Abstract
The response of grafted vegetables to stress conditions owing to the nutrient status may be different than that of self-rooted plants, depending mainly on the rootstock genotype. The aim of the present work is to determine the response of reciprocal grafts made between cherry tomato cultivars (Solanum lycopersicum) to moderate water stress, examining uptake and concentration of nutrient. The tomato cultivars Zarina (drought-tolerant) and Josefina (drought-sensitive) were reciprocal grafted and selfgrafted, and ungrafted were used as control. The total content and uptake fluxes of macro- and micronutrients were determined in leaves. Our results show that the use of drought-tolerant cv. Zarina like rootstocks (ZarxJos) showed improve ionome, with increases in nitrogen (N), phosphorus (P) and potassium (K) concentration and uptake fluxes, and an increase in iron (Fe) and copper (Cu) concentration and uptake under water stress. Besides, the vigorous root system of rootstocks is often capable of absorbing plant nutrients more efficently than scion root, and we have showed that cv. Zarina and ZarxJos develop a better radicular system. This result confirms the hypothesis that grafted plants on vigorous rootstocks can improve mineral nutrition and nutrient uptake with respect to ungrafted plants, especially under water stress conditions.
INTRODUCTION
The study of the mineral composition of a plant and the changes in this composition in response to physiological and environmental stimuli, the plant’s developmental state and genetic modifications has been recently defined as “ionome” (Salt et al. Citation2008). Therefore, the ionome can provide information on the functional state of an organism under different growth conditions. Due to the limited availability of arable land, the high demand for off-season vegetables and intensive farming practices with limited crop rotations, vegetables are often cultivated under unfavourable conditions which induce stress (Savvas et al. Citation2010). These conditions include dry environments, because water is an economically scarce resource in many areas of the world, especially in arid and semiarid regions such as the Mediterranean basin. Water stress causes various physiological disorders leading to severe crop loss. One environmentally-friendly technique for avoiding or reducing losses in production caused by abiotic stress conditions in high-yielding genotypes belonging to Solanaceae and Cucurbitaceae families would be to graft them onto rootstocks capable of reducing the negative effect of external stress on the shoot (Savvas et al. Citation2010). The cultivated area of grafted Solanaceae, including a number of important annual fruit-crop plants such as tomato (Solanum lycopersicum), eggplant (Solanum melongena) and pepper (Capsicum annuum), has increased in recent years in order to obtain resistance to soil-borne diseases (Bletsos et al. Citation2003; Davis et al. Citation2008), tolerance against abiotic stresses such as salinity, wet soils and high and low temperatures (Ahn et al. Citation1999; Rivero et al. Citation2003a, Citation2003b; Estañ et al. Citation2005; Venema et al. Citation2008; Abdelmageed and Gruda Citation2009) and to improve fruit quality (Fernández-García et al. Citation2004a, Citation2004b; Colla et al. Citation2006).
Although water absorption and nutrient uptake are independent processes in the root, the need for available water for growth and nutrient transport makes them intimately related (Viets Citation1972). Generally, drought reduces not only nutrient uptake by the root but also nutrient transport from the root to the shoot due to a restricted transpiration rate, depressed active transport and reduced membrane permeability. The overall result is that the uptake power of the plant is disminished (Kramer and Boyer Citation1995). During the formation of the graft union, the vascular connection in the rootstock-scion interface may determine water and nutrient translocation, affecting other physiological traits (Oda et al. Citation2005; Johkan et al. Citation2009). However, water and nutrient uptake could be increased in grafted plants as result of the enhancement of vigour by the rootstock root system and its effects on plant yield (Ruiz et al. Citation1997). The influence of the rootstock on the mineral content in aerial plant parts was attributed to physical characteristics of the root system, such as lateral and vertical development, which resulted in enhanced uptake of water and minerals, this being one of the main motives for the widespread use of grafted rootstocks (Heo Citation1991). However, in grafted fruit trees, no effect of the rootstock on the leaf mineral content was found and, in this case, more influence of the scion on leaf nutrient content was observed (Seki et al. Citation2008). Tagliavani et al. (Citation1993) suggested that vigour of both the scion and root system had an important role in the uptake and translocation of nutrients in grafted fruit trees, while in cucumber (Cucumis sativus), grafted plants changes of rootstock had an influence on the leaf content of certain essential minerals under salinity (Huang et al. Citation2010). Therefore, the contents of macro- and micronutrients are affected by the rootstock and scion characteristics but, depending on the element and environmental conditions, the effect of the rootstock and/or scion may change.
Given that tomato is one of the most important crops worldwide, and that its production is concentrated in semiarid regions, where water stress is frequent, it is of great interest to ascertain whether grafting is a valid strategy to improve water-stress tolerance and its ionome in this plant. In preliminary studies, we have observed that the cv. Zarina shows better water-stress tolerance and better mineral content than cv. Josefina, which is more drought sensitive (Sánchez-Rodríguez et al. Citation2010a, Citation2010b). In this light, the aim of the present work is to determine the response of reciprocal grafts made between one tolerant cultivar, Zarina, and a more sensitive cultivar, Josefina, to moderate water stress, examining uptake and concentration of nutrients.
MATERIAL AND METHODS
Plant material and growth conditions
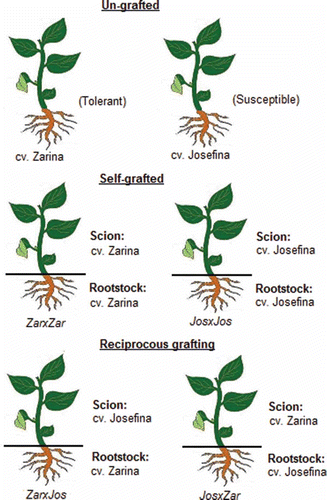
Two tomato cultivars, Zarina and Josefina, were used as scion and rootstock (). The seeds of these cultivars were germinated and grown for 30 d in a tray with wells (each well 3 cm × 3 cm × 10 cm) in the nursery Semillero Saliplant S.L. (Carchuna, Granada). Grafting was performed when seedlings had developed 3–4 true leaves. In the vermiculite trays used for germination, the seedlings were cut over the cotyledons, using the shoot as scion and the remaining plant part as rootstock. Grafts were made inmediately after cutting the plants and grafting clips were used to adhere the graft union. Self-grafted plants were included as controls. After grafting, seedlings were covered with a transparent plastic lid to maintain a high humidity level (60–70%) and to facilitate graft formation and were left in the shade for 24 h. The plastic was opened slightly every day to allow reduction in relative humidity and it was removed 6 days after grafting. Afterwards, ungrafted and grafted plants were transferred to a cultivation chamber at the Plant Physiology Department of the University of Granada under controlled conditions with relative humidity of 50 ± 10%, at 25/15°C (day/night), and a 16/8 h photoperiod with a photosynthetic photon-flux density (PPFD) of 350 µmol m−2 s−1 (measured with an SB quantum 190 sensor, LI-COR Inc., Lincoln, NE, USA). Under these conditions, the plants grew in individual pots (25 cm upper diameter, 17 cm lower diameter, and 25 cm high) of 8 L in volume and filled with a 1:1 perlite:vermiculite mixture. Throughout the experiment, the plants were grown in a complete nutrient solution containing: 4 mM potassium nitrate (KNO3), 3 mM calcium nitrate (Ca(NO3)2), 2 mM magnesium sulfate (MgSO4), 6 mM potassium phosphate monobasic (KH2PO4), 1 mM monosodium phosphate (NaH2PO4), 2 µM manganese chloride (MnCl2), 1 µM zinc sulfate (ZnSO4), 0.25 µM copper sulfate (CuSO4), 0,1 µM sodium molibdate dihydrate (Na2MoO4), 5 µM iron chelate (Fe-EDDHA), and 50 µM boric acid (H3BO3). The nutrient solution (pH 5.8) was renewed every 3 d and the substrate was partially rinsed with distilled water to avoid nutrient accumulation. The water-stress treatments began 45 d after germination and maintained for 22 d. The control treatment received 100% field capacity (FC) irrigation, whereas moderate water stress corresponded to 50% FC. The experimental design was a randomized complete block with 12 treatments (Zarina ungrafted, Josefina ungrafted, Zarina self-grafted, Josefina self-grafted, JosxZar and ZarxJos well-watered 100% FC and water stress 50%) () arranged in individual pots with six plants per treatment (one plant per pot) and three replications each.
All plants were at the late vegetative stage when harvested. Leaves (excluding petioles) and roots were harvested, frozen immediately in liquid N2, and kept at −80°C until used. To determine the relative growth rate (RGR), leaves and roots from six plants per cultivar were sampled on day 45 after germination, immediately before starting the water-stress treatment (Ti). The leaves and roots were dried in a forced-air oven at 70°C for 24 h, and the dry weight (DW) was recorded as grams per plant. The remaining plants were sampled 67 d after sowing (day 22 of treatments, Tf). The relative growth rate was calculated from the increase in DW at the beginning and at the end of the water-stress treatment, using the equation
where T is the time and the subscripts denote the final and initial sampling (i.e. days 0 and 22, respectively, after the water-stress treatment) (Bellaloui and Brown Citation1998).
Root biomass and metabolic efficiency
All plants were at the late vegetative stage when harvested. Leaves fully expanded (excluding petioles) and roots were harvested, frozen immediately in liquid nitrogen (N2), and kept at −80°C until used. The leaves and roots were dried in a forced-air oven at 70°C for 24 h, and the DW was recorded as grams per plant.
Metabolic activity in the root tips was measured by the reduction in an electron acceptor 2,3,5-triphenyltetrazolium chloride (TTC) following the method of Steponkus (Citation1971). Roots were placed on 3-mm filter paper saturated with a solution containing 0.8% TTC in 50 mM potassium phosphate buffer, pH 7.1. The filter paper pads were located on top of temperature blocks (thermoelectric cells) of a Cellular Thermoelectric Controller (CELTEC). Transparent plastic carbon dioxide (CO2)-permeable film was placed over the roots during the 30-min dark treatment to prevent water loss yet permit gas exchange. Following the 30-min treatment, the root tips (0.5 cm) were excised, rinsed with deionized water and placed in 95% ethanol. After a 24-h dark incubation period, the level of TTC reduction was determined by the absorbance of the ethanol extract at 490 nm.
Determination of mineral nutrients
The nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), iron (Fe), manganese (Mn), zinc (Zn), boron (B) and copper (Cu) were mineralized by wet digestion, following Wolf (Citation1982). For this, 0.2 g of dry roots and leaves were ground and mineralized with sulfuric acid H2SO4 12 N and hydrogen peroxide (H2O2), 30% P free, at a temperature of 275–300°C. From the resulting mineralization, and after the addition of 20 mL of deionized H2O, the mineral nutrients were determined as described in the following.
The reduced N concentration was determined by colorimetry based on the Berthelot reaction, with slight modifications (Krom Citation1980).
The total P concentration was determined using the colorimetric nitrovanadomolybdate method of Hogue et al. Citation1970 while the total K concentration was analyzed by flame photometry (Lachica et al. Citation1973).
The total Mg and Ca concentrations were quantified by atomic absorption spectrophotometry (Hocking and Pate Citation1977), as were the micronutrients, Fe, Mn and Zn. The total B concentration was determined by the colorimetric method of azomethine-H (Wolf Citation1974). Reduced sulfur (S) was extracted by mineralization with nitric/perchloric acid. For this, a quantity of 0.2 g of cherry tomato ground dry was digested with a mixture of nitric acid (HNO3)/perchloric acid (HClO4) (volume/volume) and hydrogen peroxide (H2O2) at 30%. The reduced S was determined on an aliquot of the mineralization using barium sulfate (BaSO4) in suspension by means of a surfactant agent such as gum arabic, all this against a standard curve of potassium sulfate (K2SO4) and a turbidmetric reading at 435 nm (Novozamsky and Vaneck Citation1977).
The chlorine (Cl), nitrate (NO3−), phosphate (PO43−), sulfate (SO42−), soluble potassium (K), magnesium (Mg) and calcium (Ca) concentrations in roots and leaves were determined from an aqueous extraction following the method of Cataldo et al. (Citation1975) with slight modifications (0.1 g dry material in 10 mL deionized water). The Cl concentration was determined by the method of Diatloff and Rengel (Citation2001), based on the displacement of biocyanate by chloride, which in the presence of Fe3+ forms the highly colored complex ferric thiocyanate. The determination of NO3− was based on a colorimetric reaction formed by the bonding with salicylic acid in a basic medium (Cataldo et al. Citation1975). The total N concentration was determined as the sum of the reduced N and NO3− concentrations. The PO43− was determined following the method of Hogue et al. (Citation1970). The SO42− was determined following the method of Novozamsky and Vaneck (Citation1977). The total S corresponded to the sum of the concentrations of reduced S and SO42−. Soluble Mg and Ca were quantified by atomic absorption spectrophotometry (Hocking and Pate Citation1977), while the soluble K concentration was analyzed by flame photometry (Lachica et al. Citation1973).
The soluble Fe, Mn, Cu and Zn concentrations were determined from a hydrochloric acid (HCl) 1M extraction following the method of Cataldo et al. (Citation1975) with slight modifications. These nutrients were quantified by atomic absorption spectrophotometry (Hocking and Pate Citation1977)
Determination of uptake fluxes
Over the period under study, determination of nutrient uptake fluxes was calculated from the RGR, the DW, the total nutrient concentrations and the soluble nutrient concentration contents of leaves (l) and roots (r) as folllows (Kruse et al. Citation2007):
The following fluxes were determined from experimental data:
Statistical analysis
The data compiled were subjected to a simple analysis of variance (ANOVA) at 95% confidence. A two-tail ANOVA was applied to ascertain wheter the cultivar and treatment applied significantly affected the results, and the means were compared by Fisher´s least-significant differences (LSD).
RESULTS
Root biomass and metabolic efficiency
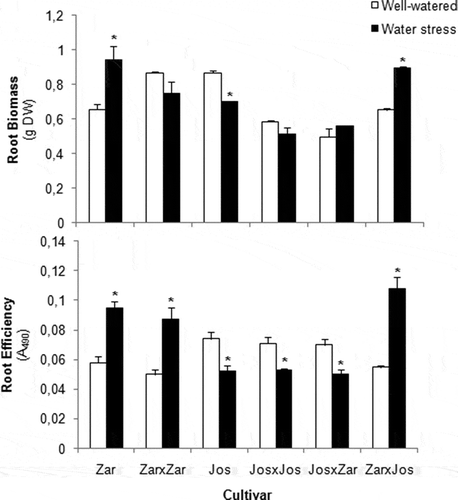
The cv. Zarina augmented its radicular biomass to 28% under stress conditions. In cv. Josefina the root biomass disminished with the water-deficit treatment, while JosxJos was not visibly affected. The reciprocal grafts behaved a different way; the radicular biomass in JosxZar was not affected under stress, and in ZarxJos it increased by 24% (). In the root metabolic efficiency, cv. Zarina and its self-graft increased to 63 and 74% under stress conditions. On the contrary, in cv. Josefina as well as JosxJos, the radicular efficiency declined under water stress (). In the reciprocal grafts, JosxZar decreased to 20% with respect control conditions, whereas ZarxJos showed a rise under water stress ().
Figure 2 Influence of moderate water stress on root biomass and root efficiency in ungrafted, grafted and self-grafted tomato (Solanum lycopersicum) plants. Columns are mean ± SE (n = 9) and differences between means were compared by Fisher’s least-significant difference test (LSD; P = 0.05). Asterisk (*) indicates significant difference with control groups. DW, dry weight.
Mineral nutrient accumulation in leaves
Mineral macronutrient concentrations (N, P, S, Ca, Mg, and K) in cherry tomato leaves for the reciprocal grafting studied are presented in . Both cv. Zarina ungrafted and its self-grafting under water stress registered a 16 and 20% increase with respect to well-watered conditions in N concentration, respectively. On the contrary, cv. Josefina ungrafted and JosxJos lowered the N concentration under water stress. In the reciprocal grafting, only ZarxJos presented an increase of 27% in N concentration under stress conditions. Identical results were found in P concentrations. Meanwhile, water deficit generally lowered (cv. Zarina, ZarxZar, JosxJos and ZarxJos) or maintained (cv. Josefina and JosxZar) the S concentration. No significant increases were detected in the Ca concentration under stress in comparison to well-watered plants. The Mg concentration was maintained in cv. Zarina, cv. Josefina and their self-grafting; however, under water stress, in JosxZar it lowered by 27% and in ZarxJos increased by 41%, with respect to well-watered conditions. Zarina and ZarxJos were the only plants to present a higher K concentration under water deficit, whereas the rest of the cultivars or grafted plants showed no differences or even declining concentrations in this element under water-stress conditions ().
Table 1 Influence of moderate water stress on foliar concentration of macronutrients (mg g−1 dry weight, DW) in ungrafted, grafted and self-grafted tomato (Solanum lycopersicum) plants.
The concentrations of mineral micronutrients (Fe, Cu, Mn, Zn, Cl and B) in leaves are reflected in . For Fe concentration, only cv. Zarina, its self-grafting and ZarxJos showed an increase of 13, 69 and 81% in water stress with respect to well-watered, respectively. Similar results were found for Cu, where cv. Zarina and ZarxJos increased its concentration under water stress. Generally, Mn, Zn and B concentrations were lowered or maintained under water stress in all plants of this experiment, especially in c. Josefina ungrafted. However, in the case of Cl, Zarina was the only cultivar to present a higher concentration under water deficit, whereas JosxJos and JosxZar showed a decline ().
Table 2 Influence of moderate water stress on foliar concentration of micronutrient (µg g−1 dry weight, DW) in ungrafted, grafted and self-grafted tomato (Solanum lycopersicum) plants.
Uptake fluxes in mineral nutrients
The data for macronutrient uptake by the grafted tomato plants studied are presented in . For both N and P, uptake diminished in cv. Josefina and its self-grafting with respect to the control, whereas in leaves of cv. Zarina, ZarxZar and ZarxJos, the transport increased under stress conditions. In the case of S and Ca, generally all cultivars and graftings registered a decline in the uptake fluxes (). Finally, no significant differences in uptake of Mg and K in cv. Zarina, its self-grafting, cv. Josefina and ZarxJos were found under stress conditions, while in the rest of the graftings values sharply fell ().
Table 3 Influence of moderate water stress on uptake fluxes of macronutrients (mg plant−1 day−1) in ungrafted, grafted and self-grafted tomato (Solanum lycopersicum) plants.
For the micronutrients (), cv. Zarina significantly increased in uptake of Fe and Cl under stress, whereas ZarxZar, JosxZar and ZarxJos only showed a increase in Fe uptake fluxes. For the rest of the micronutrients, all the cultivars and graftings registered a general decline in transport under stress with respect to control conditions ().
Table 4 Influence of moderate water stress on uptake fluxes of micronutrients (mg plant−1 day−1) in ungrafted, grafted and self-grafted tomato (Solanum lycopersicum) plants.
DISCUSSION
The foliar N concentrations have been correlated positively with the yield. So, the higher N uptake efficiency of some graft combinations can minimize or even eliminate yield losses owing to marginal soil fertility (Simonne et al. Citation2010). However, under water-stress conditions, it has been shown that N uptake diminishes in soy (Glycine max) and rice (Oryza sativa) plants (Tanguilig et al. Citation1987) and wheat (Triticum sp) (Hu et al. Citation2006). Agreeing with these results, our data reflect a decline in the N concentration associated with the worst uptake under stress conditions only in cv. Josefina susceptible and its self-grafting ( and ). On the other hand, our results show a higher N concentration and uptake in cv. Zarina tolerant, its self-grafting and ZarxJos under water stress ( and ). In a previous work, we showed that cv. Zarina and ZarxJos presented greater biomass and RGR associated with high leaf relative water content (LRWC) under water deficit conditions, indicating that these cultivars are more tolerant to this growth situation (Sánchez-Rodríguez et al. Citation2011b). These results agree with those of Ruiz et al. (Citation1997), who tested the effects of two different rootstocks on the leaf macronutrient contents of melon plants and concluded that, in general, N was influenced more by the rootstock genotype than by the scion. In addition to the rootstock-scion interaction, the N content depends on the environmental conditions in which plants develop.
P uptake can be reduced by grafting, depending mainly on the genotype of the rootstock (Kawaguchi et al. Citation2008). We found that P concentration and its uptake diminished in cv. Josefina and JosxJos under stress conditions. The low N and P concentrations observed could be ascribed to the smaller root system (). In contrast, higher P concentrations in the leaves of grafted plants, or higher translocation rates from root to shoot, in comparation with non-grafted plants were reported by Leonardi and Giuffrida (Citation2006) for grafted eggplant. Similar results were found for cucumber, watermelon (Citrullus lanatus) and grafted melon (Cucumis melo) (Rouphael et al. Citation2008; Colla et al. Citation2010; Salehi et al. Citation2010). We found higher P concentration and its uptake flux ( and ) under water stress in cv. Zarina, ZarxZar and ZarxJos. Due to the low mobility of P, a more vigorous root system in these plants () could increase active P uptake by the plants. P nutritional status is very critical for photosynthesis; C partitioning and energy transfer of plants and the energy required for ion uptake is supplied by an energy rich coenzyme, principally adenosine triphosphate (ATP) (Maathuis Citation2009).
So, increases in N and P concentrations and uptake have been considered a tolerance mechanism to salinity stress in potato (Solanum tuberosum) and strawberry (Fragaria moschata) plants (Khalifa et al. Citation2000; Kaya et al. Citation2001) and in tomato plants under water stress (Sánchez-Rodríguez et al. Citation2010b). In our work, the increased concentration and uptake of N and S could be considered as a tolerance mechanism too, as well as the fact that the use of cv. Zarina as rootstocks improves growth of cv. Josefina in grafted plants ZarxJos (Sánchez-Rodríguez et al. Citation2011a).
S intervenes in the production of glutation and forms part of the sulpholipids that are essential for stabilization of photosynthetic compounds (Maathuis Citation2009). In our work, all cultivars and grafting plants showed a lower S concentration and uptake under stress conditions with respect to well-watered plants ( and ), which was probably due to the ion antagonism between H2PO4– and SO42– in the nutrient solutions (Guo et al. Citation2004).
Enhanced Ca uptake due to grafting and higher Ca translocation rates is important in fruiting Solanaceae (Savvas et al. Citation2010). Fernández-García et al. (Citation2004c) found a significant increase in leaf Ca concentrations when the tomato cultivars “Fanny” and “Goldmar” were grafted onto the tomato rootstock hybrid “AR-9704”. Similarly, Leonardi and Giuffrida (Citation2006) found a significant increase in the leaf Ca concentrations of tomato and eggplant grafted plants. Besides, the impact of grafting on Mg uptake depends largely on the rootstock genotype. Some tomato rootstocks such as “He-Man” may decrease the leaf Mg concentration (Savvas et al. Citation2009). However, other rootstocks seem to increase significantly the Mg uptake in grafted eggplants and mini-watermelon (Leonardi and Giuffrida Citation2006; Rouphael et al. Citation2008). Our results showed no significant differences in Ca and Mg concentrations in all cultivars and grafted plants, except JosxZar and ZarxJos with a decrease and an increase in Mg concentration, respectively ( and ). These results agree with those of Colla et al. (Citation2010), who observed no differences in Ca content, but the concentration of Mg in leaves was influenced significantly by the grafting combination in grafted watermelon.
Enhancement of K uptake due to grafting has been also reported by some authors, specifically Leonardi and Giuffrida (Citation2006) for eggplants, Qi et al. (Citation2006) for grafted melon, and Rouphael et al. (Citation2008) for mini-watermelon. Our data show an increase in the K concentration under stress conditions only in cv. Zarina and ZarxJos (). Several authors hold that a greater K accumulation improves stomatal resistance, benefiting drought tolerance (Sinha Citation1978; Kafkafi and Xu Citation1999), which could explain why cv. Zarina is more tolerant to water stress than cv. Josefina. The use of cv. Zarina and rootstock in grafted plants ZarxJos increases the leaf K concentration, which could facilitate the adjustment of water stress in these plants.
Micronutrients are essential for plant growth and they are involved in virtually all metabolic and cellular functions, such as energy metabolism, primary and secondary metabolism, cell protection, gene regulation, hormone perception, signal transduction and reproduction (Hansch and Mendel Citation2009). In most cases, grafting of fruit vegetables either decreases or has no effect on the uptake of micronutrients (Edelstein et al. Citation2005; Rouphael et al. Citation2008; Savvas et al. Citation2009). However, some rootstocks increase the efficiency of grafted plants to take up and translocate Fe and/or Mn to the shoot, in comparison with the corresponding self-rooted cultivars (Rivero et al. Citation2004; Colla et al. Citation2010; Huang et al. Citation2010). Our data in relation to micronutrient concentrations and uptake fluxes in leaves showed in general no significant differences or decrease under water stress, except in the case of Fe, Cu and Cl, which increased in the cv. Zarina, and only Fe and Cu in ZarxJos ( and ). Cu and Fe participate to prevent oxidative stress binding of proteins and enzymes (Hansch and Mendel Citation2009), and this would coincide with the results reported in previous works (Sánchez-Rodríguez et al. Citation2011b), demonstrating that cv. Zarina and ZarxJos present more vigorous enzymatic antioxidant activity. In turn, a higher Cl concentration could be related to stronger stomatal resistance together with K in these same cultivars.
CONCLUSION
The use of drought-tolerant cv. Zarina as rootstocks (ZarxJos) showed improve ionome, with increases in N, P and K concentration and uptake fluxes, and an increase in Fe and Cu concentration and uptake under water stress. Besides, the vigorous root system of rootstocks is often capable of absorbing plant nutrients more efficiently than scion root, and we have shown that cv. Zarina and ZarxJos develop a better radicular system. This result confirms the hypothesis that grafted plants on vigorous rootstocks can improve mineral nutrition and nutrient uptake with respect to ungrafted plants, especially under water stress conditions.
ACKNOWLEDGMENTS
This work was financed by the PAI programme (Plan Andaluz de Investigación, Grupo de Investigación AGR161) and by a grant from the Formacion Profesor Universitario (FPU) of the Ministerio de Educación y Ciencia awarded to ESR.
REFERENCES
- Abdelmageed AHA, Gruda N 2009: Influence of grafting on growth, development and some physiological parameters of tomatoes under controlled heat stress conditions. Eur. J. Hortic. Sci., 74, 16–20.
- Ahn SJ, Im YJ, Chung GC, Cho BH, Suh SR 1999: Physiological responses of grafted-cucumber leaves and rootstock roots as affected by low root temperature. Sci. Hortic., 81, 397–408.
- Bellaloui N, Brown PH 1998: Cultivar differences in boron uptake and distribution in celery (Apium graveolens), tomato (Lycopersicon esculetum) and wheat (Triticum aestivum). Plant Soil, 198, 153–158.
- Bletsos F, Thanassoulopoulos C, Roupakias D 2003: Effect of grafting on growth, yield and Verticillium wilt of eggplant. HortScience, 38, 183–186.
- Cataldo DA, Haroon M, Schrader LE, Young VL 1975: Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Comm. Soil Sci. Plant Anal., 6, 71–80.
- Colla G, Rouphael Y, Cardarelli M, Massa D, Salerno A, Rea E 2006: Yield, fruit quality and mineral composition of grafted melon plants grown under saline conditions. J. Hortic. Sci. Biotechnol., 81, 146–152.
- Colla G, Suãrez CMC, Carderelli M, Rouphael Y 2010: Improving nitrogen use efficiency in melon by grafting. HortScience, 45, 559–565.
- Davis AR, Perkins-Veazie P, Sakata Y et al. 2008: Cucurbit grafting. Crit. Rev. Plant Sci., 27, 50–74.
- Diatloff E, Rengel Z 2001: Compilation of simple spectrophotometric techniques for the determination of elements in nutrient solutions. J. Plant Nutr., 24, 75–86.
- Edelstein M, Ben-Hur M, Cohen R, Burger Y, Ravina I 2005: Boron and salinity effects on grafted and non-grafted melon plants. Plant Soil, 269, 273–284.
- Estañ MT, Martinez-Rodriguez MM, Pérez-Alfocea F, Flowers TJ, Bolarin MC 2005: Grafting raises the salt tolerance of tomato through limiting the transport of sodium and chloride to the shoot. J. Exp. Bot., 56, 703–712.
- Fernández-García N, Carvajal M, Olmos E 2004a: Graft union formation in tomato plants. Peroxidase and catalase involvement. Ann. Bot., 93, 53–60.
- Fernández-García N, Martínez V, Carvajal M 2004c: Effect of salinity on growth, mineral composition, and water relations of grafted tomato plants. J. Plant Nutr. Soil Sci., 167, 616–622.
- Fernández-García N, Martínez V, Cerdá A, Carvajal M 2004b: Fruit quality of grafted tomato plants grown under saline conditions. J. Hortic. Sci. Biotechnol., 79, 995–1001.
- Guo WZ, Liu SF, Li DR, Zhao SS 2004: Mechanisms of soil salinization in protected cultivation. Soils, 36, 25–29.
- Hansch R, Mendel RR 2009: Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni Mo, B, Cl. Curr. Opin. Plant Biol, 12, 259–266.
- Heo YC 1991: Effects of rootstocks on exudation and mineral elements contents in different parts of Oriental melon and cucumber. MS thesis, Kyung Hee University, Seoul, South Korea, p. 53.
- Hocking PJ, Pate JS 1977: Mobilization of minerals to developing seeds of legumes. Ann. Bot., 41, 1259–1278.
- Hogue E, Wilcow GE, Cantliffe DJ 1970: Effect of soil P on phosphate fraction in tomato leaves. JASHS, 95, 174–176.
- Hu Y, Burucs Z, Schmidhalter U 2006: Short-term effect of drought and salinity on growth and mineral elements in wheat seedlings. J. Plant Nutr., 29, 2227–2243.
- Huang Y, Bie Z, He S, Hua B, Zhen A, Liu Z 2010: Improving cucumber tolerance to major nutrients induced salinity by grafting onto Cucurbita ficifolia. Environ. Exp. Bot., 69, 32–38.
- Johkan M, Mitukuri K, Yamasaki S, Mori G, Oda M 2009: Causes of defolation and low survival rate of grafted sweet pepper plants. Sci. Hortic., 119, 103–107.
- Kafkafi U, Xu GH 1999: Potassium nutrition for high crop yields. In Frontiers in Potassium Nutrition, Eds. Oosterhuis DM and Berkowitz GA, pp. 133–141. Potash and Phosphate Institute, Norcross, GA.
- Kawaguchi M, Taji A, Backhouse D, Oda M 2008: Anatomy and physiology of graft incompatibity in solanaceous plants. J. Hortic. Sci. Biotechnol., 83, 581–588.
- Kaya C, Kirnak H, Higgs D 2001: An experiment to investigate the ameliorative effects of foliar potassium phosphate sprays on salt-stressed strawberry plants. Aust. J. Agric. Res., 52, 995–1000.
- Khalifa A, Barthakur NN, Donnelly DJ 2000: Phosphorous reduces salinity stress in micropropagated potato. Am. J. Potato Res., 77, 179–182.
- Kramer PJ, Boyer JS 1995: Water relations of plants and soils, pp. 495–524. Academic Press, San Diego.
- Krom MD 1980: Spectrophotometric determination of ammonia: study of a modified berthelot reaction using salicylate and dichloroisocyanurate. Analyst, 105, 305–316.
- Kruse J, Kopriva S, Hänsch R, Krauss GJ, Mendel RR, Rennenberg H 2007: Interaction of sulfur and nitrogen nutrition in tobacco (Nicotiana tabacum) plants; significance of nitrogen source and root nitrate reductase. Plant Biol., 9, 638–646.
- Lachica M, Aguilar A, Iañez J 1973: Análisis foliar. Métodos utilizados en la estación experimental del Zaidin. Anales de Edafología y Agrobiología, 32, 1033–1047.
- Leonardi C, Giuffrida F 2006: Variation of plant growth and macronutrient uptake in grafted tomatoes and eggplants on three different rootstocks. Eur. J. Hortic. Sci., 71, 97–101.
- Maathuis FJM 2009: Physiological functions of mineral macronutrients. Curr. Opin. Plant Biol., 12, 250–258.
- Novozamsky I, Vaneck R 1977: Total sulfur determination in plant material. Fresenius' Zeitschrift für Analytische Chemie, 286, 367–368.
- Oda M, Maruyama M, Mori G 2005: Water transfer at graft union of tomato plants grafted onto Solanum rootstocks. J. Jpn. Soc. Hortic. Sci., 74, 458–463.
- Qi HY, Liu YF, Li D, Li TL 2006: Effects of grafting on nutrient absorption, hormone content in xylem exudation and yield of melon (Cucumis melo L.). Plant Physiol. Commun., 42, 199–202.
- Rivero RM, Ruiz JM, Romero L 2003a: Can grafting in tomato plants strengthen resistance to thermal stress? J. Sci. Food Agric., 83, 1315–1319.
- Rivero RM, Ruiz JM, Romero L 2004: Iron metabolism in tomato and watermelon plants: influence of grafting. J. Plant Nutr., 27, 2221–2234.
- Rivero RM, Ruiz JM, Sánchez E, Romero L 2003b: Does grafting provide tomato plants an advantage against H2O2 production under conditions of thermal shock? Physiol. Plant., 117, 44–50.
- Rouphael Y, Cardarelli M, Colla G, Rea E 2008: Yield, mineral composition, water relations, and water use efficiency of grafted mini-watermelon plants under deficit irrigation. HortScience., 43, 730–736.
- Ruiz JM, Belakbir A, López-Cantarero I, Romero L 1997: Leaf-macronutrient content and yield in grafted melon plants: a model to evaluate the influence of rootstock genotype. Sci. Hortic., 71, 227–234.
- Salehi R, Kashi A, Lee JM, Babalar M, Delshad M, Lee SG, Huh YC 2010: Leaf gas exchanges and mineral ion composition in xylem sap of Iranian melon affected by rootstocks and training methods. HortScience, 45, 766–770.
- Salt DE, Baxter I, Lahner B 2008: Ionomics and the study of the plant ionome. Annu. Rev. Plant Biol., 59, 709–733.
- Sánchez-Rodríguez E, Rubio-Wilhelmi MM, Blasco B, Leyva R, Romero L, Ruiz JM 2011b: Antioxidant response resides in the shoot in reciprocal grafts of drought-tolerant and drought-sensitive cultivars in tomato under water stress. Plant Sci., 188, 89–96.
- Sánchez-Rodríguez E, Rubio-Wilhelmi MM, Cervilla LM, Blasco B, Rios JJ, Leyva R, Romero L, Ruiz JM 2010b: Study of the ionome and uptake fluxes in cherry tomato plants under moderate water stress conditions. Plant Soil, 335, 339–347.
- Sánchez-Rodríguez E, Rubio-Wilhelmi MM, Cervilla LM, Blasco B, Rios JJ, Rosales MA, Romero L, Ruiz JM 2010a: Genotypic differences in some physiological parameters symptomatic for oxidative stress under moderate drought in tomato plants. Plant Sci., 178, 30–40.
- Sánchez-Rodríguez E, Ruiz JM, Ferreres F, Moreno DA 2011a: Phenolic metabolism in grafted versus nongrafted cherry tomatoes under the influence of water stress. J. Agric. Food Chem., 59, 8839–8846.
- Savvas D, Colla G, Rouphael Y, Schwarz D 2010: Amelioration of heavy metal and nutrient stress in fruit vegetables by grafting. Sci. Hortic., 127, 156–161.
- Savvas D, Papastavrou D, Ntatsi G, Ropokis A, Olympios C, Hartmann H, Schwarz D 2009: Interactive effects of grafting and Mn-supply level on growth, yield and nutrient uptake by tomato. HortScience, 44, 1978–1982.
- Seki T, Kawashima K, Shibata K, Inoue H, Umemiya Y 2008: Translocation of nutrients in Japanese pear 'Kosui' grafted by the 'Tree Joint' method. Acta Hortic., 800, 273–279.
- Simonne E, Hutchinson C, DeValerio J et al. 2010: Current knowledge, gaps, and future needs for keeping water and nutrients in the root zone of vegetables grown in Florida. HortTechnology, 20, 143–152.
- Sinha SK 1978: Influence of potassium on tolerance to stress. In Potassium in Soils and Crops, Ed. Sekhon GS, p. 223. Potash Res. Institute, New Delhi.
- Steponkus PL 1971: Effect of frezzing on dehydrogenase activity and reduction of triphenyltetrazolium chloride. Cryobiology, 8, 570–573.
- Tagliavani M, Bassi D, Marangoni B 1993: Growth and mineral nutrition of pear rootstocks in lime soils. Sci. Hortic., 54, 13–22.
- Tanguilig VC, Yambao EB, Toole JCO, DeDatta SK 1987: Water stress effects on leaf elongation, leaf water potential, transpiration, and nutrient uptake of rice, maize and soybean. Plant Soil, 103, 155–159.
- Venema JH, Dijk BE, Bax JM, van Haselt PR, Elzenga JTM 2008: Grafting tomato (Solanum lycopersicum) onto the rootstock of a high-altitude accession of Solamun habrochaites improves suboptimal-temperature tolerance. Environ. Exp. Bot., 63, 359–367.
- Viets Jr FG 1972: Water deficits and nutrient availability. In Water Deficits and Plant Growth. Vol. III: Plant Responses and Control of Water balance, Ed. Kozlowski TT, pp. 217–240. Academic Press, New York.
- Wolf B 1974: Improvement in the Azometine-H method for determination of boron, Comm. Soil Sci. Plant Analysis, 5, 39–44.
- Wolf B 1982: A comprehensive system of leaf analysis and its use for diagnosing crop nutrients status. Comm. Soil Sci. Plant Analysis, 13, 1035–1059.