Abstract
It has been showed that Chao’s method [extraction with 0.1 mol L−1 hydroxylamine hydrochloride (NH2OH-HCl) at pH 2.0 for 30 min], which is commonly used to extract manganese (Mn) oxides and occluded heavy metals from soil samples, is not suitable for Andisols because of low solubility, and thus low extractability, of Mn oxides in such soils. Therefore, a new method is evaluated here, for extracting Mn oxides and occluded heavy metals from Andisols, Entisols and Inceptisols. The method has three steps: (1) reduction of Mn oxides with 0.01 mol L−1 NH2OH-HCl (pH 5.0) for 16 h, (2) recovery of re-adsorbed metals by short-time extraction with 0.5 mol L−1 ammonium chloride in 0.02 mol L‒1 hydrochloric acid, and (3) washing with ultrapure water. This method achieves a higher rate of extraction of Mn oxides than does Chao’s method, especially from Andisol samples. Standard addition experiments showed that both the new method and Chao’s method can successfully extract released cadmium (Cd), cobalt (Co), nickel (Ni) and zinc (Zn) from Mn oxides with little re-adsorption. The selectivity of Mn oxide extraction by the new method, indicated by the rate of extraction of iron (Fe) oxides and the aluminum (Al)/Mn and silicon (Si)/Mn extraction ratios, is comparable to that of Chao’s method. Thus, the new method should be useful for extracting Mn oxides and occluded Cd, Co, Ni, and Zn from soil samples. Moreover, because the new method achieved nearly complete extraction of NH2OH-HCl reactive Mn oxides even from Andisol samples, the method is more applicable to Andisol samples than Chao’s method.
INTRODUCTION
Manganese (Mn) oxides have low point-of-zero-charge values, large specific surface areas, and strong pH-dependent surface charges (Healy et al. Citation1966; McKenzie Citation1989), and metal ions can be adsorbed onto surface and interlayer sites or be incorporated into their crystal lattices (Miyata et al. Citation2007). Although iron (Fe) oxides can also strongly retain heavy metals in soils (Shuman Citation1985), Fe oxides and Mn oxides have different affinities for different metals. Phosphorus (P), arsenic (As) and chromium (Cr) bind mainly to Fe oxides, whereas barium (Ba), cobalt (Co) and lead (Pb) are more likely to bind to Mn oxides (Neaman et al. Citation2004; Citation2008). Mn oxides are readily mobilized by changes in environmental conditions, such as soil flooding (Patrick and Jugsujinda Citation1992), soil drying (Makino et al. Citation2000) and soil sterilization (Suda et al. Citation2009), whereas strong reducing conditions are required to reduce Fe oxides (Patrick and Jugsujinda Citation1992).
It is desirable to determine the heavy metals bound to Mn oxides and Fe oxides separately, because of the differences in their affinities for different metals and the different solubilities of the two oxides in soils. Therefore, it is appropriate to use a selective extraction method for Mn oxides and occluded heavy metals. Chao (Citation1972) showed that Mn oxides can be extracted by shaking soil samples for 30 min with 0.1 mol L−1 hydroxylamine hydrochloride (NH2OH-HCl) (pH 2.0) without significant extraction of Fe oxides. However, although Chao’s method has been widely adopted (e.g., Means et al. Citation1978; Obrador et al. Citation2007), especially in sequential extraction procedures (e.g., Miller et al. Citation1986; Makino et al. Citation2006), Suda et al. (Citation2011) showed that Chao’s method fails to extract measurable quantities of Mn oxides from Andisols and Andisol-like soil samples (10 ≤ Alo + 1/2Feo < 20 g kg−1, where the subscript “o” means ammonium oxalate-extractable). Andisol, a kind of volcanic ash soils, is found in volcanic regions across the world. Because of the inapplicability of Chao’s method to that type of soils, an alternative method would be needed for fractionation studies of heavy metals in volcanic region.
Therefore, the objective of the present study is to establish a highly effective and selective extraction method for Mn oxides and occluded metals from soil samples, with a special emphasis on its applicability to Andisol samples.
MATERIALS AND METHODS
Soil samples and properties
Twelve samples were collected from agricultural fields (paddy and upland fields) and forests in Japan, including samples of Entisols (E), Inceptisols (I) and Andisols (Soil Survey Staff Citation1998) (). Andisol samples were divided into allophanic (AA) and non-allophanic (NA) samples, where non-allophanic samples are those with a ratio of pyrophosphate-extractable aluminum (Alp) to ammonium oxalate-extractable aluminum (Alo) of more than 0.5. Sample AA-2 was collected from the soil profile classified as Udepts by the United States Department of Agriculture (USDA) Soil Taxonomy system, but the sum of Alo and 1/2Feo was greater than 20 g kg−1 and Alp/Alo was less than 0.5, so the sample was treated as an allophanic Andisol sample in this study. Soil samples were air-dried, passed through a 2-mm mesh sieve, and then stored at room temperature.
Table 1 Brief descriptions of the soil samples
Soil pH was measured in H2O with a glass electrode pH meter (UltraBasic, Denver Instrument, Colorado, USA), with a ratio of soil sample to H2O of 1:2.5 (weight/volume). Total carbon (TC) content of the soil sample was measured by a dry combustion method (Sumigraph NC-22F, Shimadzu, Kyoto, Japan). Dithionite citrate-extractable Fe (Fed) and ammonium oxalate-extractable Al, Fe, Mn, and silicon (Alo, Feo, Mno, and Sio) were extracted as described by Blakemore et al. (Citation1987). Then the extract was centrifuged and filtrated by a 0.2-µm mesh filter (Merck Millipore, Billerica, MA, USA). Pyrophosphate-extractable Al (Alp) was extracted as described by Blakemore et al. (Citation1987); after centrifugation of the extract as described, the supernatant was filtered through a 0.025-µm mesh filter (Merck Millipore) to prevent contamination of Fe caused by dispersion of microcrystalline minerals (Schuppli et al. Citation1983). The concentrations of Al and Fe in the filtrate were measured by inductively coupled plasma optical emission spectrometry (ICP-OES) (Vista-Pro, Varian, Palo Alto, CA, USA). Clay content was determined by the pipette method (Gee and Bauder Citation1986). Pseudo-total amounts of cadmium (Cd), Co, copper (Cu), nickel (Ni), Pb, and zinc (Zn) were extracted by hot-plate aqua regia digestion by the procedure described by Chen and Ma (Citation2001) after grinding and sieving (< 150 μm) the air-dried samples. The concentrations of metals in the extracts were determined by ICP-OES and inductively coupled plasma mass spectrometry (ICP-MS) (Elan DRC-e, PerkinElmer, Waltham, MA, USA).
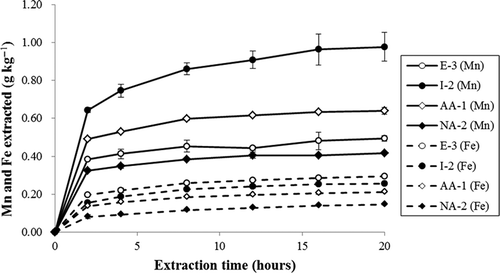
Time-course changes in the amounts of Mn during extraction with 0.01 mol L−1 NH2OH-HCl (pH 5.0) followed by washing with acidified NH4Cl
To determine the optimal time for extraction of Mn oxides from soil samples, time-course changes in the amounts of Mn and Fe extracted with NH2OH-HCl was examined. Four soil samples (E-3, I-2, AA-1 and NA-2) containing the largest amount of Mno for each soil type were selected for this experiment (). The concentration of NH2OH-HCl was set to 0.01 mol L−1, one-tenth the concentration (0.1 mol L−1) used in Chao’s method. Stoichiometrically, 0.2 mmol of NH2OH-HCl (i.e., 20 mL of 0.01 mol L−1 solution) can reduce 0.1 mmol (5.5 mg) of Mn4+. Hence, the lower NH2OH-HCl concentration is sufficient to reduce all of the Mn oxide in these soil samples. Although the rate of extraction of Fe oxides is highly dependent on the NH2OH-HCl concentration, the concentration of NH2OH-HCl does not remarkably affect the extraction of Mn oxides (Chao Citation1972). Therefore, a NH2OH-HCl concentration of 0.01 mol L−1 was adopted to reduce Mn oxides for prolonged extraction from soil samples.
Table 2 Selected properties of the soil samples
A sieved, air-dried soil sample (0.5 g) was placed in a 50-mL polyethylene centrifuge tube, and 20 mL of 0.01 mol L−1 NH2OH-HCl (pH adjusted to 5.0) was added. The mixture was shaken in a mechanical shaker for variable time periods (2, 4, 8, 12, 16 and 20 h) at 25 ± 2°C and then centrifuged for 10 min at 3500 rpm (1700 g). The supernatant was transferred to another bottle by decantation or by removal with a pipette, and 20 mL of 0.5 mol L−1 ammonium chloride (NH4Cl) in 0.02 mol L−1 hydrochloric acid (HCl) medium (acidified NH4Cl) was added to the centrifuge tube. The mixture was manually shaken and then shaken in a mechanical shaker for 10 min and centrifuged at 3500 rpm (1700 g) for 5 min. The supernatant was removed and mixed with the preceding NH2OH-HCl extract. Then 10 mL of ultrapure water was added to the residue, and the mixture was manually shaken well. The supernatant was collected by centrifugation at 3500 rpm (1700 g) for 10 min and then transferred to a bottle containing NH2OH-HCl and acidified NH4Cl extracts. The bottle of extracts was filled to a volume of 50 mL and filtered through a 0.2-μm mesh filter, and the filtrate was diluted with 1% nitric acid. Mn and Fe concentrations in the diluted filtrate were determined by ICP-OES.
Standard addition experiments for heavy metals
Addition of heavy metals to the two extractants
Standard addition experiments were carried out with six metals (Cd, Co, Cu, Ni, Pb and Zn) to evaluate their degree of re-adsorption during extraction. Recovery rates for the new method were compared those of Chao’s method. To minimize the effects of the addition of metals on the extraction conditions, a small amount (1 mL per 100 mL of extractant) of a mixed solution of Cd, Co, Cu, Ni, Pb and Zn was added to each NH2OH-HCl extractant to bring the added amounts of these metals in the range of 70–200% of the amount of metals obtained by the control extractions. The addition of the metal solutions changed the pH by less than 0.05 units. A control extraction experiment was carried out in the same way without any addition of heavy metals, and the extracts from the control experiment were used for other experiments.
0.1 mol L−1 NH2OH-HCl (pH 2.0) extraction (Chao’s method)
An air-dried soil sample (0.5 g) was placed in a 50-mL polyethylene centrifuge tube, and 25 mL of 0.1 mol L−1 NH2OH-HCl (pH 2.0) was added. The sample was shaken in a mechanical shaker for 30 min at 25 ± 2°C and then centrifuged for 10 min at 3500 rpm (1700 g). The supernatant was filtered through a 0.2-μm mesh filter, and the filtrate was diluted with 1% nitric acid. The concentrations of Cd, Co, Cu, Ni, Pb and Zn in the diluted filtrate were determined by ICP-OES or ICP-MS. All procedures were performed in triplicate.
0.01 mol L−1 NH2OH-HCl (pH 5.0) extraction followed by washing with acidified NH4Cl (new method)
The extraction, filtration and dilution were carried out as described in the preceding section; however, the first extraction was carried out for 16 h. The concentrations of Cd, Co, Cu, Ni, Pb and Zn in the diluted filtrate were determined by ICP-OES or ICP-MS. All procedures were performed in triplicate.
Calculation of the recovery rate
The recovery rate for each metal was calculated by the following formula:
where A is the amount of metal extracted from a soil sample to which the metal was added, B is the amount of the metal extracted from the control soil sample to which no metals were added and C is the amount of the metal added to the soil sample.
Selectivity of extraction for Mn oxides
Extraction of Mn, Fe, Al and Si by the new method and by Chao’s method
The amounts of Mn, Fe, Al and Si extracted by the new method and by Chao’s method were compared to determine the selectivity of Mn oxide extraction by each method. The control extraction data of standard addition experiments were also used in this experiment. The filtrate obtained by each extraction method was highly diluted with 1% nitric acid, and then the concentrations of Mn, Fe, Al and Si were determined by ICP-OES.
Calculation of the rates of extraction of Mn oxides and short-range-ordered Fe oxides
The rates of extraction of Mn oxides and Fe oxides were obtained as follows:
where MnEx and FeEx are the amounts of Mn and Fe extracted by the new method or Chao’s method, Mno and Feo are the amounts of Mn and Fe extracted by oxalate and Fed is the amount of Fe extracted with the dithionite-citrate.
Statistical analysis
A paired t-test was used to compare the differences between the amounts of Mn, Fe, Al and Si extracted using Chao’s method and those extracted using the new method. A paired t-test was also used to compare differences between the amounts of Cd, Co, Cu, Ni, Pb and Zn extracted using the two methods.
RESULTS
Soil properties
Selected properties of the soil samples are listed in . The pH (H2O) of the soil samples ranged from acidic to neutral (pH 4.43 to 7.06). The TC contents of the samples ranged from 10.4 to 211 g kg−1, and the non-allophanic Andisol samples contained much more TC than the other soil samples. The clay contents of the samples ranged from 76.5 to 291 g kg−1. The values of Fed, Feo, Alo, Alp, Mno and Sio varied widely. Samples E-3, I-2, AA-1 and NA-1 contained the largest amounts of Mno in each of their respective soil types. The allophanic Andisol samples contained abundant Alo and Fed, and Alp values for the non-allophanic Andisol samples were much higher than those for the other soil samples. Samples E-3, I-2, and I-3 contained relatively large amounts of Alo, indicating that one of the parent materials of these soils was volcanic ash.
The pseudo-total contents of Cd, Co, Cu, Ni, Pb and Zn are shown in . For comparison, the mean and median values of the contents of these metals in 514 Japanese soil samples (Takeda et al. Citation2004) are also listed. Cd, Pb and Zn in E-2, Cu in I-2, Cu and Pb in AA-2, and Pb in NA-3 were much higher than the mean or median values of the 514 Japanese soil samples.
Table 3 Pseudo-total contents of heavy metals in soil samples
Relationships between extraction time and amounts of Mn and Fe extracted with 0.01 mol L−1 NH2OH-HCl (pH 5.0) followed by washing with acidified NH4Cl
The amounts of Mn and Fe extracted from all four soil types increased with increasing extraction time (). However, relatively prompt extraction of Mn was likely finished within 16 h of shaking, even for sample I-2, which contained the largest amount of Mno of all the soil samples (). The amounts of Fe extracted were lower than those of Mn but also increased with extraction time.
Extracted amounts and recovery rates for the six heavy metals
Chao’s method extracted 0.026–1.92 mg kg−1 of Cd, 0.515–7.23 mg kg−1 of Co, 0.043–138 mg kg−1 of Cu, < 0.02–1.13 mg kg−1 of Ni, 0.094–22.3 mg kg−1 of Pb and 0.576–36.4 mg kg−1 of Zn from the control samples (). Average recovery rates for Cd, Co, Ni and Zn were nearly 100% (range 84.9–94.6%), but those for Cu and Pb were only 44.9% and 44.8%, respectively. The recovery rates for Cu and Pb differed widely among the samples.
Table 4 Extracted amounts and recovery rates of heavy metals by Chao’s method
The new method extracted 0.032–1.91 mg kg−1 of Cd, 0.557–9.64 mg kg−1 of Co, 0.120–174 mg kg−1 of Cu, < 0.02–1.09 mg kg−1 of Ni, 0.917–44.7 mg kg−1 of Pb and 0.385–38.6 mg kg−1 of Zn from the control samples (). Similar to Chao’s method, the new method recovered most of the added Cd, Co, Ni and Zn (range 86.0–98.6%), whereas just 44.7% and 63.5% of added Cu and Pb, respectively, were recovered.
Table 5 Extracted amounts and recovery rates of heavy metals by the new method
In general, the new method extracted larger amounts of metals from each soil sample, except for Ni. The differences in the amounts of metals extracted with the two methods were significant for Cd, Co, Pb and Zn (P < 0.05 or P < 0.01), but not significant for Cu.
Extraction of Mn, Fe, Al and Si by Chao’s method and the new method
The amounts of Mn and Fe extracted by Chao’s method ranged from 0.048 to 0.415 g kg−1 and from 0.063 to 0.846 g kg−1, respectively (). The amounts of extracted Mn and Fe calculated from Eq. 2, 3 and 4 were equivalent to 20.2–84.1% of total Mn oxides, 0.638–8.40% of short-range-ordered Fe oxides and 0.310–5.13% of total Fe oxides. From 0.255 to 3.91 g kg−1 of Al was extracted by Chao’s method, and the amounts extracted from Andisol samples were much higher than those extracted from non-Andisol samples. The amount of extracted Si ranged from 0.027 to 0.459 g kg−1.
Table 6 Extracted amounts of manganese (Mn), iron (Fe), aluminum (Al) and silicon (Si); rates of extraction of Mn and Fe oxides, Fe/Mn, Al/Mn and Si/Mn extraction ratios by Chao’s method
The amounts of Mn and Fe extracted with the new method ranged from 0.058 to 0.930 g kg−1 and from 0.134 to 0.838 g kg−1, respectively (). The amounts of extracted Mn were equivalent to 49.8–93.7% of total Mn oxides, and the amounts of extracted Fe were equivalent to 1.27–9.25% of short-range-ordered Fe oxides and 0.333–5.08% of total Fe oxides. The amounts of Al extracted with the new method ranged from 0.205 to 4.16 g kg−1, and larger amounts were extracted from Andisol samples than from non-Andisol samples. The new method extracted from 0.042 to 0.588 g kg−1 of Si. On average, the new method extracted significantly larger amounts of Mn and Fe (n = 12, P < 0.01), especially from Andisol samples. On the other hand, the differences between the amounts of Al and Si extracted with the new method and those extracted with Chao’s method were not significant. The average values of the Al/Mn and Si/Mn extraction ratios (mole basis) obtained with the new method were significantly lower than those obtained with Chao’s method (n = 12, P < 0.01 and P < 0.05, respectively). With both methods, Al/Mn values were much larger for Andisol samples than for non-Andisol samples.
Table 7 Extracted amounts of manganese (Mn), iron (Fe), aluminum (Al), and silicon (Si); rates of extraction of Mn and Fe oxides; Fe/Mn, Al/Mn and Si/Mn extraction ratios by the new method
DISCUSSION
Optimal time for extraction of Mn oxides
The amount of Mn extracted with non-acidified 0.01 mol L−1 NH2OH-HCl (pH 5.0) followed by washing with acidified NH4Cl increased as the extraction time increased (). However, most of the reactive Mn oxides were extracted within 16 h, even in sample I-2, which contained the largest amount of Mno among the samples. Although a much longer extraction time might achieve complete extraction of Mn oxides, the amount of Fe extracted also increased with time (). Therefore, we chose an extraction time of 16 h to maintain selectivity of the extraction method.
Heavy metal extraction and prevention of re-adsorption of released heavy metals during extraction
The new method extracted larger amounts of Cd, Co, Cu, Pb and Zn from most samples than Chao’s method ( and ). The increase in the amounts of extracted heavy metals was attributed to an increase in the release of occluded metals from the Mn and Fe oxides. The larger amounts of Pb extracted with the new method can be partially explained by less re-adsorption of Pb, indicated by the higher rate of recovery of Pb. The amounts of Ni extracted with the two methods were comparable, indicating that Ni might be eccentrically located at an easily reducible part of the Mn and Fe oxides. For both methods, the amounts of Ni extracted from AA-2 were much lower than those extracted from the other samples, although the total amounts of Ni in the samples were comparable. This result was unexpected, but might be explained by the different chemical forms of Ni among the soil samples.
In chemical fractionation studies, re-adsorption of heavy metals should be prevented during extraction, because re-adsorption not only results in underestimation of the amounts of metals in the fractions but also results in overestimation of the amounts of metals in fractions analyzed in subsequent sequential extraction procedures. Non-acidified NH2OH-HCl selectively extracted Mn without significant extraction of Fe; however, the recovery rates for the heavy metals were critically low, probably due to the high extraction pH (Suda Citation2013). The main adsorption sites for released cationic heavy metals are considered to be surface of the residual Mn and Fe oxides and Al minerals, and organic matter in the soil samples. Therefore, lowering the pH or adding chelating agents such as ethylenediaminetetraacetate might prevent re-adsorption during extraction. However, reduction of Fe oxides by NH2OH-HCl is highly accelerated by acidification (Chao Citation1972; Suda et al. Citation2011). Therefore, longer extraction in an acidic NH2OH-HCl solution will not avoid significant extraction of Fe oxides. Howard and Shu (Citation1996) reported that the addition of nitrilotriacetic acid (NTA) at low concentration counteracts re-adsorption in quartz-rich sediment. However, they also pointed out that NTA at low concentration does not prevent a considerable rate of re-adsorption of metals onto some strong sorbents such as organic matter. Addition of a concentrated or stronger chelating agent might resolve the problem, but this approach suffers from the risk of extraction of metals from non-target phases, especially from organic matter. Therefore, in the new method, after the reduction of Mn oxides, re-adsorbed metals are recovered by washing for a short time (10 min) with acidified NH4Cl. The results of standard addition experiments ( and ) strongly indicated that the new method successfully recovered Cd, Co, Ni and Zn, which were released from Mn oxides. However, the method did not prevent re-adsorption of Cu and Pb. In Chao’s method, re-adsorption of Cd, Co, Ni and Zn was also prevented, but Cu and Pb were re-adsorbed ( and ). Taken together, these results strongly indicate that neither the new method nor Chao’s method is suitable for fractionation studies of Cu and Pb in soil. It is well known that Fe and Al oxides (Kinniburgh et al. Citation1976; McKenzie Citation1980) and organic matter (Schnitzer and Skinner Citation1967) have higher affinities for Cu and Pb than other heavy metals. Recovery rates for Cu from the non-allophanic Andisol samples, which had large amounts of TC, were lower than those from the other samples for both methods, indicating Cu released from Mn oxides was mainly re-adsorbed to organic matter. For both methods, the rates of extraction of Pb from NA-3, which contained the largest amount of TC, were relatively high, whereas those from AA-3, which contained the largest amount of Alo + 1/2Feo, were the lowest among the samples. These results indicate that short-range-ordered Al minerals and Fe oxides (e.g., allophane and ferrihydrite) are important sorbents for released Pb, consistent with the fact that organic matter has a much lower affinity for Pb than does Fe oxide (Sauve et al. Citation2000).
Evaluation of extractability and extraction selectivity for Mn oxides
Because the objective of the present study was to establish a method for the selective extraction of Mn oxides and heavy metals occluded in them, the amounts of Fe oxides or Al and Si during extraction should be limited. The amounts of Mn, Fe, Al and Si extracted, and the extraction rates for the Mn oxides and Fe oxides (short-range-ordered and total), are shown in and . Larger amounts of Mn were extracted with the new method than with Chao’s method, especially from the Andisol samples. However, the rates of extraction of Mn oxides from these samples were much less than 100%, even though 16 h of extraction was likely sufficient to extract most of the reactive Mn oxides. This result indicates that part of the oxalate-extractable Mn was not reactive to non-acidified NH2OH-HCl.
Oxalate would extract Mn bound to organic matter. The soil samples containing abundant organic matter in this study (i.e., the non-allophanic Andisols) were either moderately or strongly acidic (pH 4.43–5.67). McBride (Citation1982) showed that strong binding of Mn to organic matter is unlikely at pH < 6.0, so the amount of organically bound Mn in samples would not be considerable. Therefore, Mn that is extracted by oxalate extraction but not extracted by the new method might exist as a relatively insoluble or physically isolated Mn oxide. For example, lithiophorite [(Al,Li)MnO2(OH)2] is hardly soluble in non-acidified NH2OH-HCl at room temperature (Tokashiki et al. Citation2003). Moreover, Reyes and Torrent (Citation1997) indicated that allophanic micro-aggregates inhibit reductive extraction of short-range-ordered Fe oxides. Fendorf et al. (Citation1993) showed that surface precipitation of aluminum hydroxide [Al(OH)3] formed at pH 5.0 on Mn oxide surfaces inhibits Cr(III) oxidation of Mn oxides. Reduction and extraction of Mn oxides in Andisol samples might be decelerated by a similar mechanism. Further studies are required to examine these possibilities.
The new method extracted not only larger amounts of Mn but also larger amounts of Fe than did Chao’s method ( and ). However, with the new method, the average rates of extraction of short-range-ordered Fe oxides were only 5.97% for non-Andisol samples and 1.94% for Andisol samples. These values are not very different from the values of 4.87% and 1.04%, respectively, obtained with Chao’s method. The average rates of extraction of total Fe oxides for the non-Andisol and Andisol samples were 2.05% and 1.05% (the new method) and 2.12% and 0.85% (Chao’s method). Thus, like Chao’s method, the new method could extract Mn oxides from soil samples with limited extraction of Fe oxides.
For both methods, the amounts of Al extracted from the Andisol samples were higher than the amounts extracted from other soil samples, indicating that a part of the Al in short-range-ordered Al minerals (e.g., allophane) or in Al-humus complexes was extracted. However, the amounts of Al and Si extracted with the two methods differed only slightly. In addition, the Al/Mn and Si/Mn extraction ratios obtained with the new method were less than the ratios obtained with Chao’s method, indicating that the new method extracted fewer moles of Al and Si per Mn than did Chao’s method. Thus, it is concluded that the extraction selectivity of the new method is comparable to that of Chao’s method.
CONCLUSION
A new method for selectively extracting Mn oxides and occluded metals from soil samples was developed. The new method consists of prolonged (16 h) extraction with 0.01 mol L−1 NH2OH-HCl (pH 5.0) followed by short-time extraction with acidified NH4Cl and washing with ultrapure water. The new method has the disadvantages of the long extraction time and complexity of the procedure; however, it extracted most Mn oxides reactive to NH2OH-HCl, unlike Chao’s method, even from Andisol samples. Standard addition experiments showed that both the new method and Chao’s method successfully extracted heavy metals released from Mn oxides without measurable re-adsorption of Cd, Co, Ni and Zn. On the other hand, for both methods, substantial amounts of released Cu and Pb remained in the samples after extraction. The extraction selectivity of the new method was as high as that of Chao’s method, as indicated by the rates of extraction of Fe oxides and the Al/Mn and Si/Mn extraction ratios.
The new method should be useful for the extraction of Mn oxides and some occluded metals (Cd, Co, Ni and Zn) from soil samples. Moreover, because the new method achieved nearly complete extraction of NH2OH-HCl reactive Mn oxides, even from Andisols, the new method seems to be more applicable to Andisol samples than Chao’s method.
ACKNOWLEDGMENTS
We thank S. Ono, K. Kamewada (Tochigi Prefecture Agricultural Experiment Station), A. Aoki (Tochigi Prefecture Agricultural Experiment Station) and Y. Nagasaki (National Agricultural Research Center for the Western Region) for soil sampling. We also thank J. Takahashi (University of Tsukuba) for providing some of the soil samples used in this study and for providing physicochemical data for some of the samples. We thank Y. Maejima, I. Akahane (both at the National Institute for Agro-Environmental Sciences) and K. Tamura (University of Tsukuba) for helpful comments. This work was partly supported by a Grant-in-Aid for Research Fellows of the Japan Society for the Promotion of Science (No. 23–444).
REFERENCES
- Blakemore LC, Searle PL, Darly BK 1987: Extractable iron, aluminum and silicon. In Methods for Chemical Analysis of Soils, New Zealand Soil Bureau Scientific Report 10A, Ed. Blakemore L, pp. 71–76. DISR, Lower Hutt.
- Chao TT 1972: Selective dissolution of manganese oxides from soils and sediments with acidified hydroxylamine hydrochloride. Proc. Soil Sci. Soc. Am., 36, 764–768.
- Chen M, Ma LQ 2001: Comparison of three aqua regia digestion methods for twenty Florida soils. Soil Sci. Soc. Am. J., 65, 491–499.
- Fendorf SE, Zasoski RJ, Burau RG 1993: Competing metal-ion influence on chromium(III) oxidation by birnessite. Soil Sci. Soc. Am. J., 57, 1508–1515.
- Gee GW, Bauder JW 1986: Particle-size analysis. In Methods of Soil Analysis, Part 1, Physical and Mineralogical Methods, Ed. Klute A, pp. 383–411. American Society of Agronomy, Inc.; Soil Science Society of America, Inc., Madison.
- Healy T, Herring A, Fuerstenau D 1966: The effect of crystal structure on the surface properties of a series of manganese dioxides. J. Colloid Interface Sci., 21, 435–444.
- Howard JL, Shu J 1996: Sequential extraction analysis of heavy metals using a chelating agent (NTA) to counteract resorption. Environ. Pollut., 91, 89–96.
- Kinniburgh DG, Jackson ML, Syers JK 1976: Adsorption of alkaline-earth, transition, and heavy-metal cations by hydrous oxide gels of iron and aluminum. Soil Sci. Soc. Am. J., 40, 796–799.
- Makino T, Hasegawa S, Sakurai Y, Ohno S, Utagawa H, Maejima Y, Momohara K 2000: Influence of soil-drying under field conditions on exchangeable manganese, cobalt, and copper contents. Soil Sci. Plant Nutr., 46, 581–590.
- Makino T, Sugahara K, Sakurai Y, Takano H, Kamiya T, Sasaki K, Itou T, Sekiya N 2006: Remediation of cadmium contamination in paddy soils by washing with chemicals: Selection of washing chemicals. Environ. Pollut., 144, 2–10.
- McBride MB 1982: Electron-spin resonance investigation of Mn2+ complexation in natural and synthetic organics. Soil Sci. Soc. Am. J., 46, 1137–1143.
- McKenzie RM 1980: Adsorption of lead and other heavy-metals on oxides of manganese and iron. Aust. J. Soil Res., 18, 61–73.
- McKenzie RM 1989: Manganese oxides and hydroxides. In Minerals in Soil Environments, Eds. Dixon JB,Weed SB, pp. 439–465. Soil Science Society of America, Inc., Madison.
- Means JL, Crerar DA, Borcsik MP, Duguid JO 1978: Adsorption of Co and selected actinides by Mn and Fe oxides in soils and sediments. Geochim. Cosmochim. Acta., 42, 1763–1773.
- Miller WP, Martens DC, Zelazny LW 1986: Effect of sequence in extraction of trace metals from soils. Proc. Soil Sci. Soc. Am., 50, 598–601.
- Miyata N, Tani Y, Sakata M, Iwahori K 2007: Microbial manganese oxide formation and interaction with toxic metal ions. J. Biosci. Bioeng., 104, 1–8.
- Neaman A, Martínez CE, Trolard F, Bourrié G 2008: Trace element associations with Fe- and Mn-oxides in soil nodules: Comparison of selective dissolution with electron probe microanalysis. Appl. Geochem., 23, 778–782.
- Neaman A, Mouele F, Trolard F, Bourrié G 2004: Improved methods for selective dissolution of Mn oxides: Applications for studying trace element associations. Appl. Geochem., 19, 973–979.
- Obrador A, Alvarez JM, Lopez-Valdivia LM, Gonzalez D, Novillo J, Rico MI 2007: Relationships of soil properties with Mn and Zn distribution in acidic soils and their uptake by a barley crop. Geoderma., 137, 432–443.
- Patrick WH, Jugsujinda A 1992: Sequential reduction and oxidation of inorganic nitrogen, manganese, and iron in flooded soil. Soil Sci. Soc. Am. J., 56, 1071–1073.
- Reyes I, Torrent J 1997: Citrate-ascorbate as a highly selective extractant for poorly crystalline iron oxides. Soil Sci. Soc. Am. J., 61, 1647–1654.
- Sauvé S, Martínez C, McBride M, Hendershot W 2000: Adsorption of free lead (Pb2+) by pedogenic oxides, ferrihydrite, and leaf compost. Soil Sci. Soc. Am. J., 64, 595–599.
- Schnitzer M, Skinner SIM 1967: Organo-metallic interactions in soils: 7. Stability constants of Pb++, Ni++, Mn++, Co++, Ca++ and Mg++-fulvic acid complexes. Soil Sci., 103, 247–252.
- Schuppli PA, Ross GJ, McKeague JA 1983: The effective removal of suspended materials from pyrophosphate extracts of soils from tropical and temperature regions. Soil Sci. Soc. Am. J., 47, 1026–1032.
- Shuman LM 1985: Fractionation method for soil microelements. Soil. Sci., 140, 11–22.
- Soil Survey Staff 1998: Keys to Soil Taxonomy. 8th ed. 1–326. National Resources Conservation Service, Washington.
- Suda A 2013: Improvement of the methods to extract heavy metals occluded in Mn and Fe oxides from major soils in Japan. doctoral thesis. University of Tsukuba, Tsukuba.
- Suda A, Makino T, Higashi T 2011: Applicability of selective dissolution of manganese oxide by acidified 0.1 M NH2OH–HCl in Japanese soils. Geoderma., 163, 291–295.
- Suda A, Makino T, Maejima Y, Akahane I, Higashi T 2009: Effects of sterilization on the chemical forms of heavy metals in soils. Soil Sci. Plant Nutr., 55, 623–626.
- Takeda A, Kimura K, Yamasaki S 2004: Analysis of 57 elements in Japanese soils, with special reference to soil group and agricultural use. Geoderma., 119, 291–307.
- Tokashiki Y, Shimo M, Arachchi LPV 2003: Improvement of the successive selective dissolution procedure for the separation of birnessite, lithiophorite, and goethite in soil manganese nodules. Soil Sci. Soc. Am. J., 67, 837–843.