Abstract
The concentrations of 48 trace elements (Li, Be, Sc, V, Cr, Co, Ni, Cu, Zn, Ga, As, Br, Rb, Sr, Y, Zr, Nb, Mo, Ag, In, Cd, Sn, Sb, I, Cs, Ba, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Hf, Ta, W, Tl, Pb, Bi, Th and U) in 14 soils derived from limestone, sampled at three sites, are compared with the concentrations in 500 soil samples derived from a variety of other parent materials. The 500 samples were collected from 75 sites nationwide in order to include the wide range of common soil types in Japan. Most analytical results were obtained by inductively coupled plasma mass spectrometry (ICP-MS), but Cr, As, Br, Zr, Sn, and I concentrations were determined by energy dispersive X-ray fluorescence spectrometry (EDXRF), because the acid dissolution techniques employed in this study were found to be incapable of recovering these elements completely. In order to examine the reliability of analyses, the concentrations of many elements were also determined by EDXRF, inductively coupled plasma-atomic emission spectrometry (ICP-AES), and atomic absorption spectrometry (AAS). Box and whisker diagrams (Tukey plots), constructed using log-transformed values of each element, show clearly that geometric means of nearly all the trace elements in soils derived from limestone are higher than those in soils derived from other parent materials. The only exceptions are Sr, Ag and Eu, though statistical analysis (Student’s t-test) shows that the differences for these three elements were not significant at p < 0.05. Similarly, the observed differences of geometric means for Sc, Br and Ba between limestone soils and other types of soils were also not significant at p < 0.05. It can be concluded, therefore, that the concentrations of the above-mentioned 48 trace elements in soils derived from limestone are significantly higher than those in other types of soils, with the exception of Sc, Br, Sr, Ag, and Ba, though it was necessary to exclude 81 soil samples, developed on scoriaceous (basaltic) volcanic ash from Mt. Fuji, as an exceptional group for comparisons of V and Cu, as these soils contain higher levels of these two elements. The above results can be attributed to the gradual accumulation of trace elements in the limestone soils due to the intense weathering processes.
INTRODUCTION
In recent years, the concentration levels of a wide variety of trace elements in soils have been attracting increasing attention among researchers in various fields as basic information for studying the fate of elements in the environment. To assist in this research, we have already compiled data on concentration levels of as many elements as possible in a large number of soil samples (Egashira et al. Citation1996; Yamasaki et al. Citation2001; Nanzyo et al. Citation2002; Egashira et al. Citation2004; Takeda et al. Citation2004; Egashira et al. Citation2007; Nanzyo et al. Citation2007; Kimura et al. Citation2008; Yamasaki et al. Citation2009).
Although the weathering of rock-forming minerals is one of the main sources of trace elements in soils, soil processes that accumulate or mobilize trace elements must also be considered. In this context, limestone is a unique parent material: on the one hand, the concentration levels of trace elements in limestone are generally much lower than those of silicate parent materials (Imai et al. Citation1995, Citation1996) but, on the other hand, a large part of the parent limestone (CaCO3) is lost in the process of soil formation. For example, Temur et al. (Citation2009) calculated that the percentage of “washed-away limestone” was as high as 97.76% for Terra Rossa derived from the Mortas Formation in Konya, Turkey. Our results in this work also showed that whereas the contents of calcium oxide (CaO) in sand dune soils from the Ryukyu limestone were 34~35%, those in red soils derived from the same parent material were only less than 1%. In addition, the above intensive weathering proceeds under neutral pH conditions that favor the retention of transition elements. Accordingly, it can be expected that soils derived from limestone would contain much higher levels of trace elements than soils derived from other parent materials. In fact, there have been reports that support this prediction (Bellanca et al. Citation1996; Palumbo et al. Citation2000; Terashima et al. Citation2004), though only a few elements have been examined.
In our previous work (Yamasaki et al. Citation2001), our major concern was to examine the concentrations of 45 trace elements (Li, Be, Sc, V, Cr, Co, Ni, Cu, Zn, Ga, Rb, Sr, Y, Zr, Nb, Mo, Ag, Cd, In, Sn, Sb, Cs, Ba, La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er, Tm, Yb, Lu, Hf, Ta, W, Tl, Pb, Bi, Th and U) in soils and to consider their mutual correlations. The primary aim of the present research is to examine the differences in trace element concentrations in soils derived from limestone versus other parent materials. Furthermore, data on the concentrations of As, Br, and I, obtained by energy dispersive X-ray fluorescence spectrometry (EDXRF), have been added to the above 45 elements, and previous results for Cr, Zr, and Sn (obtained using inductively coupled plasma-mass spectrometry, ICP-MS) have been replaced by new results obtained by EDXRF.
MATERIALS AND METHODS
Soil samples
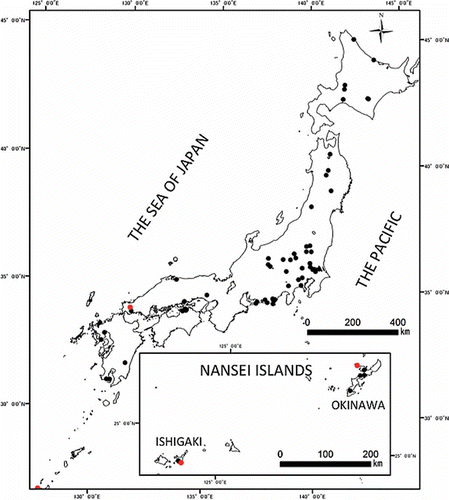
From a large number of soil samples stored at the Inventory Center of the National Institute for Agro-Environmental Sciences (NIAES), those sampled from 1976 to 1993 were used in this study. In addition, seven samples collected from two sites on Ishigaki Island were also included. Seventy-eight nationwide sampling sites were selected in order to include a wide range of soil types common to Japan (). Brief information on the sampling sites, land use and soil classification has been reported previously (Yamasaki et al. Citation2001; Takeda et al. Citation2004). Among these samples, 14 taken at three sampling sites are red soils derived from limestone, and they have been classified as limestone group soils, abbreviated as LGS. The remaining 500 samples were used as the reference samples for comparison purposes (termed as reference group soils, and abbreviated as RGS). More detailed information on each sampling site, including latitude and longitude, profile description, landscape and general chemical and physical properties of the soils collected by 2005 has been presented elsewhere (Nakai et al. Citation2006).
Analytical methods
Most of the data were obtained by ICP-MS after acid dissolution of the samples. However, Cr, As, Br, Zr, Sn and I were determined by EDXRF because the complete recovery of these elements was not achieved by the acid digestion procedure employed (Takeda et al. Citation2011: Yamasaki et al. Citation2011). However, only 490 RGS samples were analyzed with EDXRF because 10 samples were already used up for other purposes. Accordingly, the total numbers of data for Cr, As, Br, Zr, Sn and I in RGS were 490. EDXRF was also used to analyze for V, Ni, Cu, Zn, Rb, Sr, Y, Nb, Cs, Ba, La, Ce, Nd, Pb and Th (Matsunami et al. Citation2010; Yamasaki et al. Citation2011). Other techniques such as inductively coupled plasma-atomic emission spectrometry (ICP-AES) and atomic absorption spectrometry (AAS) were also used to check the reliability of the results. Analytical results using ICP-MS compared favorably with those using EDXRF, ICP-AES, and AAS in most cases, except in the cases of Co, Cr, Zr and Sn (Matsunami et al. Citation2010; Yamasaki et al. Citation2011). In contrast to Cr, Zr and Sn, discrepancies observed in Co were attributed to the overlapping of the huge iron (Fe) Kβ line on the Co Kα line, as was manifested by lower correlation coefficient of the calibration curve obtained by EDXRF (Yamasaki et al. Citation2011).
Sample dissolution for ICP-MS analyses
Half a gram of each finely ground sample was treated with 10 mL of perchloric acid (HClO4) + nitric acid (HNO3) (1:1 mixture) to decompose the organic matter in the soils, followed by a double treatment with 15 mL of HClO4 + hydrofluoric acid (HF) (1: 2 mixture) in a polytetrafluoroethylene (PTFE) beaker on a domestic hot plate. The residue was heated with 5 mL of HNO3 and dissolved by the addition of 30~50 mL of H2O with gentle boiling and finally made up to 100 mL. The final solutions were stored in plastic bottles until the measurements were made.
Sample preparation for EDXRF analyses
The samples were prepared in the form of pressed powder pellets, without adding binders. Samples were first pulverized for 10 min in a planetary ball mill made of agate at 450 rpm (pulverisette 7, Fritsch GmbH, Idar-Oberstein, Germany), and then pressed into pellets (internal diameter 32 mm) using a hydraulic press operated at 20 tonnes pressure (Specac Ltd, River House, 97 Cray Avenue, Orpington, Kent, UK).
ICP-MS measurement
After a 10~100 fold dilution, the acid digests were analyzed by ICP-MS for all the trace and ultra-trace elements. In was used as an internal standard element (Yamasaki Citation1996; Yamasaki Citation2000). For the determination of In, however, rhodium (Rh) was used as an internal standard element. The working standards were prepared from a series of SPEX Multi-Element Plasma Standards (XSTC-1, XSTC-7, XSTC-8, and XSTC-13), supplied by SPEX Industries, Inc. (New Jersey, USA). It was possible to obtain working standard solutions containing up to 70 elements in this way. A quadrupole type ICP-MS, (HP-4500, Hewlett Packard, now Agilent Technologies, Palo Alto, CA, USA), was used for most of trace elements (> 0.1 mg kg–1 soil). However, for several ultra-trace elements (< 0.1 mg kg–1) and/or trace elements susceptible to interferences due to spectral overlaps, it was necessary to use a high resolution ICP-MS, (ELEMENT, Finnigan MAT, now Thermo Scientific, Bremen, Germany) (Yamasaki et al. Citation1994). A desolvation system (MCN 6000) provided by CETAC Technologies, Inc. was used (Tao & Miyazaki Citation1995; Matoba et al. Citation1998; Prohaska et al. Citation1999) for the determination of Ag to avoid possible errors due to spectral overlaps of 91ZrO and 93NbO on 107Ag and 109Ag, respectively.
EDXRF measurements
X-ray fluorescence measurements were performed on an Epsilon 5 spectrometer (PANalytical B. V., Lelyweg, The Netherlands). The instrument was installed with an Sc/W anode X-ray tube, had a series of user-selectable secondary targets and was equipped with a liquid nitrogen-cooled Ge solid-state high-resolution detector with a Be (8 μm) window. The instrument adopted a three-dimensional design (or Cartesian geometry) to eliminate the X-ray tube spectrum by polarization.
The instrument was calibrated for 21 selected trace elements using 26 geological and environmental standard reference materials (Matsunami et al. Citation2010; Yamasaki et al. Citation2011). Compton scatter radiation was used as an internal standard to compensate for variations in sample matrix, particle size, packing density and instrumental operating characteristics.
Statistical analysis
Calculation of fundamental statistics and statistical significance test were performed using JMP Statistical Discovery Software provided by SAS Institute Inc. (SAS Campus Drive, Cary, NC 27513, USA).
RESULTS
Fundamental statistics
shows minimum, geometric mean, maximum and standard deviations of the trace element concentrations of RGS and LGS. Geometric means were adopted here because arithmetic means were shifted toward the higher direction by the small numbers of samples having exceptionally high concentrations. In fact, the ratio of arithmetic mean to geometric mean of RGS ranged from 1.08 to 3.58, and was 1.33 when averaged. Similar trends were observed in the results reported by Yanai et al. (Citation2012).
Table 1 Minimum, geometric mean, maximum and standard deviation of the trace element concentrations (mg kg–1)
Box and whisker diagram
The results obtained were summarized in , using the box and whisker diagrams (Tukey plot). In this figure, the ends of the boxes are the first and the third quartiles, and the differences between the quartiles (the vertical length of the boxes) are the interquartile range. The black and red lines across the middle of the boxes identify the median values and the geometric means, respectively. The whiskers (both lines from the boxes) extend from the ends of the boxes to the outermost data point that falls within 1.5 times the interquartile ranges. The points beyond the whiskers are regarded as outliers.
Figure 2 Box and whisker diagrams of the trace elements concentrations in the reference group soils (RGS) and the limestone group soils (LGS). Symbols of elements with red and blue colors indicate higher concentrations in LGS than in RGS samples at p < 0.01 and at p < 0.05 respectively. Significant differences are not observed at p < 0.05 for symbols with a black color. In case of V and Cu, scoriaceous soil samples were excluded for the construction of box and whisker diagrams. Raw data are available on request from the corresponding author.
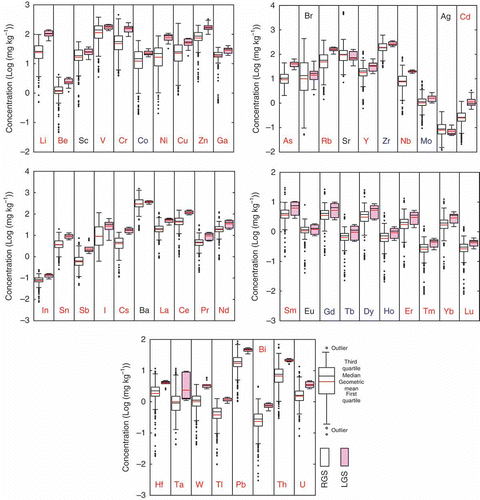
The plots were calculated using the log-transformed values of the analytical results because the frequency distributions of most trace element contents were positively skewed (Yamasaki et al. Citation2001; Kimura et al. Citation2008; Yamasaki et al. Citation2009).
By such a transformation, it became possible to detect the outliers in the lower range and, at the same time, the number of outliers in the higher range is considerably reduced. In case of Cd contents of RGS, for example, whereas the number of outliers in the lower range was as high as 33 when log-transformed values were used, there were no lower outliers if the plot was constructed using the real numbers of Cd contents. In contrast, when real numbers were used instead of log-transformed values, the number of higher outliers increased from zero to 18. Similar trends were observed for all the elements in RGS, though the effects of the log-transformation on the number of outliers differed for different elements.
shows that the geometric means of nearly all the trace elements in LGS are higher than those in RGS with the exception of Sr and Ag, though statistical analysis (Student’s t-test carried out without excluding outliers) shows that the differences of these two elements were not significant at p < 0.05. Similarly, the observed differences of geometric mean of Sc, Br and Ba between LGS and RGS were also not significant at p < 0.05. It can be concluded, therefore, that the concentrations of the all the elements in LGS were significantly higher (p < 0.05) than those in RGS with the exception of Sc, Br, Sr, Ag, and Ba.
DISCUSSION
Box and whisker diagram
shows that the median values are located near the center of boxes and the differences between the median value and geometric mean are very small in most cases. Accordingly, it would be reasonable to consider that the data appears to be obeying a nearly normal distribution after log-transformation, as has been shown by Yamasaki et al. (Citation2009), Kimura et al. (Citation2008), Yamasaki et al. (Citation2001) and Yanai et al. (Citation2012), among others.
Among the 500 soil samples used for comparative purposes (RGS), there were as many as 81 soils that had developed on scoriaceous (basaltic) volcanic ash that came from Mt. Fuji. These soils are widely distributed over the Kanto Plain (the largest plain in Japan), and they are unique in that the concentrations of the first transition elements, especially V and Cu, are much higher than in other soil types (Terashima et al. Citation2001; Okamoto & Kamiyama Citation2007). Accordingly, the concentration ranges for these two elements in RGS were obviously shifted higher as a result of those samples. Therefore, we excluded these samples on the grounds that they form an exceptional group in the box and whisker diagrams for V and Cu. When these samples were included for comparative purposes, the differences of geometric mean of V and Cu between RGS and LGS were not significant at the 5% level.
Outliers in box and whisker diagram
In the box and whisker diagrams, there are many lower outliers for most of the trace elements in the RGS. These can be attributed mainly to samples from peaty soils that are high in organic C (Dystric Histosol), sand dune soils high in SiO2 with more than 95% sand (Dystric Regosol), soils containing 34%~35% of CaO as CaCO3 (Calcaric Regosol), podzolic soils high in SiO2 that contain more than 75% sand (Orthic Podozol), and strongly acidic soils that have pH (KCl) values of 2.8~3.5 (Orthic Acrisol). In contrast, the higher outliers of the rare earth elements, and Th and U, were found in samples derived from granite (Dystric Regosol).
Accumulation of trace elements
Bellanca et al. (Citation1996) and Palumbo et al. (Citation2000) have reported the natural enrichment of heavy metals (Cr, Mn, Fe, Ni, Cu, Zn, Cd and Pb) in Terra Rossa soils from Sicily, Italy. Egashira et al. (Citation1996) also showed that the contents of the trace elements (V, Cr, Co, Ni, Cu, Zn and Sr) in paddy soils derived from limestone in the Central Region of the Mekong River, Laos, were higher than in soils derived from other parent materials. For soils on the Nansei Islands, Terashima et al. (Citation2004) determined eight major elements and 15 trace elements in dark red soils derived from the Ryukyu limestone in the Okinawa Islands, and they compared these data with those from five other different soil types sampled from the same island. They pointed out that the contents of elements such as Be, Mn, Zn, Sr and Pb, and especially Cd, were noticeably higher in the limestone-derived soils than in the others. All these data are in good agreement with those obtained by us for this paper, though the number of trace elements examined in their studies was smaller than that in our work.
As for the sources of trace elements in the Nansei Islands (including Okinawa and Ishigaki islands), Mizota and Matsuhisa (Citation1985) and Inoue and Naruse (Citation1990) pointed out that contributions from aeolian dust transported over long distances must be taken into account in addition to the local contributions from intensive weathering of carbonate rocks under neutral pH conditions. On the basis of pedological, mineralogical and geochemical data, Palumbo et al. (Citation2000) concluded that the processes of decarbonation, rubification and argillization were largely responsible for the enrichment and distribution of heavy metals in the Terra Rossa soils of Sicily. Their conclusion seems to be quite understandable from the fact that Fe and Mn oxyhydroxides and clay fraction are the major absorbent of trace elements. Terashima et al. (Citation2004) compared the trace element contents in several different soils from the Okinawa Islands with those in limestone-derived soils, and they concluded that the effects of aeolian dust would not be significant, even though the enrichment of trace elements could not be completely explained by chemical weathering processes.
It can be concluded, therefore, that the higher levels of 43 out of 48 trace elements in LGS can be attributed to the gradual accumulation of small amounts of trace elements contained in the limestone at the initial stage (Imai et al. Citation1996) during the course of intense weathering where almost all the parent material has been lost (Temur et al. Citation2009).
ACKNOWLEDGMENTS
The authors thank Dr Masami Nanzyo, Professor of the Graduate School of Agricultural Science, Tohoku University, for providing the soil samples collected from Ishigaki Island. We also thank Dr Yuji Maejima of the National Institute for Agro-Environmental Sciences (NIAES) for his information concerning red soils derived from limestone on the Kikai and Minami-Daito Islands.
REFERENCES
- Bellanca A, Hauser S, Neri R, Palumbo B 1996: Mineralogy and geochemistry of Terra Rossa soils, western Sicily: Insight into heavy metal fractionation and mobility. Sci. Total Environ., 193, 57–67.
- Egashira K, Aramaki K, Yoshimasa M, Takeda A, Yamasaki S 2004: Rare earth elements and clay minerals of soils of the floodplains of three major rivers in Bangladesh. Geoderma, 120, 7–15.
- Egashira K, Aramaki K, Yoshimura M, Takeda A, Yamasaki, S 2007: Variation of rare earth element of soils in the progress of soil formation – A case study for terrace and hill soils in Bangladesh. Clay Sci., 13, 117–124.
- Egashira K, Yamasaki S, Virakornphanich P 1996: Trace elements in paddy soils from the Central Region of the Mekong River, Laos. Soil Sci. Plant Nutr., 42, 673–676.
- Imai N, Terashima S, Itoh S, Ando A 1995: 1994 compilation of analytical data for minor and trace elements in seventeen GSJ geochemical reference samples, “Igneous rock series”, Geostandards Newslett., 19, 135–213.
- Imai N, Terashima S, Itoh S, Ando A 1996: 1996 compilation of analytical data on nine GSJ geochemical reference samples, “Sedimentary rock series”, Geostandards Newslett., 20, 165–216.
- Inoue K, Naruse, H 1990: Asian long-range eolian dust deposited on soils and Paleosols along the Japan Sea coast, Quat. Res., 29, 209–222 ( in Japanese with English summary).
- Kimura K, Motoyoshi H, Takeda A, Yamasaki S 2008: Trace elements in the arable soils of Miyagi Prefecture, northeastern Japan. Jpn. J. Soil Sci. Plant Nutr., 79, 359–364 ( in Japanese with English summary).
- Matoba S, Nishikawa M, Watanabe O, Fujii Y 1998: Determination of trace elements in an Arctic ice core by ICP/MS with a desolvated micro-concentric nebulizer. Kankyo Kagaku, 8, 421–427.
- Matsunami H, Matsuda K, Yamasaki S, Kimura K, Ogawa Y, Miura Y, Yamaji I, Tsuchiya N 2010: Applicability of polarizing energy dispersive X-ray fluorescence (EDXRF) spectrometry using pressed powder pellet for the rapid simultaneous multi-element determinations of soils and environmental samples. Soil Sci. Plant Nutr., 56, 530–540.
- Mizota C, Matsuhisa Y 1985: Eolian additions to soils and sediments of Japan. Soil Sci. Plant Nutr., 31, 369–382.
- Nakai M, Obara H, Togami K 2006: Catalog and data of soil monolith in NIAES. Misc. Publ. Natl. Agro-Environ. Sci., 29, pp 1–118 ( in Japanese).
- Nanzyo M, Yamasaki S, Honna T 2002: Changes in content of trace and ultratrace elements with an increase in noncrystalline materials in volcanic ash soils of Japan. Clay Sci., 12, 25–32.
- Nanzyo M, Yamasaki S, Honna T, Yamada I, Shoji S, Takahashi T 2007: Changes in element concentrations during Andsol formation on tephra in Japan. Euro. J. Soil Sci., 58, 465–477.
- Okamoto T, Kamiyama K 2007: Agriculture, soil science and plant nutrition in Kanagawa Prefecture. Jpn. J. Soil Sci. Plant Nutr., 78, 547–548 ( in Japanese with English summary).
- Palumbo B, Angelone M, Bellanca A, Dazzi C, Hauser S, Neri R, Wilson J 2000: Influence of inheritance and pedogenesis on heavy metal distribution in soils of Sicily, Italy. Geoderma, 95, 247–266.
- Prohaska T, Hann S, Latkoczy C, Stingeder G 1999: Determination of rare earth elements U and Th in environmental samples by inductively coupled plasma double focusing sectorfield mass spectrometry (ICP-SMS). J. Anal. Atomic. Spectrom., 14, 1–8.
- Takeda A, Kimura K, Yamasaki S 2004: Analysis of 57 elements in Japanese soils, with special reference to soil group and agricultural use. Geoderma, 119, 291–307.
- Takeda A, Yamasaki S, Tsukada H, Takaku Y, Hisamatsu S, Tsuchiya N 2011: Determination of total contents of bromine, iodine and several trace elements in soil by polarizing energy-dispersive X-ray fluorescence spectrometry. Soil Sci. Plant Nutr., 57, 19–28.
- Tao H, and Miyazaki A, 1995: Decrease of solvent water loading in inductively coupled plasma mass spectrometry using a membrane separator, J. Anal. Atomic. Spectrom., 10, 1–5.
- Temur S, Orhan, H, Deli A 2009: Geochemistry of the limestone of Martas Formation and related terra rossa, Seydisehir, Konya, Turkey. Geochem. Int., 47, 67–93.
- Terashima S, Imai N, Okai T 2001: Elemental distribution in the volcanic ash soils from the Kanto District, Japan: Preliminary study for the soil geochemical mapping. Bull. Geol. Surv. Japan, 52, 9–40 ( in Japanese with English summary).
- Terashima S, Ohta, A, Okai T, Imai N, Ujiie Mikoshiba M 2004: Geochemistry of elements and parent materials of non-alluvial soils from Tokai and Okinawa district, Japan. Earth Sci. (Chikyu Kagaku), 58, 317–336 ( in Japanese with English summary).
- Yamasaki S 1996: Inductively coupled plasma mass spectrometry. In Mass Spectrometry of Soils, Eds. Boutton T, Yamasaki S, pp. 459–491. Marcel Dekker, New York.
- Yamasaki S 2000: Inductively coupled plasma mass spectrometry in environmental analysis. In Encyclopedia of Analytical Chemistry, Ed. Meyer RA, pp. 2672–2692. John Wiley & Sons Ltd, Chiechester.
- Yamasaki S, Kimura K, Motoyoshi H, Takeda A, Nanzyo M 2009: Cadmium concentrations in soils of Japan. Jpn. J. Soil Sci. Plant Nutr., 80, 30–36 ( in Japanese with English summary).
- Yamasaki S, Matsunami H, Takeda A, Kimura K, Yamaji I, Ogawa Y, Tsuchiya N 2011: Simultaneous determination of trace elements in soils and sediments by polarizing energy dispersive X-ray fluorescence spectrometry. Bunseki Kagaku, 60, 315–323 ( in Japanese with English summary).
- Yamasaki S, Takeda A, Nanzyo M, Taniyama I, Nakai M 2001: Background levels of trace and ultra-trace elements in soils of Japan. Soil Sci. Plant Nutr., 47, 755–765.
- Yamasaki S, Tsumura A, Takaku Y 1994: Ultratrace elements in terrestrial water as determined by high-resolution ICP-MS. Microchem. J., 49, 305–318.
- Yanai J, Okada T, Yamada H 2012: Elemental composition of agricultural soils in Japan in relation to soil type, land use and region. Soil Sci. Plant Nutr., 58, 1–10.