Abstract
Tobacco (Nicotiana tabacum L.) leaf starch is degraded to sugars through curing (42°C/82.3% relative humidity/72 h). Total carbohydrate content remained almost constant, starch content decreased markedly, and soluble sugar content (mostly glucose) increased. α-Amylase and starch phosphorylase activities increased sixfold and threefold, respectively, whereas β-amylase activity was unaltered and isoamylase activity decreased. Increased α-amylase activity was accompanied by increased α-amylase protein levels. Although tobacco has four α-amylase gene members, only NtAMY1 mRNA levels increased. For other starch degradation genes, such as NtBAM1 and NtBMY2 (β-amylase), NtISO1 and NtISO2 (isoamylase) and NtGWD1 and NtGWD3 (glucan water dikinase), the mRNA levels remained unaltered during the first 48 h of curing. NtAMY1 expression was induced by osmotic stress but was unaffected by high temperature and/or injury stresses. Similarly, soluble sugar contents were largely increased by osmotic stress. This suggests that starch is degraded by α-amylase during curing and that α-amylase is coded by NtAMY1, induced by osmotic stress.
INTRODUCTION
Tobacco (Nicotiana tabacum L.) leaves are cured after harvest. Curing is a drying process by which the biochemical ingredients are altered and is managed under controlled temperature and moisture conditions (Frankenburg, Citation1946; Bacon et al. Citation1952). In this process, the alteration of starch to sugars is one of the most important determinants of the quality of cigarette materials.
One of the features of cured tobacco leaf is its sweet roast smell. It is unclear which is more effective—the kind or the amount of the sugars in good tobacco smell. Leaves with a high starch content sometimes produce no good smell. Therefore, we first should be clear about the degradation in leaves during the curing process. In order to improve leaf tobacco smell and fragrance, we need to elucidate what kinds of sugars increase during the process.
It is largely unknown how starch is degraded in tobacco leaves during the curing process. There are two proposed pathways for starch degradation in tobacco leaves, the first being that it is first phosphorylated by glucan water dikinase, following which the phosphorylated starch is catalyzed by β-amylase and isoamylase (Ritte et al. Citation2002; Stanley et al. Citation2005; Ediner et al. 2007). The second is that starch is directly degraded into glucose by α-amylase (Beck and Ziegler Citation1989). Although starch phosphorylase is also regarded as an enzyme involved in starch breakdown, its activity in leaves corresponds to only 10% of α-amylase activity and its contribution is minimal (Lin et al. Citation1988).
Glucan water dikinase is activated during the daytime (Reimann et al. Citation2004) and β-amylase activity is regulated by the redox state in plastids (Sparla et al. Citation2006). Therefore, these enzymes are considered to be closely related to circadian starch metabolism (Caspar et al. Citation1991; Lloyd et al. Citation2005; Orzechowski Citation2008). A deficiency of glucan water dikinase and/or downregulation of β-amylase leads to excess accumulation of starch (Yu et al. Citation2001; Scheidig et al. Citation2002). α-amylase is also targeted by chloroplasts and plastids (Chen et al. Citation2004; Asatsuma et al. Citation2005; Yu et al. Citation2005). Although Yu et al. (Citation2005) reported that α-amylase is not essential for the transitory breakdown of starch, some reports have shown that α-amylase activity is upregulated in dark-treated, senescent leaves and that its overexpression leads to a decrease in leaf starch content (Saeed and Duke, Citation1990; Li et al. Citation1992; Kakefuda and Preiss, Citation1997).
Thus, the degradation mechanisms of starch in leaves are still uncertain, but it is expected that glucan water dikinase and β-amylase are involved in circadian starch metabolism, whereas α-amylase may play an important role in starch degradation under environmental stress conditions such as prolonged dark-induced senescence.
In this study, we first examined the activities of several starch degradation enzymes including α-amylase, β-amylase, isoamylase and starch phosphorylase, and then determined the transcript levels for α-amylase, β-amylase and glucan water dikinase in tobacco leaves during the curing process. Having discovered an increase in α-amylase activity and in a gene member of α-amylase, NtAMY, we concentrated on the relationship between α-amylase and starch degradation during the curing process.
MATERIALS AND METHODS
Plant growth conditions and curing treatments
Tobacco plants (Nicotiana tabacum L.) were grown for 8 weeks in a greenhouse and then transplanted into a growth chamber at 25°C, 60% relative humidity (RH), under a 12-h light/12-h dark photoperiod of irradiance, and at 600 µmol m−2 s−1. At flowering, the plants were topped to approximately 20 leaves and the mature leaves were harvested.
The harvested leaves were cured in a temperature and humidity chamber in darkness at 42°C and 82% RH. The cured leaves were then frozen at −80°C until assay.
Carbohydrate assay
Frozen leaves were dried in a freeze-dry chamber and ground to a fine powder with a mill crusher. Powder samples were extracted by water and centrifuged at 12,000 × g for 15 min at 4°C. The precipitate fractions contained starch, and the supernatant fractions contained polyglucan and sugars. To separate polyglucan and sugars, the fractions were added at a fourfold volume of ethanol before re-centrifugation. The ethanol-insoluble fractions contained polyglucan, and the ethanol-soluble fractions contained total sugars. The soluble fractions were heated to 65°C to evaporate alcohol, and the residue was re-solubilized in water. All carbohydrates were determined using F-kit (Roche Diagnostics, Basel, Switzerland) according to the manufacturer’s instructions.
Protein extraction, enzyme analysis, and immunoblotting
Protein was extracted from the ground powder in 100 mM sodium (Na)-phosphate buffer (pH 6.5) containing 5 mM dithiothreitol (DTT), 2 mM calcium chloride (CaCl2), and a protease inhibitor cocktail (complete mini, Roche Diagnostics). Extracts were centrifuged at 12,000 × g for 10 min at 4°C, and the supernatant was applied to a desalted column (PD-10 Desalting Columns; GE Healthcare, Amersham, UK). The eluate was immediately used for enzyme assay. Soluble protein contents were determined by the Bradford method (Bradford Citation1976) using bovine serum choride (BSA) as a standard.
α-amylase and β-amylase activities were measured using a Megazyme kit (Megazyme, Bray, Ireland) for α-amylase and β-amylase assay, respectively. Isoamylase and starch phosphorylase were assayed by the method of Li et al. (Citation1992).
Proteins were separated by sodium dodecyl sulfate-gel electrophoresis (SDS-PAGE) with 10% weight/volume (w/v) polyacrylamide gel. After electrophoresis, the gels were transferred electrophoretically to nitrocellulose membranes (Hybond-ECL; GE Healthcare) according to the manufacturer’s instructions. After blocking with 5% (w/v) fat-free milk powder dissolved in phosphate buffered saline (PBS) for 1 h, the membranes were incubated with rabbit antisera directed against tobacco α-amylase1 protein which was diluted at the ratio of 1:2000 in PBS. The blot was then washed with PBS and incubated with horseradish peroxidase-coupled goat anti-rabbit Immunoglobulin G (IgG) (Stressgen Bioreagents, British Columbia, Canada). Immunoreactive proteins in blots were detected using ECL Plus reagent (GE Healthcare). Polyclonal antibody against α-amylase1 was generated in immunizing rabbits with the synthesized peptide as NH2–CDHFYDWGNSTHDQI–CONH2.
Total RNA extraction and qRT-PCR
Total RNA was isolated from leaf samples, which had been ground in liquid N2, using an RNeasy Plant Mini kit (Qiagen, Hilden, Germany). Genomic DNA was eliminated by DNase (TURBO DNA-free Kit, Foster City, CA, USA). Total RNA (2 µg) was reverse-transcribed to cDNA using a cDNA Synthesis Kit (Takara, Shiga, Japan) according to the manufacturer’s instructions. cDNA corresponding to 10 ng of total RNA was used in 20 μL of reaction mixture (TaqMan Universal PCR Master Mix, Foster City, CA, USA). Expression of target genes was quantified in triplicate using Applied Biosystems 7,300 Real-Time PCR system (Foster City, CA, USA) and was determined by normalizing to β-actin (Livak and Schmittgen Citation2001). The primers used for quantitative reverse transcription polymerase chain reaction (qRT-PCR) are shown in
Table 1 Sequences of primers and probe used for qRT-PCR analysis
RESULTS
Changes in carbohydrate contents
Starch content decreased from 57% to 22%, during 72 h of curing at 42°C and 60% RH (). In contrast, soluble sugar content increased and total carbohydrate content remained almost constant, implying that carbon loss by respiration is limited during the curing process. Water content decreased by 40% during this period (), whereas the increase in soluble sugars was mainly derived from glucose (> 70%) and fructose (). The sucrose and galactose contents remained constant, whereas maltose content decreased rapidly immediately after curing until it could not be detected. Raffinose content, which is an index of drying stress (Black et al. Citation1999), increased during the first 3 h of curing, representing only 0.6% of total sugar contents.
Figure 1 Changes in carbohydrate content (% dry weight) in tobacco (Nicotiana tabacum L.) leaves during the curing process. Starch (open circles), total main sugars (glucose, fructose and sucrose; closed circles), and total carbohydrates (open triangles). Vertical bars, standard error (SE) (n = 6).
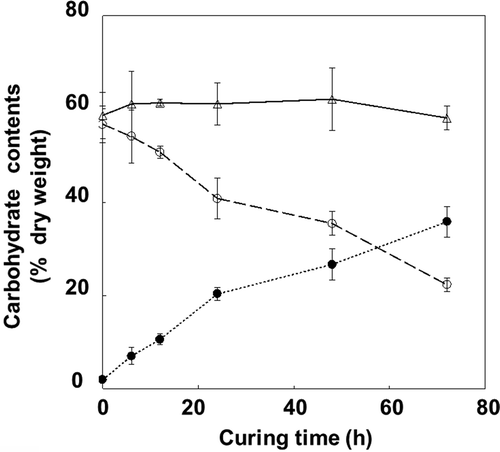
Figure 2 Changes in water content levels in tobacco (Nicotiana tabacum L.) leaves during the curing process. Data were expressed to 100 at harvesting (time point 0). Leaves just after harvest contained 9-fold water content relative to dry materials. Vertical bars, standard error (SE) (n = 6).
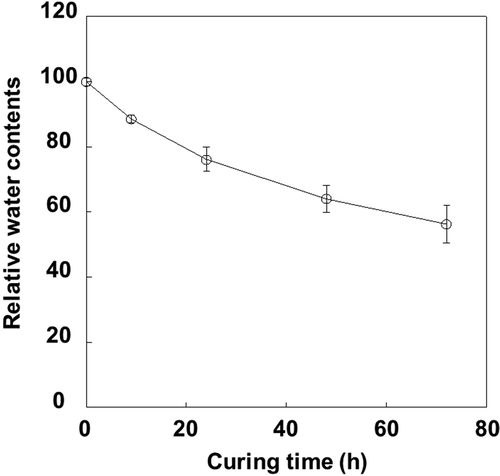
Figure 3 Changes in major (a) and minor (b) tobacco (Nicotiana tabacum L.) leaf sugar contents (% dry weight) during the curing process. (a) Glucose (open circles), fructose (closed circles) and sucrose (open triangles). (b) Raffinose (open circles), galactose (closed circles), polyglucan (open triangles) and maltose (closed triangles). Vertical bars, standard error (SE) (n = 4).
Changes in starch degradation enzymes
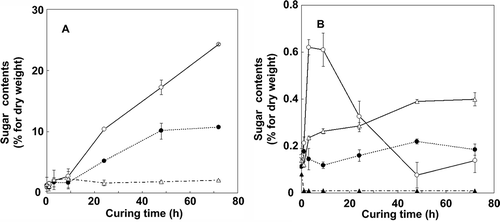
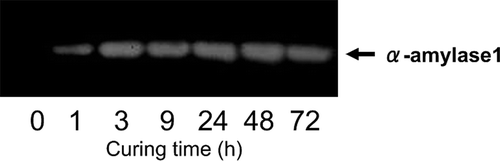
α-Amylase, β-amylase, isoamylase, and starch phosphorylase activities were examined with regard to their role as starch degradation enzymes ( and ). During the curing process, the α-amylase activity increased predominantly, whereas starch phosphorylase and β-amylase activities also increased but at lower incremental rates than that of α-amylase, and isoamylase activity decreased. Western blot analysis of α-amylase suggested that increased enzyme activity was accompanied by an increase in protein levels ().
Figure 4 Changes in α-amylase, β-amylase, starch phosphorylase, and isoamylase activities in tobacco (Nicotiana tabacum L.) leaves during the curing process. (a) α-amylase (open circles), β-amylase (closed circles) and starch phosphorylase (open triangles). (b) Isoamylase (closed triangles). All enzyme activities were measured at 25°C. Vertical bars, standard error (SE) (n = 4).
Figure 5 Immunoblot analysis of α-amylase1 protein in tobacco (Nicotiana tabacum L.) leaves during the curing process. Zero curing time indicated at harvesting. The other time points indicated at curing time after start. The arrow indicates α-amylase1 protein at MW 46.6 kDa.
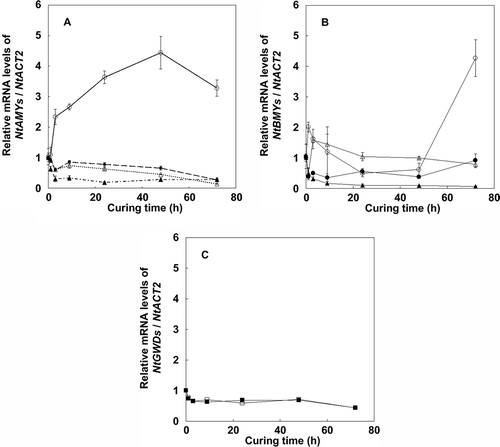
The transcript levels of key enzymes for starch degradation, including α-amylase, β-amylase and glucan water dikinase were also examined, and were normalized to that of the β-actin gene (NtACT2) (). Although there are four α-amylase gene members in tobacco, only NtAMY1 mRNA levels increased during the curing process. In contrast, the mRNA levels of the other three genes, NtAMY2, NtAMY3 and NtAMY4, decreased. For β-amylase, the total transcript levels of NtBMY1 increased suddenly at 72 h after initiation of the curing process, whereas the NtBMY2 mRNA levels remained almost constant. For isoamylase, NtISO1 and NtISO2, and glucan water dikinase, NtGWD1 and NtGWD3, mRNA levels also remained constant during the curing process. From the results, we considered the possibility that starch degradation in tobacco leaves during the curing process is mainly caused by α-amylase, which is coded by NtAMY1.
Figure 6 Changes in transcript levels of α-amylase (a) and other starch degradation enzyme genes (b) and (c) in tobacco (Nicotiana tabacum L.) leaves during the curing process. (a) NtAMY1 (open circles), NtAMY2 (closed circles), NtAMY3 (open triangles) and NtAMY4 (closed triangles). (b) NtBAM1 (open circles), NtBAM2 (closed circles), NtISO1 (open triangles) and NtISO2 (closed triangles). (c) NtGWD1(open squares) and NtGWD3 (closed squares). Relative levels were analyzed by comparative threshold cycle (CT) using the β-actin gene (NtACT2). Transcript levels were set to 1 at harvesting (time point 0) for all genes. Vertical bars, standard error (SE) (n = 6).
Induction of NtAMY1 expression and increase in sugar content following osmotic stress
Injury stress occurs in harvested tobacco leaves. During the curing process, tobacco leaves are assumed to be exposed to injury, high temperature and osmotic stresses. Therefore, we examined which stress most strongly affected the induction of NtAMY1 expression and increased sugar contents during the curing process. In order to avoid injury stress, leaves attached to whole plants were treated with each stress (see legend). Major induction of NtAMY1 expression was observed only under osmotic stress. However, minor induction of NtAMY2 was observed following injury and osmotic stress, which was of a much lower order. The NtGWDs expression was not affected by stress (data not shown). shows changes in sugar content under various stressors. Osmotic stress led to the greatest increase in sugar content. Injury and high-temperature stresses also led to an increase in sugar content, but these effects were much less than those caused by osmotic stress.
Figure 7 Changes in transcript levels of individual NtAMYs in leaves attached to tobacco (Nicotiana tabacum L.) whole plants under various stress conditions: NtAMY1 (closed bars), NtAMY2 (stripe bars), NtAMY3 (dot bars) and NtAMY4 (opened bars). Whole plants were kept in a temperature and humidity chamber in darkness at 25°C and 82% relative humidity (RH) for 24 h. Injury stress was applied by topping under the same conditions. High-temperature stress was applied at 42°C, whereas osmotic stress was applied by subjecting the plants to 20% (w/v) polyethylene glycol 6000 under the same conditions. Each transcript level was normalized to that of the β-actin gene (NtACT2), and shown as a relative value to control. Different letters designate statistically different values for each treatment [analysis of variance (ANOVA) with Tukey’s highly significant difference (HSD) test, P < 0.05]. Vertical bars, standard error (SE) (n = 6).
![Figure 7 Changes in transcript levels of individual NtAMYs in leaves attached to tobacco (Nicotiana tabacum L.) whole plants under various stress conditions: NtAMY1 (closed bars), NtAMY2 (stripe bars), NtAMY3 (dot bars) and NtAMY4 (opened bars). Whole plants were kept in a temperature and humidity chamber in darkness at 25°C and 82% relative humidity (RH) for 24 h. Injury stress was applied by topping under the same conditions. High-temperature stress was applied at 42°C, whereas osmotic stress was applied by subjecting the plants to 20% (w/v) polyethylene glycol 6000 under the same conditions. Each transcript level was normalized to that of the β-actin gene (NtACT2), and shown as a relative value to control. Different letters designate statistically different values for each treatment [analysis of variance (ANOVA) with Tukey’s highly significant difference (HSD) test, P < 0.05]. Vertical bars, standard error (SE) (n = 6).](/cms/asset/c21cf968-4957-46ca-b04a-99eff40759f9/tssp_a_842884_f0007_oc.jpg)
DISCUSSION
Our results suggest that α-amylase plays an important role in starch degradation in tobacco leaves during the curing process. Though glucan water dikinase and β-amylase were not found to be key enzymes in this process in the present study, many other studies have reported the importance of these enzymes in leaf starch metabolism under natural growth conditions. According to these researchers, starch is first phosphorylated by glucan water dikinase, and then the phosphorylated soluble starch is catalyzed by β-amylase and isoamylase. In fact, because β-amylase produces maltose, substantial amounts of maltose are frequently observed in darkened leaves and/or isolated chloroplasts (Weise et al. Citation2005; Samojedny and Orzechowski Citation2007; Fulton et al. Citation2008). However, in our studies of tobacco leaves during the curing process, maltose was not detected even when starch levels decreased rapidly (). We found a marked increase in glucose content with decreasing starch content. Because sucrose content remained constantly low during the curing process, α-amylase may be regarded as the sole enzyme producing glucose at this time. In addition, β-amylase does not directly degrade insoluble starch (Maeda et al. Citation1978). Although gelatinization temperature decreases with increase in phosphorylated starch level (Rooke et al. Citation1949; Lund Citation1984; Hoover Citation2001; Karim Citation2007), starch was not gelatinized in tobacco leaves at 42°C (Kakie Citation1973). Therefore, it is unlikely that β-amylase and glucan water dikinase are active during the curing process.
During the curing process, α-amylase activity was found to be enhanced sixfold () and glucose content was markedly increased (). However, the physiological and biological implications for such phenomena are not yet known. When plants are exposed to water stress, the expression of several genes encoded by carbohydrate metabolism enzymes is induced and soluble sugar content increases (Yang et al. Citation2002; Silva and Arcrabaca Citation2004). The cellular osmotic pressure rises because starch degradation in leaves occurs during the curing process (). It is thus possible that osmotic stress in chloroplasts is also enhanced by raised sugar content. Thus, starch degradation may function in maintaining osmotic pressure between chloroplasts and the cytoplasm. In fact, only osmotic stress was found to induce NtAMY1 expression () and enhance soluble sugar content ().
Figure 8 Total sugar content (glucose, fructose, and sucrose) of leaves attached to tobacco (Nicotiana tabacum L.) whole plants under the same stress conditions as in . As an additional treatment in , curing was applied to detached in darkness at 42°C and 82% relative humidity (RH) for 24 h. Different letters designate statistically different values for each treatment [analysis of variance (ANOVA) with Tukey’s highly significant difference (HSD) test, P < 0.05]. Vertical bars, standard error (SE) (n = 4).
![Figure 8 Total sugar content (glucose, fructose, and sucrose) of leaves attached to tobacco (Nicotiana tabacum L.) whole plants under the same stress conditions as in Fig. 7. As an additional treatment in Fig. 7, curing was applied to detached in darkness at 42°C and 82% relative humidity (RH) for 24 h. Different letters designate statistically different values for each treatment [analysis of variance (ANOVA) with Tukey’s highly significant difference (HSD) test, P < 0.05]. Vertical bars, standard error (SE) (n = 4).](/cms/asset/89d7e1be-7f77-44ab-8150-19fe050058af/tssp_a_842884_f0008_b.gif)
Another implication of our findings is that an increase in soluble sugar content is required for increased respiration at high temperatures during the curing process. However, because total carbohydrate content remained almost constant during the curing process () and NtAMY1 expression was not induced at high temperatures (), it was unlikely that an increased sugar content due to starch degradation could be utilized for respiration under high temperatures.
For the experiments on the effects on various stressors including injury, high temperature and osmotic stresses, we used attached leaves (Figs. 7 and 8). However, since osmotic stress led to increases in NtAMY1 expression and sugar accumulation while injury and high temperature stresses did not affect the experiments, we think that an induction of NtAMY1 expression was caused by osmotic stress during the process. In attached leaves, sugar contents were abundantly increased with osmotic stress, the same as detached leaves were during the curing process (). Therefore, we found that NtAMY1 is strongly induced in both attached and detached leaves with osmotic stress.
There is a continuous increase in amylase activity compared to amylase protein in this experiment. Therefore, there is the possibility of an existing active control system of amylase protein. There were reports that rice (Oryza sativa L. cv. Nipponbare) Amylase1 (accession number M24286) was ornamented with sugar chain through the Golgi body and then transferred to chloroplasts with activity. But, in this experiment, we did not acquire data about this system. The first object of this report is to understand the key enzyme in starch metabolism. We need to determine the regulation system for future work, because this mechanism can impact our future research.
Circadian starch turnover is regarded as a daily photosynthetic function. In contrast, starch degradation in tobacco leaf during the curing process occurs under severe environmental conditions where tobacco plants are dying. Therefore, a powerful starch degradation enzyme such as α-amylase is induced, and plants may attempt to maintain osmotic pressure in leaf cells.
With regard to cigarette materials, the sugars within the tobacco leaves are important because mellowness and fragrance are enhanced by the combustion of various sugars. In this study, we demonstrated that α-amylase1 is the main candidate for starch degradation during leaf curing, which directly yields glucose from starch, and this glucose can be easily converted to the C-skeleton for various sugars. Therefore, our results suggest that the highly inducible expression of NtAMY1 during leaf curing and the selection of plants with the high α-amylase activity line are important for improvement of tobacco quality as cigarette material. In addition, the osmotic stress treatment may be also useful for maintaining the volatile components and sugars because some volatile components are lost by heat treatment during the curing process.
ACKNOWLEDGMENTS
We thank Dr. Toshiaki Mitsui of Niigata University, Japan for his valuable review and comments on the manuscript. We also thank Drs. Tadahiko Mae and Tomoyuki Yamaya of Tohoku University, Japan, for their valuable discussion and comments on the manuscript.
REFERENCES
- Asatsuma S, Sawada C, Itoh K, Okito M, Kitajima A, Mitsui T 2005: Involvement of α-amylase I-1 in starch degradation in rice chloroplasts. Plant Cell Physiol., 46, 858–869.
- Bacon C, Wenger R, Bullock J 1952: Chemical changes in tobacco during flue-curing. Ind. Eng. Chem., 44, 292–296.
- Beck E, Ziegler P 1989: Biosynthesis and degradation of starch in higher plants. Ann. Rev. Plant Physiol. Plant Mol. Biol., 40, 95–117.
- Black M, Corbineau F, Gee H, Come D 1999: Water content, raffinose, and dehydrins in the induction of desiccation tolerance in immature wheat embryos. Plant Physiol., 120, 463–472.
- Bradford M 1976: Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem., 72, 248–254.
- Caspar T, Lin T, Kakefuda G, Benbow L, Preiss J, Somerville C 1991: Mutants of arabidopsis with altered regulation of starch degradation. Plant Physiol., 95, 1181–1188.
- Chen M, Huang L, Li H, Chen Y, Yu S 2004: Signal peptide-dependent targeting of a rice a-amylase and cargo proteins to plastids and extracellular compartments of plant cells. Plant Physiol., 135, 1367–1377.
- Edner C, Li J, Albrecht T, et al. 2007: Glucan, water dikinase activity stimulates breakdown of starch granules by plastidial b-amylases1. Plant Physiol., 145, 17–28.
- Frankenburg W 1946: Chemical changes in the harvested tobacco leaf. Part I. Chemical and enzymic conversions during the curing process. Advan. Enzymol., 6, 309–387.
- Fulton C, Stettler M, Mettler T, et al. 2008: b-AMYLASE4, a noncatalytic protein required for starch breakdown, acts upstream of three active b-amylases in Arabidopsis chloroplasts. Plant Cell., 20, 1040–1058.
- Hoover R 2001: Composition, molecular structure, and physicochemical properties of tuber and root starches, review. Carbohydr. Polym., 45, 2053–2267.
- Kakefuda G, Preiss J 1997: Partial purification and characterization of a diurnally fluctuating novel endoamylase from Arabidopsis thaliana leaves. Plant Physiol. Biochem., 35, 907–913.
- Kakie T 1973: Degradation of starch granules of tobacco leaves during flue-curing. Nippon Nogeikagaku Kaishi (in Japanese with English summary), 11, 667–672.
- Karim A 2007: Effects of phosphorus contents on the gelatinization and retrogradation of potato starch. J. Food Sci., 72, 132–138.
- Li B, Servaites J, Geiger D 1992: Characterization and subcellular localization of debranching enzyme and endoamylase from leaves of sugar beet. Plant Physiol., 98, 1277–1284.
- Lin T, Spilatro S, Preiss J 1988: Subcellular localization and characterization of amylases in Arabidopsis leaf. Plant Physiol., 86, 251–259.
- Livak K, Schmittgen T 2001: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods, 25, 402–408.
- Lloyd J, Kossmann J, Ritte G 2005: Leaf starch degradation comes out of the shadows. Trends Plant Sci., 10, 130–137.
- Lund D 1984: Influence of time, temperature, moisture, ingredients, and processing conditions on starch gelatinization. Crit. Rev. Food Sci. Nutr., 20, 249–273.
- Maeda I, Kiribuchi S, Nakamura M 1978: Digestion of barley starch granules by the combined action of. ALPHA.-and. BETA.-amylases purified from barley and barley malt. Agric. Biol. Chem., 42, 259–267.
- Orzechowski S 2008: Starch metabolism in leaves. Acta Biochim. Polpnica, 55, 435–445 (Review).
- Reimann R, Hippler M, Machelett B, Appenroth K 2004: Light induces phosphorylation of glucan water dikinase, which precedes starch degradation in turions of the duckweed Spirodela polyrhiza. Plant Physiol., 135, 121–128.
- Ritte G, Lloyd J, Eckermann N, Rottmann A, Kossmann J, Steup M 2002: The starch-related R1 protein is an alpha -glucan, water dikinase. Proc. Natl. Acad. Sci. USA, 99, 7166–7171.
- Rooke H, Lampitt L, Jackson E 1949: The phosphorus compounds of wheat starch. Biochem. J., 45, 231–236.
- Saeed M, Duke S 1990: Amylases in pea tissues with reduced chloroplast density and/or function. Plant Physiol., 94, 1813–1819.
- Samojedny D, Orzechowski S 2007: New look at starch degradation in Arabidopsis thaliana L. chloroplasts. Postepy Biochem., 53, 74–83.
- Scheidig A, Fröhlich A, Schulze S, Lloyd, Kossmann J 2002: Downregulation of a chloroplast-targeted β-amylase leads to a starch-excess phenotype in leaves. Plant J., 30, 581–591.
- Silva J, Arcrabaça M 2004: Contributions of soluble carbohydrates to the osmotic adjustment in the C4 grass Setaria sphacelata: a comparison between rapidly and slowly imposed water stress. J. Plant Physiol., 161, 551–555.
- Sparla F, Costa A, Lo Schiavo F, Pupillo P, Trost P 2006: Redox regulation of a novel plastid-targeted beta-amylase of Arabidopsis. Plant Physiol., 141, 840–850.
- Stanley D, Farnden K, MacRae A 2005: Plant α-amylases: functions and roles in carbohydrate metabolism. Rev. Biol. Bratislava, 60, 65–71.
- Weise S, Kim K, Stewart R, Sharkey T 2005: beta-Maltose is the metabolically active anomer of maltose during transitory starch degradation. Plant Physiol., 137, 756–761.
- Yang J, Zhang J, Wang Z, Zhu Q, Liu L 2002: Abscisic acid and cytokinins in the root exudates and leaves and their relationship to senescence and remobilization of carbon reserves in rice subjected to water stress during grain filling. Planta, 215, 645–652.
- Yu T, Kofler H, Häusler R, et al. 2001: The Arabidopsis sex1 mutant is defective in the r1 protein, a general regulator of starch degradation in plants, and not in the chloroplast hexose transporter. Plant Cell, 13, 1907–1918.
- Yu T, Zeeman S, Thomeycroft D, et al. 2005: alpha-Amylase is not required for breakdown of transitory starch in Arabidopsis leaves. J. Biol. Chem., 280, 9773–9979.