06 mol L−1, (I: 0.06 mol L−1) for NaCl, and 0.1 mol L−1, (I: 0.3 mol L−1) for CaCl2 solutions. Considering the result, it can be suggested that another force, in addition to the increasing surface tensions of electrolyte solutions, in the three-phase boundary of the drop is changing with the electrolyte concentration and acting in favor of spreading. When a drop of electrolyte solution is placed on a soil surface, the cations in the solution would be adsorbed on the soil surface. From a thermodynamic viewpoint, adsorption of ions at the interface will decrease the solid-liquid interfacial free energy (Chaudhuri and Paria Citation2009), which will result in decreasing contact angle (EquationEq. 2(2)). However, the cohesion of superficial particles acts with full force in producing pressure when a fluid is surrounded by gas/vacuum, whereas this action will be partially destroyed when it is in contact with another material depending on the attractive power of the material (Young Citation1805). Accordingly, the liquid surface tension would be considerably higher than the solid-liquid interfacial free energy, and the increase in contact angle with increasing surface tension of the solutions will be more pronounced compared with the effect of lowered solid-liquid interfacial free energy. This might be the reason for the decreasing slopes of the contact angle-electrolyte concentration curves.
The response of contact angle to the increasing electrolyte concentration appeared to be low at high stearic acid contents for Andisol (). Hydrophobicity and the surface free energy of samples change with the stearic acid content. To examine this relation, the difference between contact angles measured at the lowest and the highest electrolyte concentrations was plotted against the soil surface free energy. The soil surface free energy, γsg (mN m−1), was determined using the contact angle of each sample observed with pure water. Carrillo et al. (Citation1999) related surface free energy, γsg, to the contact angle by combining Good and Girifalco's (Citation1960) equation and Young's equation (Young Citation1805).
Figure 4 Relation between soil surface free energy and the response of contact angle to the electrolyte concentration for Japanese Andisol. Error bars indicate ± standard deviation. NaCl, sodium chloride; CaCl2, calcium chloride.
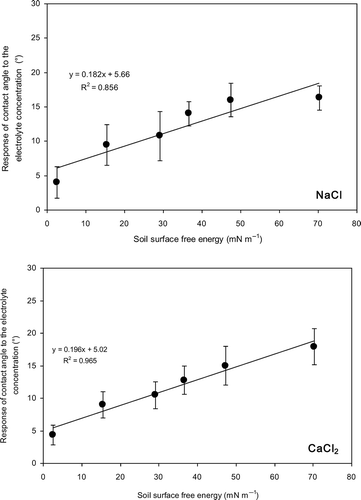
Figure 5 Relation between soil surface free energy and the response of contact angle to the electrolyte concentration for silica sand. Error bars indicate ± standard deviation. NaCl, sodium chloride; CaCl2, calcium chloride.
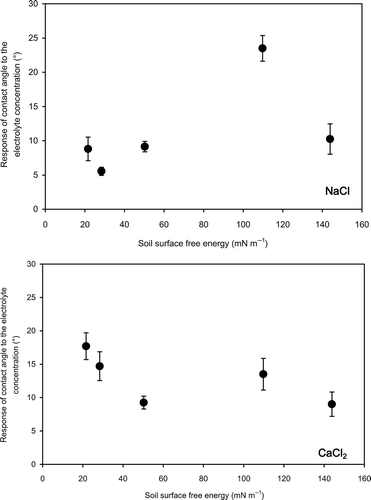
The effect of NaCl and CaCl2 on the initial increase in contact angle was compared at the same ionic strengths. Up to an ionic strength of 0.015 mol L−1, increase in contact angle with increasing ionic strength of CaCl2 was lower than that of NaCl according to the slopes of linear trend lines (). This might be explained using the adsorption of univalent and divalent cations at the solid-liquid interfaces. Adsorption of ions at the solid-liquid interface will decrease the interfacial energy (Chaudhuri and Paria Citation2009), which will result in decreasing contact angle as explained in EquationEq. 2(2). The higher the adsorption at the interface, the lower the solid-liquid interfacial free energy and the contact angle. According to the cation adsorption principles, Ca2+ will be more strongly adsorbed on soil surfaces due to its greater charge compared with Na+. Therefore, it can be considered that the effect of CaCl2 on lowering the free energy of the solid-liquid interface, and consequently the contact angle, is more prominent than that of NaCl. This might be the reason for lower initial increase in contact angle with increasing ionic strength of CaCl2 compared with that of NaCl.
Table 2 Slopes of trend lines for the initial linear increase in contact angle with increasing ionic strength (up to 0.015 mol L−1) of calcium chloride (CaCl2) and sodium chloride (NaCl)
Opposite results for the effect of CaCl2 and NaCl on drop penetration time are reported by Graber et al. (Citation2009) at high salt concentrations. They found a higher effect of CaCl2 than of NaCl at 1 M salt concentration. Their explanation for the higher effect of CaCl2 was the decrease of acid dissociation constant at high counterion concentration (1 mol L−1 CaCl2) and formation of additional Ca-fatty acid complexes, which is not applicable to our results as we tested the effect of electrolyte concentration only up to the hazardous limit for plants.
CONCLUSIONS
The effects of the electrolyte concentration on contact angle of different model soils were examined using aqueous solutions of NaCl and CaCl2. The contact angle of all the samples increased with increasing electrolyte concentration. The relationship was non-linear, where the increase of contact angle was more prominent at low electrolyte concentrations. The addition of electrolytes into pure water increases the surface tension of the solutions, which will oppose the spreading of the liquid on the soil surface. Accordingly, the increase in contact angle with increasing aqueous electrolyte concentration was attributed to the increasing surface tension of the solutions. The response of contact angle to the electrolyte concentration was positively correlated with soil surface free energy fitting into a linear relationship for Andisol, but not the silica sand, showing that the response of contact angle did not consistently correlate with the surface free energy, or the hydrophobicity, of soils.
Surface tension of aqueous electrolyte solutions such as NaCl and CaCl2 increases with electrolyte concentration up to high salinities showing a linear correlation. The contact angle, being positively related to surface tension of the solution, can also be expected to increase with increasing electrolyte concentration in a corresponding pattern. The relationship observed in the present study was non-linear, where a more prominent increase of contact angle was observed at low electrolyte concentrations. The lowered solid-liquid interfacial free energy and its effects on contact angle with increasing adsorption of ions at the interface were considered to be the reason for the decreasing slopes of the curves. Initial increase in contact angle with increasing ionic strength of CaCl2 was lower than that of NaCl. This might be because the adsorption of ions on soil surfaces, and the consequent effects on lowering the free energy of the solid-liquid interface and the contact angle, are more pronounced for Ca2+ than for Na+.
It was clear that a low electrolyte concentration has large effect on contact angle, and small changes in contact angle may affect infiltration in soils. Therefore, it can be concluded that a slight change in the salinity of irrigation water might strongly affect the soil moisture dynamics.
ACKNOWLEDGMENTS
The Japan Society for the Promotion of Science is gratefully acknowledged for providing an Invitation Fellowship for Research in Japan (Long-Term).
REFERENCES
- Abramzon , A and Gaukhberg , RD . 1993 . Surface tension of salt solutions . Russian J. Appl. Chem , 66 : 1473 – 1480 .
- Ayers R, Westcot DW 1985: Water Quality for Agriculture. FAO Irrigation and Drainage Paper 29 Rev. 1. Food and Agriculture Organization of the United Nations. Rome. (December, 2012). http://www.fao.org/docrep/003/T0234E/T0234E00.htm#TOC (http://www.fao.org/docrep/003/T0234E/T0234E00.htm#TOC)
- Bachmann , J , Horton , R , van der Ploeg , RR and Woche , S . 2000 . Modified sessile drop method for assessing initial soil–water contact angle of sandy soil . Soil Sci. Soc. Am. J , 64 : 564 – 567 .
- Bostrom , M , Williams , DRM and Ninham , BW . 2001 . Surface tension of electrolytes: Specific ion effects explained by dispersion forces . Langmuir , 17 : 4475 – 4478 .
- Carrillo , MLK , Letey , J and Yates , SR . 1999 . Measurement of initial soil-water contact angle of water repellent soils . Soil Sci. Soc. Am. J , 63 : 433 – 436 .
- Chaudhuri , RG and Paria , S . 2009 . Dynamic contact angles on PTFE surface by aqueous surfactant solution in the absence and presence of electrolytes . J. Colloid Interface Sci , 337 : 555 – 562 .
- De Jonge , LW , Jacobsen , OH and Moldrup , P . 1999 . Soil water repellency: Effects of water content, temperature and particle size . Soil Sci. Soc. Am. J , 63 : 437 – 442 .
- Dekker , LW , Ritsema , CJ , Oostindie , K and Boersma , OH . 1998 . Effect of drying temperature on the severity of soil water repellency . Soil Sci , 163 : 780 – 796 .
- Deng , Y and Dixon , JB . 2002 . “ Soil organic matter and organic-mineral interactions ” . In Soil Mineralogy with Environmental Applications , Edited by: Dixon , JB and Schulze , DG . 69 – 107 . Madison , WI : SSSA . InSSSA Book Series No. 7
- Doerr , SH , Llewellyn , CT and Douglas , P . 2005 . Extraction of compounds associated with water repellency in sandy soils of different origin . Aust. J. Soil Res , 43 : 225 – 237 .
- Gilboa , A , Bachmann , J , Woche , SK and Chen , Y . 2006 . Applicability of interfacial theories of surface tension to water-repellent soils . Soil Sci. Soc. Am. J , 70 : 1417 – 1429 .
- Gomari , KAR and Hamouda , AA . 2006 . Effect of fatty acids, water composition and pH on the wettability alteration of calcite surface . J. Pet. Sci. Eng , 50 : 140 – 150 .
- Good , RJ and Girifalco , LA . 1960 . A theory for the estimation of surface and interfacial energies. III. Estimation of surface energies of solids from contact angle data . J. Phys. Chem , 64 : 561 – 565 .
- Graber , ER , Tagger , S and Wallach , R . 2009 . Role of divalent fatty acid salts in soil water repellency . Soil Sci. Soc. Am. J , 73 : 541 – 549 .
- Grattan SR 2002: Irrigation water salinity and crop production. Publication 8066. FWQP Reference Sheet 9.10. Agriculture and Natural Resources. University of California. (December, 2012). http://anrcatalog.ucdavis.edu/pdf/8066.pdf (http://anrcatalog.ucdavis.edu/pdf/8066.pdf)
- Hamlett , CA , Shirtcliffe , NJ , McHale , G , Ahn , S , Bryant , R , Doerr , SH and Newton , MI . 2011 . Effect of particle size on droplet infiltration into hydrophobic porous media as a model of water repellent soil . Environ. Sci. Technol , 45 : 437 – 442 .
- Holthusen , D , Haas , C , Peth , S and Horn , R . 2012 . Are standard values the best choice? A critical statement on rheological soil fluid properties viscosity and surface tension . Soil Tillage Res , 125 : 61 – 71 .
- Horne , RA and Young , RP . 1972 . Cation exclusion by interfacial water structure . Electrochim. Acta , 17 : 763 – 767 .
- Jones , G and Ray , WA . 1941 . The surface tension of solutions of electrolytes as a function of the concentration II . J. Am. Chem. Soc , 63 : 288 – 294 .
- Lam , CNC , Kim , N , Hui , D , Kwok , DY , Hair , ML and Neumann , AW . 2001 . The effect of liquid properties to contact angle hysteresis . Colloids Surf., A , 189 : 265 – 278 .
- Leelamanie , DAL and Karube , J . 2012 . Drop size dependence of soil-water contact angle in relation to the droplet geometry and line tension . Soil Sci. Plant Nutr , 58 : 675 – 683 .
- Leelamanie , DAL , Karube , J and Yoshida , A . 2008a . Characterizing water repellency indices: Contact angle and water drop penetration time of hydrophobized sand . Soil Sci. Plant Nutr , 54 : 179 – 187 .
- Leelamanie , DAL , Karube , J and Yoshida , A . 2008b . Relative humidity effects on sessile drop contact angle and water drop penetration time of sand with hydrophobic coatings . Soil Sci. Plant Nutr , 54 : 695 – 700 .
- Leroy , P , Lassin , A , Azaroual , M and André , L . 2010 . Predicting the surface tension of aqueous 1-1 electrolyte solutions at high salinity . Geochim. Cosmochim. Acta , 74 : 5427 – 5442 .
- Manciu , M and Ruckenstein , E . 2003 . Specific ion effects via ion hydration: I. Surface tension . Adv. Colloid Interface Sci , 105 : 63 – 101 .
- Manciu , M and Ruckenstein , E . 2005 . On the interactions of ions with the air/water interface . Langmuir , 21 : 11312 – 11319 .
- Matubayasi , N , Takayama , K , Ito , R and Takata , R . 2011 . Thermodynamic quantities of surface formation of aqueous electrolyte solutions X. Aqueous solution of 2:1 valence-type salts . J. Colloid Interface Sci , 356 : 713 – 717 .
- Ozdemir , O , Karakashev , SI , Nguyen , AV and Miller , JD . 2009 . Adsorption and surface tension analysis of concentrated alkali halide brine solutions . Miner. Eng , 22 : 263 – 271 .
- Pratt , PF and Suarez , DL . 1996 . “ Irrigation water quality assessments ” . In Agricultural Salinity Assessment and Management , Edited by: Tanji , KK . 220 – 237 . New York : American Society of Civil Engineers . ASCE Manuals and Reports on Engineering Practice No. 71
- Ralston , J and Healy , TW . 1973 . Specific cation effects on water structure at the air-water and air-octadecanol monolayer-water interfaces . J. Colloid Interface Sci , 42 : 629 – 644 .
- Sghaier , N , Prat , M and Nasrallah , SB . 2006 . On the influence of sodium chloride concentration on equilibrium contact angle . Chem. Eng. J , 122 : 47 – 53 .
- Weissenborn , PK and Pugh , RJ . 1996 . Surface tension of aqueous solutions of electrolytes: Relationship with ion hydration, oxygen solubility, and bubble coalescence . J. Colloid Interface Sci , 184 : 550 – 563 .
- Young , T . 1805 . An essay on the cohesion of fluids . Phil. Trans. R. Soc. London , 95 : 65 – 87 .