Abstract
Boron (B) is an essential micronutrient for plants, but a high concentration of B is toxic. Typical B toxicity symptoms include necrosis of marginal leaves and inhibition of root elongation. Reduction of crop productivity has been reported in high-B contaminated soils, present especially in semi-arid areas in the world. We have previously reported that overexpression of BOR4, an Arabidopsis thaliana (L.) Heynh. efflux-type B transporter, conferred high B tolerance in A. thaliana. In the present study, we characterized physiological roles and expression patterns of endogenous BOR4 in A. thaliana. Decreased shoot growths and increased B concentrations in shoots were found in A. thaliana T-DNA insertion mutants of BOR4 under toxic levels of B supply, compared to the wild type plants. β-glucuronidase (GUS) staining in the transgenic ProBOR4-GUS plants was predominately detected in root meristems and endodermis of mature portion of the roots. Furthermore, mRNA levels of BOR4 in roots were increased by 2-fold upon 3-day treatment of the high B condition and analysis of the transgenic ProBOR4-GUS plants showed that this high B-dependent induction is controlled by the 5' flanking sequences of BOR4 ORF. We concluded that endogenous BOR4 is a high-B inducible gene that functions in high-B tolerance
INTRODUCTION
Boron (B) is an essential micronutrient for plants, but high concentrations of B cause disorders of plant growth. Typical B toxicity symptoms include necrosis in margins of leaves and inhibition of root elongation (Nable et al. Citation1997). High-B soils are distributed over semi-arid areas of the world and reduced quality and quantity of crop has been reported in such areas (Gupta et al. Citation1995; Nable et al. Citation1997).
It has been revealed over the last 10 years that plants have B transporters and regulation systems of their expression to maintain B homeostasis in plant bodies. Under limited B conditions, a boric acid channel NIP5;1 and a boric acid/borate exporter BOR1 are essential for B uptake and xylem loading in Arabidopsis thaliana (L.) Heynh., respectively (Takano et al. Citation2002, Citation2006). Transcript levels of NIP5;1 are increased by more than 10-fold by low B treatment (Takano et al. Citation2006) and are regulated through mRNA degradation (Tanaka et al. Citation2011). The transcript levels of BOR1 are not greatly affected by B status but protein accumulation of BOR1 is increased under B limitation (Takano et al. Citation2005).
Under high B conditions, homologous genes to A. thaliana BOR1 were identified as key determinants for high-B tolerance in Hordeum vulgare L. (barley) and Triticum aestivum L. (wheat), which encode B exporters to reduce B concentrations in roots and to alter cellular distribution of B in shoots (Reid Citation2007; Sutton et al. Citation2007; Reid and Fitzpatrick Citation2009). Sutton et al. (Citation2007) and Reid (Citation2007) found the positive correlations between mRNA levels of HvBor2 (Bot1) and TaBor2, and reduction of root B concentrations or plant growth among cultivars exhibiting different sensitivities to toxic B. However, regulation of these genes’ expression in response to B conditions has not been well characterized.
We have found that BOR4, a homologous gene of BOR1 in A. thaliana, encodes an efflux-type B transporter localized to the plasma membrane, and overexpression of BOR4 conferred high-B tolerance in A. thaliana (Miwa et al. Citation2007; Miwa and Fujiwara Citation2011), mainly through B efflux in roots. Amino acid sequences encoded by HvBOR2 and TaBor2 are turned out to be more similar to A. thaliana BOR4 than to AtBOR1 (Nakagawa et al. Citation2007; Reid Citation2007; Sutton et al. Citation2007), supporting the idea that endogenous BOR4 functions in high-B tolerance. In the present study, we studied the physiological function of endogenous BOR4 and B-dependent regulation of transcript levels in A. thaliana.
MATERIALS AND METHODS
Plant materials and culture
Arabidopsis thaliana (L.) Heynh. Col-0 and Ler were used. Plants were grown in solid media (Fujiwara et al. Citation1992) containing 2% sucrose and 1.5% gellan gum (Wako Pure Chemicals, Osaka, Japan) in which the B concentrations were adjusted by changing the concentration of boric acid. For growth test in , the media contained 5 mM potassium chloride (KCl). Surface-sterilized seeds were sown on the solid media and incubated for 2 d at 4°C. The plates were then placed vertically and incubated at 22°C under a 16-h light/8-h dark cycle.
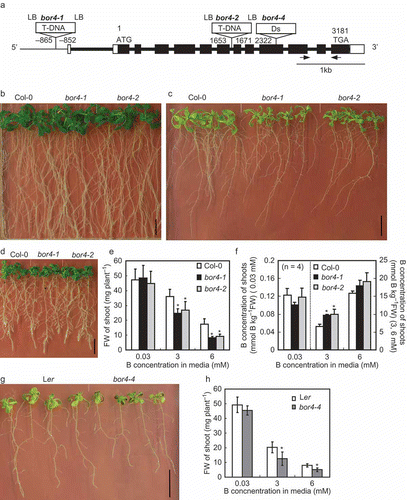
Figure 1 Growth characteristics of Arabidopsis thaliana mutants of BOR4. (a) The exon-intron structures of the BOR4 gene, and schematic representation of insertions of T-DNA in bor4-1, bor4-2 and Ds transposon in bor4-4. Filled boxes, open boxes and thick bars indicate exons encoding a protein, untranslated regions (UTR) and introns, respectively. The arrows indicate the position of the primers for real-time polymerase chain reaction (PCR). (b–d) Growth of wild type Col-0 plants, bor4-1 and bor4-2 under 0.03 mM (b), 6 mM (c), and 0.1 µM (d) boric acid supply. Four-day-old plants grown under 0.03 mM boric acid were transferred to the media containing 0.1 µM, 0.03 mM and 6 mM boric acid and then they were incubated for 14 d. Bars show 10 mm. (e, f) Fresh weights (e) and boron (B) concentrations (f) of aerial portions of bor4-1 and bor4-2. Four-day-old plants grown under 0.03 mM boric acid were transferred to the media containing 0.03, 3 and 6 mM boric acid and then they were incubated for 14 d. Means ± standard deviation (SD) are shown (n = 7–8 for fresh weight, n = 4 for B concentrations). Asterisks show a significant difference compared to wild type Col-0 under the same condition (Student’s t-test, p < 0.05). (g) Growth of wild type Ler plants and bor4-4 under 6 mM boric acid supply. Four-day-old plants grown under 0.03 mM boric acid were transferred to the media containing 6 mM boric acid and then they were incubated for 14 d. A bar shows 10 mm. (h) Fresh weights of aerial portions of bor4-4. Four-day-old plants grown under 0.03 mM boric acid were transferred to the media containing 0.03, 3 and 6 mM boric acid and then they were incubated for 14 d. Means ± SD are shown (n = 8). Asterisks indicate a significant difference compared to wild type Ler under the same condition (Student’s t-test, p < 0.05). FW, fresh weight.
Establishment of T-DNA or Ds insertional mutants of BOR4 (At1g15460)
T-DNA insertion lines bor4-1 (SALK_135095) were provided by the Salk Institute (Alonso et al. Citation2003). bor4-2 was developed by the plant genome project of RIKEN Genomic Sciences Center and provided by RIKEN BioResource Center (Nakazawa et al. Citation2003). bor4-4 (CSHL_ET13769) carrying Ds transposon was obtained from Cold Spring Harbor Laboratory. bor4-1 and bor4-2 are Col-0 background and bor4-4 is Ler background. Homozygous lines were selected by polymerase chain reaction (PCR). The primers used are 5'-CCATTGACGTGGAGTGATAT-3' (A. thaliana genome forward), 5'- AACTCGGAAATTTGAGGCGT-3' (reverse) and 5'-GATGGCCCACTACGTGAACCAT-3' (SALK LBb1) for bor4-1, 5'-TGATCGCAGGGCTTTACTTC-3' (for-ward), 5'-TGGTATGCATAGGAGACTGAGG-3' (reverse) and 5'-ATAACGCTGCGGACATCTAC-3' (LB) for bor4-2, 5'-AGCCAAAGAAAGCATCAGGA-3' (forward), 5'-CTCCCTTGTCGTGCTCATGTCCCC-3' (reverse) and 5'-TACCGACCGTTTTCATCCCT-3' (Ds transposon) for bor4-4.
Determination of B concentration in shoots
Seedlings were harvested and dried at 60°C for more than 48 h, followed by dry weight measurements. Plant samples were placed in 8-mL Teflon tubes and 1 mL of concentrated nitric acid was added. The samples were digested at 130°C for 2 h and the pellets were dissolved with 3 mL of 0.08 N nitric acid containing 5 ppb beryllium (Be) as an internal standard. B concentrations were determined by ICP-MS (SPQ9000, SII, Tokyo, Japan).
Generation of transgenic A. thaliana line expressing GUS under the control of BOR4 promoter
A plasmid for the Promoter-GUS (β-glucuronidase) construct was generated by replacing the GFP ORF of the ProBOR1-GFP construct (Takano et al. Citation2002) with a GUS open reading frame (ORF). The six extra nucleotides ATGGTA, which encode Met-Val, were inserted at the 5' end of the original GUS ORF (Jefferson et al. Citation1986) to generate an NcoI site at the 5' end of the GUS ORF. The resulting plasmid was named as pTF538 (ProBOR1-GUS-tNOS in pUC119). A 3-kb fragment upstream from the BOR4 start codon, corresponding to the nucleotides 5316437 to 5313377of chromosome 1, was used as a BOR4 promoter. This fragment was amplified with KOD plus DNA polymerase (TOYOBO, Tokyo, Japan) with BAC F9L1 as a template. The primers 5'-CGCGGATCCCTAGGAACATAGTTTCCCCAT-3' and 5'-TAAGACCATGGCCAGAATCCCTCAAAGACTC-3' were used to introduce a BamHI and a NcoI site, respectively. The amplified DNA fragment was digested with BamHI and NcoI followed by cloning into pTF538 (ProBOR1-GUS-tNOS in pUC119) to replace the ProBOR1 fragment. Then, they were cloned into binary vector pBIN19 for plant transformation. The resulting plasmid (pTF564, ProBOR4-GUS-tNOS in pBIN19) was used for Agrobacterium-mediated in planta transformation of A. thaliana Col-0 by the floral dip method (Clough and Bent Citation1998).
GUS histochemical staining
GUS staining was carried out with transgenic plants carrying ProBOR4-GUS in the T2 generation. Plants were grown for 7 d in medium containing 0.03 mM boric acid. GUS staining was conducted as described in Shibagaki et al. (Citation2002). Optical images were taken with a light microscope (BX50WI; Olympus, Tokyo, Japan).
Quantification of mRNA levels by real-time PCR
Real-time polymerase chain reaction was conducted to quantify mRNA levels of endogenous BOR4 in wild type Col-0 plants and transgene GUS in T2 bulk plants of transgenic lines carrying ProBOR4-GUS. Plants were first grown with 0.03 mM boric acid supply for 7–8 d and then transferred to the media containing different concentrations of boric acid and incubated for 3 d. Total RNA was extracted from roots tissues by RNeasy Plant Mini kit (QIAGEN, Germany) from three independent plant populations. RNA was subjected to reverse transcription with Primescript RT reagent kit (TAKARA, Japan). cDNAs were subjected to quantitative PCR with Dice (TAKARA) using SYBR Premix Ex TaqII (TAKARA). The target transcript levels were standardized to the levels of β-tublin or elongation factor 1α (EF1α) transcript. The sequences of the primers used in PCR were 5'-GGAACTGTCTTTCCGGTCGAA-3' (exon11–exon12) and 5'-CTTGGGATAAATCTGGTTGCCT-3' (exon13–exon12) to amplify 148 bp of the 3' portion of BOR4, and 5'-GATGTGGAGTATTGCCAACG-3' 5'-TGAGCGTCGCAGAACATTAC-3' for 138 bp of GUS. The primers for β-tublin were 5'-GCTCGCTAATCCTACCTTTGG-3' and 5'-AGCCTTGGGAATGGGATAAG-3' for 141 bp. The primers for EF1α are described in Takano et al. (Citation2006). To detect splicing of the first intron of BOR4 gene, the primers 5'-TCGGCTCCGGCTATTCTCCT-3' (exon1) and 5'-GTGGGTGCTAAAATCCCGAA-3' (exon3–exon2) were used. The primers used for detecting the BOR4 transcript contain sequences that encompass splice junctions in the genome.
Quantification of GUS activities
Seven-day-old transgenic lines carrying ProBOR4-GUS under 0.03 mM boric acid were treated with 0.03 and 3 mM boric acid for 3 d. The bulk of 20–24 T2 plants were harvested as one sample. GUS activities were measured based on Jefferson (Citation1987). To extract proteins, roots were homogenized with an extraction buffer (50 mM sodium phosphate buffer (pH 7), 10 mM disodium ethylenediaminetetraacetate (EDTA), 0.1% sodium sarcosyl, 0.1% Triton X-100, 10 mM 2-mercaptoethanol. After centrifugation, the supernatant was subjected to measurement of protein concentration and GUS activities. The concentrations of total protein were determined with Quick Start Bradford Protein Assay (Bio-Rad Laboratories, USA). To measure GUS activities, the supernatant samples were incubated in the extraction buffer containing 1 mM 4-methylumbelliferyl-beta-D-glucuronide (MUG) at 37°C. The aliquot of the samples were put into 0.2 M sodium carbonate (Na2CO3) to terminate the reaction at multiple time points. The concentrations of 7-hydoxy-4-methylcoumarin (4-MU) in the samples were determined with a fluorescence spectrophotometer.
RESULTS
Shoot growth reduction in T-DNA or Ds insertion mutants of BOR4 under toxic levels of B
To examine the roles of endogenous BOR4 in A. thaliana plants, three independent T-DNA or Ds insertional mutants of BOR4 were obtained and established (). bor4-1 and bor4-2 are Col-0 background and carry T-DNA in the promoter and in an intron of BOR4, respectively. mRNA levels of BOR4 in roots of bor4-1 and bor4-2 were decreased to 2% of those of wild type plants under 0.03 mM boric acid supply. When 4-day-old bor4-1 and bor4-2 were transferred to normal (0.03 mM), toxic (6 mM) and deficient (0.1 µM) levels of B conditions, shoot growth was reduced under 6 mM boric acid in bor4-1 and bor4-2 compared to the wild type plants () whereas plant growths did not apparently differ under 0.03 mM () and 0.1 µM () B. Shoot fresh weights were decreased by 31 and 25% under 3 mM B and by 53 and 48% under 6 mM B in bor4-1 and bor4-2, respectively (). Total B concentrations in shoot tissues in bor4-1 and bor4-2 were significantly increased at 3 mM compared to the wild type Col-0 (). bor4-4, carrying a Ds transposon in the exon10 of BOR4 in Ler background, also displayed shoot growth reduction under toxic levels of B supply ( and ). The fact that three independently generated insertional mutants exhibited a similar growth reduction under high B suggests that endogenous BOR4 functions in high B tolerance in A. thaliana.
Cell-specific expression of BOR4 in roots
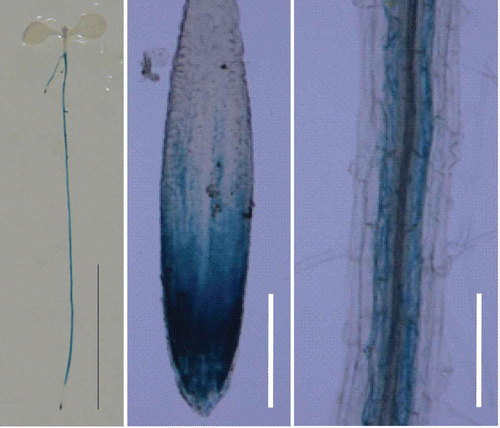
Accumulation of BOR4 mRNA was detected both in shoots and roots by RT-PCR. To reveal cell-specificity of BOR4, T2 transgenic A. thaliana lines expressing GUS under the control of the BOR4 promoter were grown in solid media containing 0.03 mM boric acid for 7 d. Representative GUS staining patterns are depicted in . GUS staining was predominantly observed in roots and hardly detected in shoots ( left). In roots, GUS staining was observed in root meristem and in endodermis, but not in the epidermis and cortex of mature portions of the roots ( middle and right). Predominant expression in root tips is consistent with BOR4-GFP expression in the plants carrying ProBOR4-BOR4-GFP as BOR4-GFP was detected in epidermis of root tips (Miwa et al. Citation2007).
Figure 2 GUS histochemical staining of the transgenic Arabidopsis thaliana expressing β-glucuronidase (GUS) under the control of the BOR4 promoter. Transgenic plants were grown in the media containing 0.03 mM boric acid for 7 d. GUS histochemical staining of a whole plant (left), root tip (middle) and root hair zone (right) are shown. Bars show 10 mm (left) and 100 µm (middle and right), respectively.
Up-regulation of BOR4 mRNA upon high B treatment
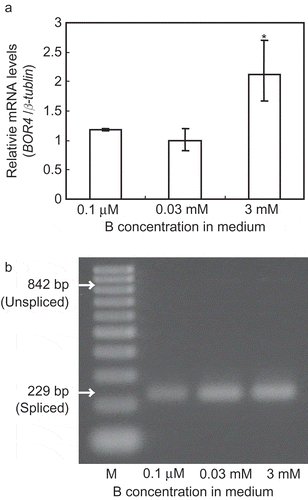
To determine effects of B nutrition status on BOR4 mRNA accumulation, we performed quantitative RT-PCR on plants transferred from medium containing sufficient B (0.03 mM) to medium with deficient (0.1 µM), sufficient (0.03 mM) and toxic (3 mM) levels of B concentrations (). BOR4 transcript levels in roots were not significantly changed by low B treatment, but increased by 2-fold upon high B supply (). Since BOR4 gene contains the first intron of 613 bp in the 5' untranslated region (UTR) () and the length of the 5' UTR may affect mRNA stability depending upon B conditions, splicing of the first intron was examined by RT-PCR. We detected spliced transcripts but not unspliced transcripts under the three different B conditions, suggesting that the first intron was completely spliced out in the BOR4 transcripts irrespective of B conditions ().
Figure 3 Changes of BOR4 transcript levels in roots after high boron (B) treatment. Arabidopsis thaliana wild type Col-0 plants were grown in the solid media supplemented with 0.03 mM boric acid for 8 d. Then the plants were transferred to the media containing 0.1 µM, 0.03 mM and 3 mM boric acid, incubated for 3 d and RNA was extracted from roots. (a) Quantification of BOR4 mRNA levels. BOR4 transcript levels were standardized by the levels of β-tublin. The values shown are those relative to the mean value at 0.03 mM boric acid, which was defined as 1. Means ± standard deviation (SD) are shown (n = 3, from independent plant materials). An asterisk shows a significant difference compared to the values at 0.03 mM boric acid (Student’s t-test, p < 0.05). (b) Splicing of the first intron of BOR4 transcripts. cDNA was amplified with the primers corresponding to the exon1 and exon3-exon2. The size of the spliced and unspliced product is expected to be 229 and 842 bp, respectively. 100 bp ladder DNA size marker is shown on the left lane as M.
High-B induction of reporter gene expression in the transgenic ProBOR4-GUS plants
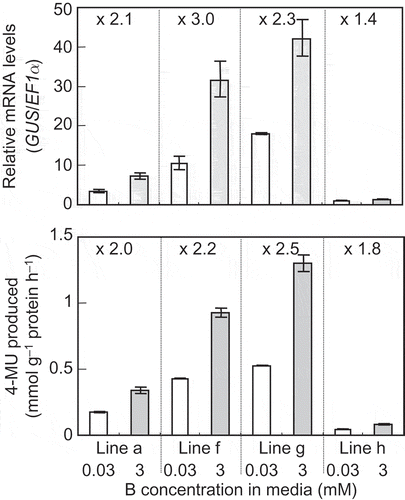
To investigate whether the promoter region affects BOR4 expression in response to high B, GUS mRNA levels and GUS activities were measured in roots of the transgenic lines carrying ProBOR4-GUS after 3-day incubation in the media containing 0.03 or 3 mM boric acid. In the four independent transgenic lines, GUS mRNA levels were increased by 1.4- to 3.0-fold at 3 mM relative to those at 0.03 mM (, upper) similar to the case of mRNA of endogenous BOR4 gene. GUS activities were elevated by 1.8- to 2.5-fold at 3 mM in a similar manner to GUS mRNA (, lower). The four transgenic lines exhibited different levels of transgene expression, but the responses to high B and relative accumulation of GUS mRNA to GUS activity were consistent among the lines.
Figure 4 Response of β-glucuronidase (GUS) mRNA and GUS activity in the transgenic Arabidopsis thaliana expressing GUS under the control of the BOR4 promoter upon high boron (B) treatment. Four independent transgenic A. thaliana lines carrying ProBOR4-GUS (Lines a, f, g and h) were first grown in the solid media containing 0.03 mM boric acid for 7 d, and were transferred to the media supplied with 0.03 or 3 mM boric acid. Then they were incubated for 3 d, and mRNA levels of the GUS gene and GUS activities were determined in the roots. mRNA levels of GUS were standardized by the levels of elongation factor 1α transcript. The values shown are those relative to the mean value of Line h under 0.03 mM boric acid, which was defined as 1. GUS activities are shown as production of 4-MU (mmol g−1 protein h−1). Means ± standard deviation (SD) are shown (n = 3, three measurements). Fold changes of mRNA levels or GUS activities under 3 mM boric acid relative to those under 0.03 mM boric acid are shown above the bars in the graph.
DISCUSSION
We have previously demonstrated that overexpression of BOR4 decreases B concentrations in plant tissues and improves growth under B toxicity, mainly through B exclusion in roots under high B conditions (Miwa et al. Citation2007; Miwa and Fujiwara Citation2011). In the present study, we reveal that endogenous BOR4 is predominately expressed in roots () and is also involved in high B tolerance (). Furthermore, mRNA levels of endogenous BOR4 are induced upon high-B treatment (), and this induction is mediated by the 5' flanking sequence of BOR4 ORF ().
Shoot growth reduction was observed in bor4-1 and bor4-2 with increased B concentrations in tissues under toxic levels of B supply (), suggesting that endogenous BOR4 also functions in reduction of B concentrations in plant tissues for high-B tolerance in A. thaliana. GUS staining derived from ProBOR4-GUS is predominately detected in endodermis of root hair zone and meristems of the roots (). Endodermis in A. thaliana roots is the cell type where Casparin strips are developed as apoplastic barriers (Geldner Citation2013). Expression of an efflux-type B transporter in endodermis of mature portion of the roots and its likely polar localization to outer domain of plasma membrane (Miwa et al. Citation2007) is possibly effective to reduce B flux into xylem. Reduced B concentrations in root meristem could be beneficial for alleviation of B toxicity to sustain root elongation under high B conditions (Sakamoto et al. Citation2011), although inhibition of root elongation was not evident in bor4-1 and bor4-2 in our study. Considering that overexpression of BOR4 greatly improves high-B tolerance in A. thaliana, it can be assumed that contribution of endogenous BOR4 for high-B tolerance is minor in wild type plants.
The transcript levels of BOR4 were increased by 2-fold upon 3-day treatment of high B concentration (). GUS mRNA levels in the transgenic A. thaliana lines carrying ProBOR4-GUS were induced upon high B, which reflects the patterns of endogenous BOR4 (). Since the 3-kb 5' flanking sequence of BOR4 ORF is included in the ProBOR4-GUS construct, it is demonstrated that the corresponding sequences, including promoter and 5' UTR, control up-regulation of BOR4 mRNA under the high-B treatment. In addition, GUS activities were in clear parallel with GUS mRNA levels under normal and high B conditions among the lines, suggesting that translational efficiency is not significantly changed in response to B conditions in this ProBOR4-GUS construct.
Among BOR genes in plants, TaBOR1.2 in wheat is reported to be induced under toxic levels of B supply (Leaungthitikanchana et al. Citation2013), although the physiological function of TaBOR1.2 is still unclear. It has been also reported that transcript levels of a homologous gene to BOR (Bot1) is induced by high-B treatment in shoots of Puccinellia distans, an extremely high-B tolerant plant species (Padmanabhan et al. Citation2012). High-B induction of BOR4 mRNA in A. thaliana is consistent with the physiological function of endogenous BOR4 against B toxicity. It should be noted that GUS activities in the transgenic ProBOR4-GUS plants were not greatly changed under continuous exposure to high B for 10 d. It is possible to assume that transient induction of BOR4 mRNA has a significant impact to adapt to high B environments.
In conclusion, we demonstrate that endogenous BOR4 functions in high-B tolerance and mRNA of BOR4 is induced upon high-B treatment by upstream sequence of BOR4 ORF.
ACKNOWLEDGMENTS
We appreciate Ms. Chie Kobayashi for her technical assistance. We appreciate the Japanese Society for the Promotion of Science Research Fellowships for young scientists and Hokkaido University Leader Development System in the Basic Interdisciplinary Research to K.M. This work was supported in part by a Grant-in-Aid for Young Scientists (A) to K.M. (No. 22688005), a Grant-in-Aid for Scientific Research (S) to T.F. (No. 25221202) and a Grant-in-Aid for Scientific Research on Innovative Areas to T.F. (No. 22119002) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
REFERENCES
- Alonso JM, Stepanova AN, Leisse TJ 2003: Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science, 301, 653–657.
- Clough SJ, Bent AF 1998: Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J., 16, 735–743.
- Fujiwara T, Hirai MY, Chino M, Komeda Y, Naito S 1992: Effects of sulfur nutrition on expression of the soybean seed storage protein genes in transgenic petunia. Plant Physiol., 99, 263–268.
- Geldner N 2013: The endodermis. Annu. Rev. Plant. Biol., 64, 531–558.
- Gupta UC, Jame YM, Campbell CA, Leyshon AJ, Nicholaichuk W 1995: Boron toxicity and deficiency: a review. Can. J. Soil Sci., 65, 381–409.
- Jefferson RA 1987: Assaying chimeric genes in plants: The GUS fusion system. Plant Mol. Biol. Rep., 5, 387–405.
- Jefferson RA, Burgess SM, Hirsh D 1986: β-glucuronidase from Escherichia coli as a gene-fusion marker. Proc. Natl. Acad. Sci. USA, 83, 8447–8451.
- Leaungthitikanchana S, Fujibe T, Tanaka M, Wang S, Sotta N, Takano J, Fujiwara T 2013: Differential expression of three BOR1 genes corresponding to different genomes in response to boron conditions in hexaploid wheat (Triticum aestivum L.) Plant Cell Physiol., 54, 1056–1063.
- Miwa K, Fujiwara T 2011: Role of overexpressed BOR4, a boron exporter, in tolerance to high level of boron in shoots. Soil Sci. Plant Nutr., 57, 558–565.
- Miwa K, Takano J, Omori H, Seki M, Shinozaki K, Fujiwara T 2007: Plants tolerant of high boron levels. Science, 318, 1417.
- Nable RO, Bañuelos GS, Paull JG 1997: Boron toxicity. Plant Soil, 193, 181–198.
- Nakagawa Y, Hanaoka H, Kobayash M, Miyoshi K, Miwa K, Fujiwara T 2007: Cell-type specificity of the expression of Os BOR1, a rice efflux boron transporter gene, is regulated in response to boron availability for efficient boron uptake and xylem loading. Plant Cell, 19, 2624–2635.
- Nakazawa M, Ichikawa T, Ishikawa A, Kobayashi H, Tsuhara Y, Kawashima M, Suzuki K, Muto S, Matsui M 2003: Activation tagging, a novel tool to dissect the functions of a gene family. Plant J., 34, 741–750.
- Padmanabhan P, Babaoğlu M, Terry NA 2012: Comparative transcriptomic analysis of the extremely boron tolerant plant Puccinellia distans with the moderately boron tolerant Gypsophila arrostil. Plant Cell Rep., 31, 1407–1413.
- Reid R 2007: Identification of boron transporter genes likely to be responsible for tolerance to boron toxicity in wheat and barley. Plant Cell Physiol., 48, 1673–1678.
- Reid R, Fitzpatrick K 2009: Influence of leaf tolerance mechanisms and rain on boron toxicity in barley and wheat. Plant Physiol., 151, 413–420.
- Sakamoto T, Inui YT, Uraguchi S, Yoshizumi T, Matsunaga S, Mastui M, Umeda M, Fukui K, Fujiwara T 2011: Condensin II alleviates DNA damage and is essential for tolerance of boron overload stress in Arabidopsis. Plant Cell, 23, 3533–3546.
- Shibagaki N, Rose A, McDermott JP, Fujiwara T, Hayashi H, Yoneyama T, Davies JP 2002: Selenate-resistant mutants of Arabidopsis thaliana identify Sultr1;2, a sulfate transporter required for efficient transport of sulfate into roots. Plant J., 29, 475–486.
- Sutton T, Baumann U, Hayes J et al. 2007: Boron-toxicity tolerance in barley arising from efflux transporter amplification. Science, 318, 1446–1449.
- Takano J, Miwa K, Yuan L, von Wirén N, Fujiwara T 2005: Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc. Natl. Acad. Sci. USA, 102, 12276–12281.
- Takano J, Noguchi K, Yasumori M, Kobayashi M, Gajdos Z, Miwa K, Hayashi H, Yoneyama T, Fujiwara T 2002: Arabidopsis boron transporter for xylem loading. Nature, 420, 337–340.
- Takano J, Wada M, Ludewig U, Schaaf G, von Wirén N, Fujiwara T 2006: The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell, 18, 1498–1509.
- Tanaka M, Takano J, Chiba Y, Lombardo F, Ogasawara Y, Onouchi H, Naito S, Fujiwara T 2011: Boron-dependent degradation of NIP5;1 mRNA for acclimation to excess boron conditions in Arabidopsis. Plant Cell, 23, 3547–3559.
