Abstract
Boron (B) cross-links the pectin polysaccharide rhamnogalacturonan II (RG-II) and thus is important for cell wall structure in plants. B deficiency is an agricultural problem that causes significant losses of crop productivity worldwide. To address this, B deficiency-tolerant plants have been generated using B transporters. With the goal of further improving plant tolerance to low-B conditions, we generated transgenic Arabidopsis thaliana (L.) Heynh. with enhanced expression of BOR2, a B transporter that promotes cross-linking of RG-II and root elongation under low B supply. We generated a DNA construct containing the cauliflower mosaic virus 35S RNA promoter, a native promoter of BOR2, a BOR2 gene and green fluorescent protein (GFP) (Pro35S-BOR2:BOR2-GFP), and obtained three independent transgenic lines with relatively high levels of BOR2-GFP expression. In the transgenic lines, BOR2-GFP was expressed mainly in the lateral root caps in the meristem zone and in the epidermal cells in the elongation zone, similar to its expression when driven only by its native promoter. In the Pro35S-BOR2:BOR2-GFP lines, BOR2-GFP was also expressed in various cells in the maturation zone of roots and epidermal cells of shoots, where the expression was hardly detectable in ProBOR2:BOR2-GFP lines. The transgenic lines were cultured under various B concentrations on solid media and it was found that root growth of three lines and shoot growth of two lines were enhanced compared to wild-type plants under low-B conditions. This finding established that enhanced expression of BOR2 leads to improved root growth under low-B conditions. We also examined growth of the transgenic lines under hydroponic conditions. One of the three lines showed better growth and fertility under a low-B condition, while the wild type did not set seeds under the same condition, suggesting the potential utility of BOR2 expression in agricultural applications.
INTRODUCTION
Boron (B) is an essential element for higher plants (Marschner Citation1995). B, as borate, cross-links the pectic polysaccharide rhamnogalacturonan II (RG-II) and is important for the structure of plant cell walls (Ishii and Matsunaga Citation1996; Kobayashi et al. Citation1996; O’Neill et al. Citation1996). B deficiency is a widespread agricultural problem reported in over 80 countries and in 132 crop plants (Shorrocks Citation1997). In general, B deficiency first appears in rapidly growing tissues and causes cessation of growth (Dell and Huang Citation1997). Even at concentrations that do not affect vegetative growth, B deficiency often causes sterility, suggesting that the requirement for B is higher for reproductive growth. In contrast, excess accumulation of B is toxic to plants, causing necrosis of leaves and inhibition of root elongation (Nable et al. Citation1997). B toxicity is also an agricultural problem, especially in semi-arid areas. The range of concentration between B deficiency and toxicity is narrow and, thus, careful management is needed for the application of B fertilizers (Marschner Citation1995), and crop plants with high B-use efficiency are required.
In Arabidopsis thaliana (L.) Heynh., two types of transport protein are responsible for B transport under B limitation. The boric acid channel NIP5;1, a major intrinsic protein (MIP, the aquaporin family), functions as a boric acid importer for B uptake from soil into roots under low-B conditions (Takano et al. Citation2006). NIP6;1, the most similar protein to NIP5;1, also functions as a boric acid channel and is required for efficient B distribution to young leaves (Tanaka et al. Citation2008). The boric acid/borate exporter, BOR1, is required for efficient translocation of B into xylem in roots and for preferential translocation of B into young growing parts of shoots under low-B conditions (Takano et al. Citation2001, Citation2002). A. thaliana has six paralogs of BOR1 protein that play different roles in B transport (Miwa et al. Citation2005). Among them, BOR4 is involved in B-toxicity tolerance by excluding boric acid/borate from tissues; overexpression of BOR4 conferred high-B tolerance to A. thaliana (Miwa et al. Citation2007; Miwa and Fujiwara Citation2011). BOR2 is the closest homolog to BOR1, and analysis of its loss-of-function mutants revealed that BOR2 is required for root elongation under low-B conditions (Miwa et al. Citation2013). BOR2 is also a boric acid/borate exporter localized to plasma membranes and provides boric acid/borate from the cytosol to the cell wall or into secretion vesicles for efficient cross-linking of RG-II under low-B conditions (Miwa et al. Citation2013).
Modulation of transporters is an attractive approach for generating plants with improved nutrient use efficiency. To generate plants that are tolerant to B deficiency, the B exporter BOR1 was expressed under the control of the cauliflower mosaic virus (CaMV) 35S RNA promoter (35S promoter) in A. thaliana (Miwa et al. Citation2006). Overexpression of BOR1 improved shoot growth and fertility by enhancing B transport toward shoots under low-B conditions. In addition, enhanced expression of NIP5;1 by inserting the 35S promoter 1357 bp upstream of the NIP5;1 gene was reported to improve root growth under low B supply (Kato et al. Citation2009). Kato et al. (Citation2009) also demonstrated that the combination of enhanced expression of BOR1 and NIP5;1 can confer higher tolerance to B deficiency.
Here, we attempted to generate B deficiency-tolerant plants by enhancing expression of BOR2. We constructed an artificial gene consisted of the 35S promoter, the promoter region of the BOR2 gene, the BOR2 gene (exon and intron) and green fluorescent protein (GFP). Introduction of this construct into Arabidopsis plants led to expression of BOR2-GFP in roots at relatively high levels. We found growth improvement in roots, at a minimum, in the three independent lines in solid media under B-limited conditions.
MATERIALS AND METHODS
Plant material and plasmid constructions
Arabidopsis thaliana (L.) Heynh. ecotype Col-0 was used from our laboratory stocks. The ProBOR2:BOR2-GFP transgenic plant was described (Miwa et al. Citation2013). Pro35S-BOR2:BOR2-GFP was constructed as follows. The genomic fragment containing the 2805-bp region upstream of the start codon of BOR2 and the BOR2 gene sequence without the stop codon was amplified by polymerase chain reaction (PCR) and subcloned into a pENTRTM/D-TOPO® vector by directional TOPO® cloning (Life Technologies, Carlsbad, CA, USA; Miwa et al. Citation2013). This entry vector was used for Gateway® LR reaction (Life Technologies) with pGWB505 (35S promoter/sGFP; Nakagawa et al. Citation2007). The pGWB505 vector was kindly provided by Dr. Nakagawa (Shimane University). The sequences of the BOR2 exon and gene-GFP junction were confirmed by sequencing.
In planta transformation was performed using the Agrobacterium-mediated floral dip method (Clough and Bent Citation1998).
Plant growth conditions
Plant growth media were prepared according to Takano et al. (Citation2005) and supplemented with various concentrations of boric acid (0.03–100 µM B). For observation of vegetative growth, plants were grown on vertically-placed solid media containing 2% [weight/volume (w/v)] sucrose and 1.5% (w/v) Gellan gum (Wako Pure Chemicals, Osaka, Japan). Surface-sterilized seeds were sown on the solid media and incubated at 4°C for 2 d, and then at 22°C under a 16-h light/8-h dark photoperiod in a growth chamber. For observation of reproductive growth, plants were grown hydroponically as described by Takano et al. (Citation2001), with slight modifications. The seeds were sown on rockwool and incubated at 4°C for 2 d. Hydroponic culture was initiated after seedlings were grown for 2 weeks. Liquid culture media were prepared according to Takano et al. (Citation2005) and supplemented with various concentrations of boric acid; nutrient solution was replaced weekly. The plants were grown at 22°C with constant light.
GFP imaging
For investigation of relative fluorescence intensity, GFP images were obtained by a confocal laser scanning microscope TCS-SP8 equipped with a water-immersed 20 × lens (Leica Microsystems, Wetzlar, Germany) with an excitation wavelength of 488 nm and detection wavelengths of 500–530 nm. The obtained images were shown as color-coded heat maps. For observation of cell-type specificity and intracellular localization of BOR2-GFP, confocal images were taken with a water-immersed 40 × lens (Leica Microsystems).
Preparation of microsomal proteins and immunoblot analysis
Transgenic T3 plants were grown for 14 d on solid media containing 0.3 µM boric acid. All manipulations for preparation of proteins were conducted at 4°C or on ice. Shoot tissues from 10–15 plants were homogenized with 1 mL of homogenization buffer [250 mM tris(hydroxymethyl)amino methane (Tris, pH 8.5), 290 mM sucrose, 25 mM ethylenediaminetetraacetic acid (EDTA), 75 mM 2-mercaptoethanol, 2 mM Pefabloc SC (Roche, Mannheim, Germany), and one tablet/10ml protease inhibitor mixture (Roche cOmplete ULTRA, EDTA-free)] and centrifuged at 10,000 × g for 15 min at 4°C. The resultant supernatant was transferred to new tubes and centrifuged again. The resultant supernatant was centrifuged at 100,000 × g for 30 min at 4°C. The pellet, representing the microsomal fraction, was resuspended in 30 µL of storage buffer [50 mM potassium phosphate (KPi) buffer (pH 6.3), 1 mM magnesium sulfate (MgSO4), 20% glycerol, 2 mM Pefabloc SC, and 1 × protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA)] using a pestle. The protein concentration was estimated by Pierce 660 nm Protein Assay (Thermo Scientific, Rockford, IL, USA). The samples were stored at –30°C.
Microsomes containing 10 µg of protein were added with 4 × NuPAGE® lithium dodecyl sulfate (LDS) Sample Buffer (Life Technologies) with 50 mM dithiothreitol (DTT) and incubated at 70°C for 10 min. The NuPAGE® 4–12% Bis-Tris System (Invitrogen Life Technologies) was used for electrophoresis and Western transfer. The membranes were processed by the SNAP i.d. Protein Detection System as described in the user’s guide (Millipore, Billerica, MA, USA). Blocking was done with 0.25% skim milk in Tris-buffered saline [20 mM Tris-HCl (pH 7.5), 150 mM sodium chloride (NaCl)) with 0.1% Tween 20 surfactant. The anti-GFP mouse monoclonal antibody ab1218 (Abcam, Cambridge, UK) was used at 1:4000 dilution (1.9 µg/mL). Horseradish peroxidase (HRP)-conjugated goat antibody to mouse Immunoglobulin G (IgG) was used at 1:20,000 dilution. The Can Get Signal Immunoreaction Enhancer Solutions 1 and 2 (TOYOBO, Osaka, Japan) were used for dilution of the primary and secondary antibodies, respectively. For detection, Luminata Forte Western HRP substrate (Millipore) was used with an LAS-3000 mini imaging system (Fujifilm, Tokyo, Japan).
RESULTS
Generation of transgenic Arabidopsis plants with enhanced expression of BOR2
Our objective in this study was to enhance BOR2 expression for improvement of plant growth under low-B conditions. For this purpose, we generated a DNA construct that contained the CaMV 35S promoter, the 2805-bp region upstream of the start codon of BOR2, the BOR2 gene sequence without the stop codon, and GFP (Pro35S-BOR2:BOR2-GFP, ). This construct was introduced into A. thaliana Col-0 plants, and 51 T1 transgenic plants were selected by resistance to hygromycin B. The hygromycin-resistant T1 plants were transferred onto solid media supplemented with 0.3 µM B and grown for 4 d. We then observed the GFP signals in roots under epifluorescence microscopy and selected 30 plants with relatively strong GFP intensities. In the T2 progenies of the 30 T1 plants, segregation of hygromycin resistance was examined and 18 lines that presumably carried a single T-DNA insertion were selected (Table S1). As a preliminary growth test, we grew plants of these 18 T2 lines on solid media supplemented with a low concentration of B (0.03 µM B; Fig. S1). Among these 18 lines, two (Lines 13 and 16) demonstrated better root growth; one line (Line 5) showed better shoot growth; and 5 lines (Lines 1, 2, 9, 14, 17) exhibited better growth of both roots and shoots. Growth of roots and shoots was poorer in Line 22 than in the wild type in seven of the eight plants. This was probably due to gene silencing of BOR2-GFP and to endogenous BOR2, because the GFP signal was barely detectable in these plants. These data suggested that enhanced expression of BOR2 improved plant growths under low B supply. To verify this inference by detailed analysis, we further selected three lines (Lines 1, 6, 9) with a single T-DNA insertion site (Table S2) and with relatively high levels of BOR2-GFP expression in roots. shows imaging of BOR2-GFP in the three homozygous T3 lines at the same laser power and gain by confocal microscopy. These three lines exhibited stronger fluorescence than the ProBOR2:BOR2-GFP lines in roots.
Figure 1 Expression levels and localization of transgenic lines as indicated by green fluorescent protein (GFP) fluorescence. (A) Schematic representation of the DNA construct, which contains the CaMV 35S RNA promoter (Pro35S), the 2805-bp region upstream of the start codon (ATG) of BOR2, the BOR2 gene sequence lacking a stop codon, GFP with a stop codon (TAA), and the nopaline synthase terminator (t-NOS). (B-D) Transgenic plants carrying the Pro35S-BOR2:BOR2-GFP or ProBOR2:BOR2-GFP constructs were grown for 5 d on solid media containing 1 µM boric acid. (B) Intensities of GFP fluorescence in roots are shown as color-coded heat maps. Upper panels show the meristem zone. Lower panels show the maturation zone. Scale bars indicate 75 µm. (C) Localization of GFP fluorescence in roots. Upper panels show the meristem zone. Lower panels show the maturation zone. (D) Localization of GFP fluorescence in epidermal cells of rosette leaves. Left upper panel shows background signal from the wild-type plants. Scale bars indicate 25 µm. (C, D).
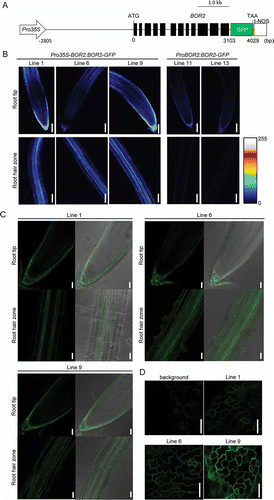
We further analyzed the cell-type specificity and localization of BOR2-GFP in the roots of three Pro35S-BOR2:BOR2-GFP lines (). BOR2-GFP was observed in the periphery of all cells that showed GFP signals, suggesting localization of the protein in the plasma membrane (). In the three Pro35S-BOR2:BOR2-GFP lines, BOR2-GFP was expressed strongly in lateral root-cap cells and less strongly in epidermal, cortical, endodermal and protovascular cells in the meristem zone ( and ). Expression of BOR2-GFP was negligible in the transition zone but was relatively strong in epidermal cells in the elongation zone (). These patterns of expression were similar to those of plants carrying ProBOR2:BOR2-GFP (Miwa et al. Citation2013), indicating that expression of BOR2-GFP was enhanced in cells in which BOR2 was originally expressed. In the root-hair zone of Pro35S-BOR2:BOR2-GFP lines, BOR2-GFP was expressed in various cell layers, including the epidermis, cortex and endodermis ( and ). In the three T3 lines, BOR2-GFP was localized preferentially in the inner (proximal) plasma-membrane domain of the endodermis, similar to BOR1-GFP (Takano et al. Citation2010). In the root-hair zone, BOR2-GFP controlled by the native promoter alone was scarcely detectable (), and BOR2-GUS was detected in the cortex (Miwa et al. Citation2013). Therefore, the cell-type specificity of BOR2-GFP expression in the root-hair zone might be altered from that of wild-type BOR2 by using the Pro35S-BOR2 chimeric promoter. We then analyzed accumulation and localization of BOR2-GFP in shoots. A western blotting analysis using an anti-GFP antibody detected BOR2-GFP in the three Pro35S-BOR2:BOR2-GFP lines at different levels whereas it was hardly detectable in ProBOR2:BOR2-GFP lines (Fig. S2). In epidermal cells of rosette leaves, BOR2-GFP was localized in the plasma membrane in the three Pro35S-BOR2:BOR2-GFP lines (). These results suggest that the Pro35S-BOR2 chimeric promoter drove strong expression in shoots where BOR2 is not or little expressed in the wild-type plants.
Enhanced expression of BOR2 affects plant growth under low B supply
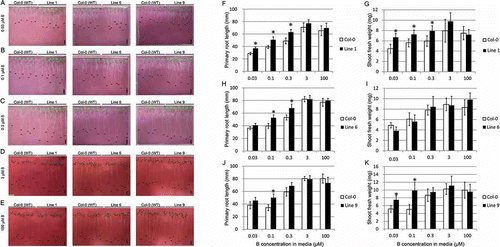
To determine whether enhanced expression of BOR2 would improve plant growth under low B supply, we grew the three Pro35S-BOR2:BOR2-GFP lines on solid media under low- to sufficient-B conditions (0.03–100 µM B) (). Line 1 showed better growth of roots and shoots under low-B conditions (0.03–0.3 µM B) (–). The primary root lengths were 1.3-, 1.3-, and 1.3-fold longer than those of wild-type Col-0 plants at 0.03, 0.1 and 0.3 µM B supply, respectively (); shoot fresh weights were 1.5-, 1.3- and 1.3-fold larger than those of wild-type Col-0 plants at 0.03, 0.1 and 0.3 µM B supply, respectively (). The differences in root length and shoot fresh weight were statistically significant (Student’s t-test, p < 0.01). Line 6 also tended to show better root growth under low-B conditions (–, , ). Root lengths in Line-6 plants were 1.1-, 1.3- and 1.3-fold greater than those of wild-type Col-0 plants at 0.03, 0.1 and 0.3 µM B supply, respectively (); these differences were significant at 0.1 and 0.3 µM B. Line 9 exhibited better growth of roots and shoots than wild-type Col-0 under low-B conditions (–, , ). Root length in Line 9 was 1.2-, 1.5- and 1.2-fold greater than that of wild-type Col-0 at 0.03, 0.1 and 0.3 µM B supply, respectively (); shoot fresh weights were 1.5-, 2.0-, 1.1- and 1.1-fold larger than those of wild-type Col-0 plants at 0.03, 0.1, 0.3 and 3 µM B supply, respectively (). Differences between Line-9 and wild-type plants were statistically significant for root length at 0.1 µM B and for shoot fresh weight at 0.03 and 0.1 µM B.
Figure 2 Vegetative growth of transgenic lines on solid media. Transgenic T3 plants were grown for 12 d on solid media containing (A) 0.03, (B) 0.1, (C) 0.3, (D) 3, and (E) 100 µM boric acid. The photographs of plants were taken on solid media (A, B, C) or on paper after transfer from solid media (D, E). Scale bars indicate 10 mm. Primary root length (F, H, J) and shoot fresh weight (G, I, K) were measured. Means ± standard deviation (SD) are shown. Student’s t-test, *p < 0.01 (n = 12–35). B, boron.
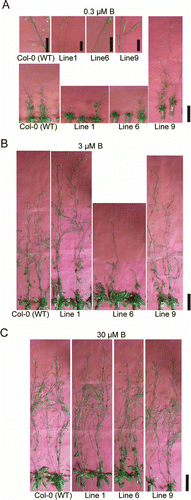
We then investigated the effects of high BOR2 expression on reproductive growth in hydroponic culture supplemented with low- to sufficient-B concentrations (0.3–30 µM B) (). At 0.3 µM B supply, wild-type plants did not set seeds (, S3A and B), which is a typical symptom of B deficiency in A. thaliana (Miwa et al. Citation2006; Kato et al. Citation2009). Given 0.3 µM B, growth of Line-9 plants was apparently better than that of wild-type plants and fertility was partially recovered, while growth of Lines 1 and 6 was apparently reduced compared to that of the wild-type (, S3A and B). At 3 µM B supply, Lines 1 and 9 showed similar growth to the wild-type and were fertile, while Line 6 showed apparently reduced growth and was sterile (, S3A and C). When provided 30 µM B, all lines showed similar growth and fertility (, S3A and D). In summary, enhanced expression of BOR2 affected plant reproductive growth only under low-B conditions.
Figure 3 Reproductive growth of transgenic lines in hydroponic culture. Transgenic plants were grown for 49 d in hydroponic cultures supplemented with (A) 0.3, (B) 3, and (C) 30 µM boric acid. Upper panels in (A) show seed pods or flowers. Scale bars indicate 10 and 50 mm in the upper and lower panels, respectively.
DISCUSSION
For sustainable agriculture with low input of fertilizers, it is important to generate crop plants with high nutrient use efficiency. Plants can generally regulate transporters involved in nutrient uptake and translocation in response to various external conditions. Thus, artificial enhancement of transporter expression is an attractive approach, although there are only few successful examples to date. In rice (Oryza sativa L.), overexpression of the iron transporter OsIRT1 by a maize ubiquitin promoter improved tolerance to iron deficiency at the seedling stage, but also caused reduced yield (Lee and An Citation2009). Overexpression of magnesium transporter AtMGT1 using the 35S promoter improved tolerance to magnesium deficiency and aluminum toxicity in Nicotiana benthamiana L. (Deng et al. Citation2006). However, this overexpression reduced plant size under normal conditions. These studies indicate the merit of constitutive overexpression of transporters in nutrient-limited conditions, and the detrimental effects of overexpression under normal conditions. Importantly, overexpression of BOR1 by the 35S promoter improved B-deficiency tolerance without affecting growth under normal-to-high B conditions in Arabidopsis (Miwa et al. Citation2006). We assume that the success obtained by overexpression of BOR1 is largely dependent on the nature of post-translational regulation of the protein. In wild-type plants, BOR1 is expressed in various root cells and is localized in plasma membrane with polarity toward the inner portion of roots (Takano et al. Citation2010). Overexpression of the polarized B exporter in many cell types is likely beneficial for directional transport of B toward root xylem. In addition, under high B supply, BOR1 is transferred from plasma membranes to vacuoles, where it is degraded (Takano et al. Citation2005). This regulation of endocytic degradation can efficiently limit over-transport of B under normal- and high-B conditions. Since BOR2 is also degraded in vacuoles under high-B conditions (Miwa et al. Citation2013), BOR2 could represent another tool for improving plant growth in low-B conditions without negative effects.
In this study, we demonstrated that enhanced expression of BOR2 by the Pro35S-BOR2 chimeric promoter can improve B-deficiency tolerance of Arabidopsis without affecting plant growth under normal conditions, at least during the vegetative growth stage on solid media. Three transgenic lines with relatively high expression of BOR2-GFP tended to show better growth than the wild type under low B supply on solid media ( and ). However, the growth patterns in hydroponic culture differed among the transgenic lines. Lines 1 and 6 showed greater sensitivity to low B supply in the reproductive phase, while Line 9 showed greater tolerance to B deficiency during reproductive growth (). The differences observed in hydroponic culture may be attributable to contrasting patterns of B distribution resulting from different patterns of BOR2 expression. BOR2 is thought to promote cross-linking of RG-II by transport of B from the cytosol to the cell wall or into secretion vesicles and contributes to root cell elongation under low-B conditions (Miwa et al. Citation2013). Enhanced expression of BOR2 in vegetative tissues might lead to increased B utilization for cell elongation in vegetative tissues, and at the same time cause reduced transport of B to inflorescence stems where a large amount of B is required. It should be noted that expression levels of BOR2-GFP in the root-hair zone were much stronger in plants with the Pro35S-BOR2 chimeric promoter than in plants with the BOR2 promoter (). The strong expression in endodermal or stellar cells of BOR2 in Line 9 might increase xylem loading of B and thus confer higher tolerance to B deficiency in reproductive growth, as is the case for BOR1 overexpression by the 35S promoter (Miwa et al. Citation2006). This view is supported by the finding that the localization of BOR2-GFP in the plasma membrane of mature endodermis was polarized toward the side of the stele (inner side of root), where the BOR1 boric acid/borate exporter mediates the loading of boric acid to steler apoplasm (; Alassimone et al. Citation2010; Takano et al. Citation2010). In addition, the strong expression in shoots of Line 9 (, Fig. S2) might result in different B distribution. Because we could not detect BOR2-GFP in shoots of ProBOR2:BOR2-GFP lines (Fig. S2), the level of BOR2 expression in shoots of wild-type plants seems to be low. However, the close paralog BOR1 is significantly expressed in shoot (Takano et al. Citation2005) and has function in distribution of B toward younger leaves and reproductive organs in low B conditions (Noguchi et al. Citation1997; Takano et al. Citation2001). It is possible that BOR2-GFP in shoots of Line 9 behaved similar to BOR1 and supported reproductive growth.
In this study, at least in the root meristem, transition and elongation zones, the chimeric Pro35S-BOR2 promoter enhanced expression of BOR2-GFP several-fold relative to the native BOR2 promoter without changing cell-type specificity ( and ). We thus propose expression of nutrient transporters using 35S-native chimeric promoters as a promising approach to improving nutrient use efficiency. We also propose application of this method to investigation of intracellular localization of proteins whose expression under the control of the native promoter is too low to be analyzed. Utilization of 35S-native chimeric promoters can avoid the disadvantages of the 35S promoter, which often causes undesirably high levels of gene expression.
In conclusion, in light of the results presented here and in our previous study using the NIP5;1 boric acid importer (Kato et al. Citation2009), we suggest that enhanced expression of nutrient transporters using the 35S-native chimeric promoters results in generation of plants with high nutrient use efficiency. In both approaches, the levels of growth enhancement under low-B conditions were different among independent transgenic lines. The expression of GFP-fused transporters is useful for selection of transgenic lines with higher expression of transgenes at desirable cell types, which can lead to higher nutrient use efficiency.
Figure_S3.tif
Download TIFF Image (3.5 MB)Figure S2: BOR2-GFP accumulation in shoots of transgenic lines.
Download TIFF Image (4.4 MB)Figure S1: Vegetative growth of transgenic lines in T2 generation on solid media.
Download TIFF Image (153 MB)Table S2: Segregation of T2 transgenic lines harboring Pro35S-BOR2:BOR2-GFP determined by GFP observation in T3 progenies
Download PDF (29.6 KB)Table S1: Number of T2 transgenic line plants with hygromycin resistance
Download PDF (45.7 KB)ACKNOWLEDGMENTS
The authors greatly appreciate Dr. T. Nakagawa (Shimane University) for providing plasmid vector pGWB505. The authors thank K. Konishi and T. Shimizu for excellent technical assistance. This work was supported in part by the Funding Program for Next Generation World-Leading Researchers (NEXT) program from the Japan Society for the Promotion of Science (JSPS, to J. T.).
REFERENCES
- Alassimone J, Naseer S, Geldner N 2010: A developmental framework for endodermal differentiation and polarity. Proc. Natl. Acad. Sci. USA, 107, 5214–5219.
- Clough SJ, Bent AF 1998: Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J., 16, 735–743.
- Dell B, Huang L 1997: Physiological response of plants to low boron. Plant Soil, 193, 103–120.
- Deng W, Luo K, Li D, Zheng X, Wei X, Smith W, Thammina C, Lu L, Li Y, Pei Y 2006: Overexpression of an Arabidopsis magnesium transport gene, AtMGT1, in Nicotiana benthamiana confers Al tolerance. J. Exp. Bot., 57, 4235–4243.
- Ishii T, Matsunaga T 1996: Isolation and characterization of a boron-rhamnogalacturonan-II complex from cell walls of sugar beet pulp. Carbohydr. Res., 284, 1–9.
- Kato Y, Miwa K, Takano J, Fujiwara WM 2009: Highly boron deficiency-tolerant plants generated by enhanced expression of NIP5;1, a boric acid channel. Plant Cell Physiol., 50, 58–66.
- Kobayashi M, Matoh T, Azuma J 1996: Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol., 110, 1017–1020.
- Lee S, An G 2009: Over-expression of OsIRT1 leads to increased iron and zinc accumulations in rice. Plant Cell Environ., 32, 408–416.
- Marschner H 1995: Nutritional Physiology, In Mineral Nutrition of Higher Plants, 2nd edn., Ed. Marschner H, pp. 379–396. Academic Press, San Diego, CA.
- Miwa K, Fujiwara T 2011: Role of overexpressed BOR4, a boron exporter, in tolerance to high level of boron in shoots. Soil Sci. Plant Nutr., 57, 558–565.
- Miwa K, Takano J, Fujiwara T 2005: Roles of BOR1 paralog in boron transport in Arabidpsis thaliana. In Plant Nutrition for Food Security, Human Health and Environmental Protection, Ed. Chunjian LI, pp. 124–125. Tsinghua University Press, Beijing.
- Miwa K, Takano J, Fujiwara T 2006: Improvement of seed yields under boron-limiting conditions through overexpression of BOR1, a boron transporter for xylem loading, in Arabidopsis thaliana. Plant J., 46, 1084–1091.
- Miwa K, Takano J, Omori H, Seki M, Shinozaki K, Fujiwara T 2007: Plants tolerant of high boron levels. Science, 318, 1417.
- Miwa K, Wakuta S, Takada S, Ide K, Takano J, Naito S, Omori H, Matsunaga T, Fujiwara T 2013: Roles of BOR2, a boron exporter, in crosslinking of rhamnogalacturonan II and root elongation under boron limitation in Arabidopsis thaliana. Plant Physiol., 163, 1699–1709.
- Nable RO, Banuelos GS, Paull JG 1997: Boron toxicity. Plant Soil, 193, 181–198.
- Nakagawa T, Suzuki T, Murata S et al. 2007: Improved Gateway binary vectors: high-performance vectors for creation of fusion constructs in transgenic analysis of plants. Biosci. Biotechnol. Biochem., 71, 2095–2100.
- Noguchi K, Yasumori M, Imai T, Naito S, Matsunaga T, Oda H, Hayashi H, Chino M, Fujiwara T 1997: bor1-1, an Arabidopsis thaliana mutant that requires a high level of boron. Plant Physiol., 115, 901–906.
- O‘Neill MA, Warrenfeltz D, Kates K, Pellerin P, Doco T, Darvill AG, Albersheim P 1996: Rhamnogalacturonan-II, a pectic polysaccharide in the walls of growing plant cell, forms a dimmer that is covalently cross-linked by borate ester. In vitro conditions for the formation and hydrolysis of the dimmer. J. Biol. Chem., 271, 22923–22930.
- Shorrocks V 1997: The occurrence and correction of boron deficiency. Plant Soil, 193, 121–148.
- Takano J, Miwa K, Yuan L, von Wirén N, Fujiwara T 2005: Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proc. Natl. Acad. Sci. USA, 102, 12276–12281.
- Takano J, Noguchi K, Yasumori M, Kobayashi M, Gajdos Z, Miwa K, Hayashi H, Yoneyama T, Fujiwara T 2002: Arabidopsis boron transporter for xylem loading. Nature, 420, 337–340.
- Takano J, Tanaka M, Toyoda A, Miwa K, Kasai K, Fuji K, Onouchi H, Naito S, Fujiwara T 2010: Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proc. Natl. Acad. Sci. USA, 107, 5220–5225.
- Takano J, Wada M, Ludewig U, Schaaf G, von Wirén N, Fujiwara T 2006: The Arabidopsis major intrinsic protein NIP5; 1 is essential for efficient boron uptake and plant development under boron limitation. Plant Cell, 18, 1498–1509.
- Takano J, Yamagmi M, Noguchi K, Hayashi H, Fujiwara T 2001: Preferential translocation of boron to young leaves in Arabidopsis thaliana regulated by the BOR1 gene. Soil Sci. Plant Nutr., 47, 345–357.
- Tanaka M, Wallace IS, Takano J, Roberts DM, Fujiwara T 2008: NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. Plant Cell, 20, 2860–2875.
Table S1 Number of T2 transgenic line plants with hygromycin resistance. Chi-square test for 3:1 segregation of the resistant plant to the sensitive plants was done and p-values are shown.
Table S2 Segregation of T2 transgenic lines harboring Pro35S-BOR2:BOR2-GFP determined by GFP observation in T3 progenies.
